Abstract
The presence of cytoplasmic sialyl glycoproteins is a conspicuous feature in chromophobe renal cell carcinoma (RCC). We compared the immunohistochemical expression of sialyl glycoproteins in chromophobe RCC with that in other types of renal tumors. Formalin-fixed, paraffin-embedded tissues of surgically resected renal tumors (chromophobe RCC, 14 cases [10 cases of classic type and 4 cases of eosinophilic variant]; oncocytoma, 7 cases; and clear cell RCC, 9 cases) and kidneys from immature infants (4 cases) were immunostained with antibodies against sialyl glycoproteins (anti-KL-6 and anti-sialyl MUC1 antibodies). Cytoplasmic expression of KL-6 and sialyl MUC1 was distinctive in the chromophobe RCC and renal oncocytoma cells, and in the intercalated cells in collecting duct epithelia. Apical-surface staining of these sialyl glycoproteins was predominantly observed in clear RCC, in the epithelia of the distal tubule and collecting duct, and in the neonatal renal proximal tubule, but not in those of the adult renal proximal tubule. The above-mentioned observations provide additional evidence for similar phenotypic profiles of chromophobe RCC and renal oncocytoma, and the intercalated cells in collecting ducts and the oncofetal expression of sialyl glycoproteins in clear cell RCC. KL-6 is a potential tumor marker for renal tumors.
Keywords: chromophobe renal cell carcinoma, immunohistochemistry, KL-6, MUC1, renal cell tumor
I. Introduction
The classification of renal cell tumors has expanded markedly to include entities other than the traditional clear- and granular-cell carcinomas [16]. Chromophobe renal cell carcinoma (RCC) is a distinct subtype of renal carcinoma, so designated in 1985 by Thoenes et al. [20] because of its resemblance to chromophobe adenoma experimentally induced in rats. Chromophobe RCC is composed of voluminous, polygonal cells with a highly opaque and finely reticular cytoplasm [20]. In addition to this classic chromophobe RCC, an eosinophilic variant was recognized in which tumor cells are smaller and markedly eosinophilic, and often exhibit a perinuclear halo [21]. Ultrastructurally, chromophobe RCC contains numerous invaginated vesicles within the cytoplasm that resemble those of intercalated cells of collecting ducts [8, 17]. In addition to these conspicuous morphological characteristics, chromophobe RCCs display an intense cytoplasmic reaction with colloidal iron [20]. Lectin histochemistry and immunohistochemistry of chromophobe RCC showed cytoplasmic expression of sialyl glycoproteins, possibly corresponding to its reactivity with colloidal iron [12, 22]. In addition, chromophobe RCCs frequently demonstrate claudin 7 and parvalbumin, which are expressed in the distal tubules and collecting ducts [2]. Based on these morphological, histochemical, and immunohistochemical studies, it has been suggested that chromophobe RCCs are related to renal distal tubules and collecting ducts [23].
Renal oncocytomas are benign renal tumors composed of large neoplastic cells with intensely eosinophilic granular cytoplasm rich in mitochondria. This tumor is thought to be histogenetically related to the intercalated cells of the collecting duct system [25].
Clear RCCs are typically composed of malignant epithelial cells with clear cytoplasm. Clear RCCs share similar immunohistochemical characteristics with the normal renal proximal tubule epithelia [23].
The KL-6 antigen recognized by the KL-6 antibody is a MUC1-bearing sialylated oligosaccharide on the cell membrane [5]. It is known to be a marker for diffuse lung disorders, such as idiopathic interstitial pneumonia [6], and serum concentrations of the KL-6 antigen increase in patients with malignant tumors, especially lung, breast, and pancreatic cancer [5], and hepatocellular carcinoma [3]. Furthermore, KL-6 expression in the distal renal tubules and elevated KL-6 expression in the renal tissues and serum of patients with diabetes mellitus have been reported [18].
This study was undertaken to compare the expression of sialylated glycoproteins in chromophobe RCC with that in other renal tumors by using the antibodies against sialylated glycoproteins: KL-6 antibody and anti-sialyl MUC1 antibody. The distinct cellular expression pattern of KL-6 in chromophobe RCC cells and its potential as a tumor marker for RCC were shown.
II. Materials and Methods
Surgically resected renal tumors (chromophobe RCC, 14 cases [10 cases of classic type and 4 cases of eosinophilic variant]; oncocytoma, 7 cases; and clear RCC, 9 cases) and kidneys obtained from the autopsies of 4 premature infants (aged 21, 21, 22, and 26 weeks) were retrieved from the pathology files of the Department of Laboratory Medicine, Shinshu University Hospital and Aizawa Hospital, Matsumoto, Japan. All tissues had been fixed in formalin, processed, and embedded in paraffin. Histological subtypes of the renal tumors were classified according to the World Health Organization histologic classification [16].
Serial paraffin sections of 3-µm thickness were stained with hematoxylin and eosin for histological examination or following immunohistochemical staining for investigating the expression of sialylated carbohydrate epitope of the MUC1 glycoprotein using KL-6 antibody (Sanko Junyaku, Tokyo, Japan) [4, 5, 7] and anti-sialyl MUC1 antibody (NCL-MUC1, Novocastra Laboratories, Newcastle upon Tyne, UK). NCL-MUC1 antibody (Novocastra Laboratories) recognizes a sialylated carbohydrate epitope of the MUC1 glycoprotein [11]. Details of the antibodies and antigen retrieval method used in this study are summarized in Table 1. Immunohistochemical staining was performed using the immuno-enzyme polymer method (Envision+- HRP kit; Dako, Carpinteria, CA) with 3,3'-diaminobenzidone as the chromogen. Before immunostaining, antigen retrieval was performed using a microwave (600 W) for 25 min with 0.01 M Tris-HCl buffer containing 1 mM EDTA-2Na (pH 8.6) for sialyl MUC1.
Table 1.
Details of the antibodies used in the immunohistochemical evaluation
| Antigen | Clone | Species | Origin | Dilution | Antigen retrieval |
|---|---|---|---|---|---|
| KL-6 | KL-6 | Mouse | Sanko Junyaku Tokyo, Japan | ×100 | Not done |
| Sialyl MUC1 | NCL-MUC1 | Mouse | Novocastra Laboratories Newcastle upon Tyne, UK | ×50 | Microwave 0.01 M TB containing 1 mM EDTA-2Na (pH 8.6) |
Digestion with neuraminidase from Arthrobacter ureafaciens (Nakarai Chemical Co., Tokyo, Japan) was used to detect the presence of N-acetylneuraminic acid (NeuAc) followed by immunostaining with anti KL-6 and anti-sialyl MUC1 antibodies. NeuAc was removed by incubating the tissue sections in a 1 U/mL enzyme solution in 0.05 M phosphate buffer (pH 7.0), overnight at 37°C.
Histologically normal renal tissues were used as internal positive controls. Negative controls were obtained by omitting the primary antibodies.
Evaluation of immunostaining
Grading of the immunoreactivity of the differential markers was estimated semiquantitatively as 0 (negative), 1 (less than one-third of the cells were positive), 2 (one-third to two-thirds of the cells were positive), or 3 (more than two-thirds of the cells were positive). The grading was performed by a single observer (MF). To validate the grading method, all specimens were graded twice, on 2 separate occasions, and no significant intraobserver variation was observed.
This study was approved by the ethics committee of Shinshu University, Japan.
III. Results
Normal adult renal tissues
Immunostaining for KL-6 and sialyl MUC1 showed similar distributions; the proximal tubule epithelia and renal glomeruli were unstained (Fig. 1), whereas the distal tubule epithelia and collecting duct epithelia showed strong staining (Fig. 2). The immunoreaction was confined to the apical surface of the epithelia in a continuous linear manner; however, in one type of cell in the collecting ducts, diffuse, cytoplasmic staining was observed (Fig. 2). The distribution pattern of this particular cell type resembled that of intercalated cells.
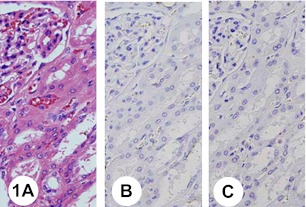
Fig. 1.
Normal renal tissue (cortex). Hematoxylin and eosin staining (H&E) (A). The proximal tubule epithelia and renal glomeruli are negative with anti-KL-6 (B) and anti-sialyl mucin 1 (MUC1) (C) antibodies.
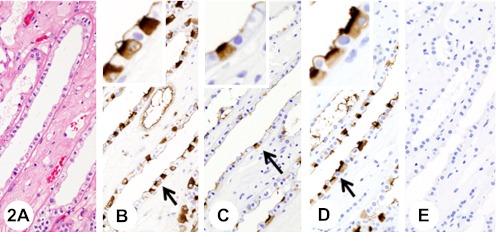
Fig. 2.
Normal renal tissue (medulla). Insets show higher-magnification views of the regions indicated by arrows. H&E (A). The immunoreaction with anti-KL-6 antibody is confined to the apical surface of the epithelia of distal tubules and collecting ducts in a continuous linear manner (B); however, in intercalated cells in the collecting ducts, diffuse staining with anti-KL-6 antibody is observed in the cytoplasm (B). Pretreatment with neuraminidase decreases immunoreactivity with anti-KL-6 antibody (C). The immunoreaction with anti-sialyl MUC1 is confined to the apical surface of the epithelia of distal tubules and collecting ducts in a continuous linear manner (D); however, in intercalated cells in the collecting ducts, diffuse staining with anti-sialyl MUC1 antibody is observed in the cytoplasm (D). Pretreatment with neuraminidase abolishes immunoreactivity with anti-sialyl MUC1 antibody (E).
Immature renal tissues
Immunostaining for KL-6 and sialyl MUC showed reactivity similar to that in the adult kidney, except in neonatal kidneys where strong apical-surface staining was also observed in the epithelia of proximal tubule (Fig. 3).
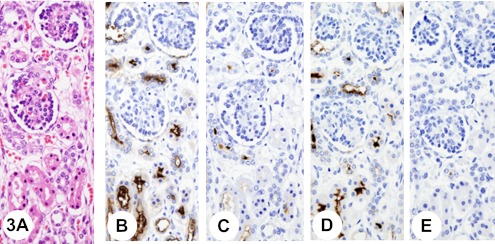
Fig. 3.
Immature kidney. Histology of the renal cortex of a kidney from a 26-week-old infant (A). Immunostaining with anti-KL-6 antibody shows strong apical-surface staining in the proximal tubule epithelia (B). Pretreatment with neuraminidase decreases immunoreactivity with anti-KL-6 antibody (C). Immunostaining with anti-sialyl MUC1 antibody shows strong apical-surface staining in the proximal tubule epithelia (D). Pretreatment with neuraminidase abolishes immunoreactivity with anti-sialyl MUC1 antibody (E).
Renal tumors
Differences in cellular staining patterns for KL-6 and sialyl MUC1 were found among the histological subtypes. The results of the immunohistochemical staining of the histological subtypes are summarized in Table 2.
Table 2.
Immunoreactivity and effect of neuraminidase treatment for KL-6 and sialyl-MUC1
| Tumor type | Makers | ||||
|---|---|---|---|---|---|
| KL-6 | Sialyl MUC1 | ||||
| Before neuraminidase treatment | After neuraminidase treatment | Before neuraminidase treatment | After neuraminidase treatment | ||
| Chromophobe renal cell carcinoma classic type (n=10) | 100a3 (3–3)b | 1001 (1–2) | 1002 (2.5–3) | 100 (0–0) | |
| Chromophobe renal cell carcinoma eosinophilic variant (n=4) | 1003 (2–3) | 1002 (1–3) | 1002 (1.5–2.5) | 250 (0–0.5) | |
| Oncocytoma (n=7) | 1003 (2.5–3) | 1002 (1.25–2) | 1003 (2.25–3) | 28.60 (0–0.75) | |
| Clear renal cell carcinoma (n=9) | 1003 (2.5–3) | 55.61 (0–1) | 1001 (1–2) | 00 (0–0) | |
a Frequency (%) of positive specimens.
b Median score with the interquartile range in parenthesis.
Chromophobe RCC
The classic chromophobe RCC was mainly composed of voluminous, polygonal cells with a highly opaque and finely reticular cytoplasm. These cells were mixed with several smaller cells with granular eosinophilic cytoplasm and a central round to oval or irregular nucleus with a perinuclear halo. They were arranged in compact nests having various degrees of tubules (Fig. 4A). The tumor cells often predominantly showed diffuse fine granular cytoplasmic staining with anti-KL-6 (Fig. 4B) and anti-sialyl MUC1 (Fig. 4D) antibodies. In addition, immunostaining was observed at the luminal surface of tubules (Fig. 4B and 4D). Single positive cells or positive cells in small groups were distributed unevenly throughout the tumor tissues.
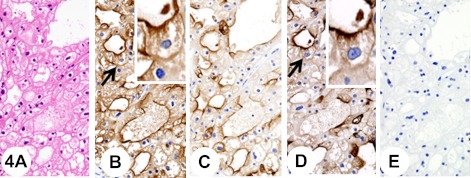
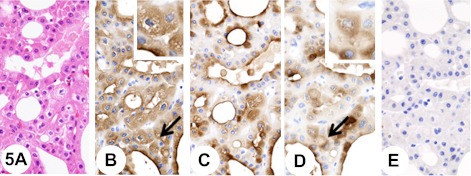
Fig. 4.
Chromophobe renal cell carcinoma, classic type. Insets show higher-magnification views of the regions indicated by arrows. The tumor is composed of voluminous, polygonal cells with a highly opaque and finely reticular cytoplasm (A). The tumor cells show diffuse fine granular cytoplasmic staining with anti-KL-6 antibody (B). In addition, immunostaining with anti-KL-6 antibody is observed at the luminal surface of tubules (B). Pretreatment with neuraminidase decreases immunoreactivity with anti-KL-6 antibody (C). The tumor cells show diffuse fine granular cytoplasmic staining with anti-sialyl MUC1 antibody (D). In addition, immunostaining with anti-sialyl MUC1 antibody is observed at the luminal surface of tubules (D). Pretreatment with neuraminidase abolishes immunoreactivity with anti-sialyl MUC1 antibody (E).
Eosinophilic variant chromophobe RCC was mainly composed of tumor cells that were smaller and markedly eosinophilic, and often showed a perinuclear halo (Fig. 5A). The tumor cells also often showed a diffuse fine granular cytoplasmic staining reaction with anti-KL-6 (Fig. 5B) and anti-sialyl MUC1 (Fig. 5D) antibodies. In addition, immunostaining was occasionally seen at the luminal surface of tubules (Fig. 5B and 5D).
Fig. 5.
Chromophobe renal cell carcinoma, eosinophilic variant. Insets show higher-magnification views of the regions indicated by arrows. The tumor is mainly composed of tumor cells that are smaller and markedly eosinophilic, and often showed a perinuclear halo (A). The tumor cells show diffuse fine granular cytoplasmic staining reaction with anti-KL-6 antibody (B). In addition, immunostaining is seen at the luminal surface of tubules (B). Pretreatment with neuraminidase decreases immunoreactivity with anti-KL-6 antibody (C). The tumor cells show diffuse fine granular cytoplasmic staining reaction with anti-sialyl MUC1 antibody (D). In addition, immunostaining is seen at the luminal surface of tubules (D). Pretreatment with neuraminidase abolishes immunoreactivity with anti-sialyl MUC1 antibody (E).
Renal oncocytoma
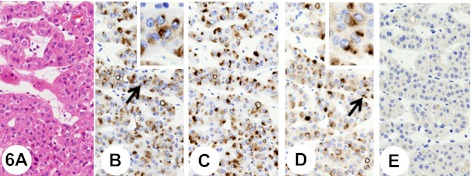
The oncocytomas were composed of large, well-differentiated, polygonal, eosinophilic cells with a cytoplasm that was rather granular. The tumor cells were arranged in solid nests and/or in cords separated by loose edematous, fibrous, or hyalinised stroma (Fig. 6A). The tumor cells showed a polarized cytoplasmic staining reaction close to the nucleus with anti-KL-6 (Fig. 6B) and anti-sialyl MUC1 (Fig. 6D) antibodies.
Fig. 6.
Renal oncocytoma. Insets show higher-magnification views of the regions indicated by arrows. The tumor is composed of large, well-differentiated, polygonal, eosinophilic cells with a cytoplasm that is rather granular. The tumor cells are arranged in solid nests and/or in cords separated by loose edematous stroma (A). The tumor cells show a polarized cytoplasmic staining reaction close to the nucleus with anti-KL-6 antibody (B). After the pretreatment with neuraminidase, the immunoreactivity with anti-KL-6 antibody remains (C). The tumor cells show a polarized cytoplasmic staining reaction close to the nucleus with anti-sialyl MUC1 antibody (D). Pretreatment with neuraminidase abolishes immunoreactivity with anti-sialyl MUC1 antibody (E).
Clear cell RCC
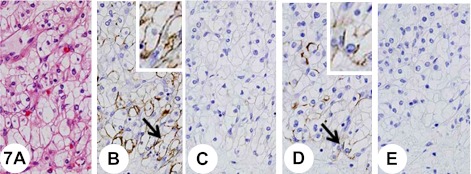
The carcinoma tissue revealed a compact, alveolar, tubular, and cystic architecture composed of clear cytoplasm with a low nuclear-cytoplasmic ratio (Fig. 7A).
Fig. 7.
Clear renal cell carcinoma. Insets show higher-magnification views of the regions indicated by arrows. The tumor shows a compact, alveolar, tubular, and cystic architecture composed of clear cytoplasm with a low nuclear-cytoplasmic ratio (A). Some tumor cells show cytoplasmic membranous staining with anti-KL-6 (B). Pretreatment with neuraminidase abolishes immunoreactivity with anti-KL-6 antibody (C). Some tumor cells show cytoplasmic membranous staining with anti-sialyl MUC1 (D). Pretreatment with neuraminidase abolishes immunoreactivity with anti-sialyl MUC1 antibody (E).
The tumor cells showed cytoplasmic membranous staining with anti-KL-6 (Fig. 7B) and anti-sialyl MUC1 (Fig. 7D) antibodies in a complete or partially circumferential manner.
Effect of neuraminidase treatment
In normal and immature kidneys, the immunoreactivity with anti-KL-6 (Figs. 2C and 3C) and anti-sialyl MUC1 (Figs. 2E and 3E) antibodies was drastically decreased or abolished at the apical surface of the renal tubular epithelia; whereas, the cytoplasmic reactivity with anti-KL-6 antibody in intercalated cells in the renal collecting ducts partially remained after the neuraminidase treatment (Fig. 2C). In classic chromophobe RCC, some tumor cells retained cytoplasmic immunoreactivity with anti-KL-6 antibody (Fig. 4C), whereas, tumor cells abolished immunoreactivity with anti-sialyl MUC1 antibody (Fig. 4E). On the other hand, although in eosinophilic variant chromophobe RCC and oncocytomas cytoplasmic staining reaction with anti-KL-6 antibody remained (Figs. 5C and 6C), tumor cells lost immunoreactivity with anti-sialyl MUC1 antibody (Figs. 5E and 6E). In clear RCC, immunoreactivity with anti-sialyl MUC1 and anti-KL-6 antibodies was abolished by the neuraminidase treatment (Figs. 7C and 7E). The results are summarized in Table 2.
IV. Discussion
This study showed distinct, diffuse cytoplasmic immunoreactivity with anti-KL-6 and anti-sialyl MUC1 antibodies in chromophobe RCC. Furthermore, this pattern of expression was similar to that in intercalated cells located in the renal collecting tubules, confirming the concept that chromophobe RCC is histologically related to the renal distal nephron epithelium, especially the intercalated cells [13].
In the normal renal parenchyma, immunoreactivity with anti-sialyl MUC1-antibody was observed at the apical (luminal) cytoplasmic membranes of the distal renal tubules and collecting ducts in a continuous linear pattern, and in the cytoplasm of the intercalated cells of the collecting ducts. By pretreatment with neuraminidase, this reactivity with anti-sialyl MUC1 antibody was abolished. These results are in accordance with those of previous studies performed with anti-MUC1 antibody [9] and lectins binding specifically to the sialic acid residues in the terminal position [12]. On the other hand, the immunoreactivity with anti-KL-6 antibody, which was reported to recognize MUC1-bearing sialylated oligosaccharides [7], showed neuraminidase-resistant cytoplasmic reactivity in intercalated cells in the collecting ducts, suggesting that anti-KL-6 antibody might react with oligosaccharides other than sialylated oligosaccharides in renal intercalated cells. Interestingly, O-linked glycans containing 6'sulfo-Gal/GalNAc of MUC1 was reported to be another possible carbohydrate epitope of anti-KL-6 antibody [14].
In this study, the cells of chromophobe RCC and oncocytomas demonstrated cytoplasmic immunoreactivity with anti KL-6 and anti-sialy MUC1 antibodies similar to that of intercalated cells, supporting the hypothesis that the cells of chromophobe RCC and oncocytomas are histogenetically related to the distal nephrons, because they express claudin-7 and parvalbumin, which are selectively expressed in distal nephron epithelium [2, 23], and aquaporin 6, which is expressed in intercalated cells in collecting ducts [24] and more frequently expressed in oncocytomas than chromophobe RCCs [19].
Furthermore, in this study, neuraminidase-resistant cytoplasmic immunoreactivity with anti-KL-6 antibody was frequently recognized in eosinophilic variant chromophobe RCC and oncocytomas as well as in intercalated cells. Interestingly, the eosinophilic variant chromophobe RCCs were reported to show a range of appearances at both the light microscopic and the ultrastructural levels, from features similar to the classic chromophobe RCC through to a neoplasm that is phenotypically similar to renal oncocytoma [10]. These results suggest that eosinophilic variant chromophobe RCC and oncocytomas are phenotypically more closely related to intercalated cells than classic chromophobe RCCs.
The diffuse cytoplasmic reactivity with anti-KL-6 and anti-sialyl MUC1 antibodies in the intercalated cells of the normal renal collecting ducts and in the chromophobe RCC, and the polarized cytoplasmic staining with the same antibodies in the oncocytoma cells, may reflect their cellular structure. Ultrastructurally, intercalated cells in the collecting duct have characteristic invaginated membrane vesicles [1]. In addition, lectin-binding sites along the membranes of the apical surface and the invaginated vesicles were observed in this type of cell [1]. Chromophobe RCC contains membrane-bound microvesicles throughout the cytoplasm that resemble the invaginated vesicles of the intercalated cells in the collecting ducts [17]. On the other hand, oncocytomas contain a focal collection of microvesicles, which were morphologically indistinguishable from those in chromophobe RCC [15]. KL-6 antigen and sialyl MUC1 may be integrated in the membranes of these cytoplasmic vesicles of intercalated cells, chromophobe RCC, and renal oncocytomas.
In this study, the expression of neuraminidase-sensitive KL6 and sialyl MUC1 was observed in clear RCCs, which show phenotypic profiles of renal proximal tubules. This expression of the above-mentioned antigens could be considered an oncofetal expression because these antigens were frequently expressed in the proximal tubules of immature kidneys, but not in those of the normal adult kidneys.
Serum concentrations of the KL-6 antigen increase in patients with diffuse lung disorders such as idiopathic interstitial pneumonia [6]; malignant tumors, especially lung, breast, and pancreatic cancers [5]; and hepatocellular carcinoma [3]. Overexpression of the KL-6 antigen in renal tumors, especially in chromophobe RCC, may elevate serum and urinary KL-6 concentrations in patients with RCC. That might point to KL-6 antigen as a potential tumor marker for renal tumors.
In conclusion, KL-6 antigen and sialyl MUC1 were expressed in renal tumors. Cytoplasmic expression of KL-6 antigen and sialyl MUC1 is a distinct feature of chromophobe RCC and renal oncocytoma, providing further evidence for the similarity of these tumor cells with the intercalated cells in collecting ducts. In addition, apical-surface staining of KL-6 antigen and sialyl MUC1 in clear RCC can be considered to be an indication of oncofetal expression. Further assessment of KL-6 antigen as a tumor marker in patients with RCC is necessary.
V. Acknowledgments
We are grateful to Professor Takayuki Honda (Department of Laboratory Medicine, Shinshu University Graduate School of Medicine) for his advice and encouragement.
VI. References
- 1.Brown D., Roth J., Orci L. Lectin-gold cytochemistry reveals intercalated cell heterogeneity along rat kidney collecting ducts. Am. J. Physiol. 1985;248:C348–356. doi: 10.1152/ajpcell.1985.248.3.C348. [DOI] [PubMed] [Google Scholar]
- 2.Choi Y. D., Kim K. S., Ryu S., Park Y., Cho N. H., Rha S. H., Jang J. J., Ro J. Y., Juhng S. W., Choi C. Claudin-7 is highly expressed in chromophobe renal cell carcinoma and renal oncocytoma. J. Korean Med. Sci. 2007;22:305–310. doi: 10.3346/jkms.2007.22.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gad A., Tanaka E., Matsumoto A., Wahab M. A., Serwah Ael H., Attia F., Ali K., Hassouba H., el-Deeb Ael R., Ichijyo T., Umemura T., Muto H., Yoshizawa K., Kiyosawa K. Assessment of KL-6 as a tumor marker in patients with hepatocellular carcinoma. World J. Gastroenterol. 2005;11:6607–6612. doi: 10.3748/wjg.v11.i42.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohno N. Serum marker KL-6/MUC1 for the diagnosis and management of interstitial pneumonitis. J. Med. Invest. 1999;46:151–158. [PubMed] [Google Scholar]
- 5.Kohno N., Akiyama M., Kyoizumi S., Hakoda M., Kobuke K., Yamakido M. Detection of soluble tumor-associated antigens in sera and effusions using novel monoclonal antibodies, KL-3 and KL-6, against lung adenocarcinoma. Jpn. J. Clin. Oncol. 1988;18:203–216. [PubMed] [Google Scholar]
- 6.Kohno N., Awaya Y., Oyama T., Yamakido M., Akiyama M., Inoue Y., Yokoyama A., Hamada H., Fujioka S., Hiwada K. KL-6, a mucin-like glycoprotein, in bronchoalveolar lavage fluid from patients with interstitial lung disease. Am. Rev. Respir. Dis. 1993;148:637–642. doi: 10.1164/ajrccm/148.3.637. [DOI] [PubMed] [Google Scholar]
- 7.Kohno N., Inoue Y., Hamada H., Fujioka S., Fujino S., Yokoyama A., Hiwada K., Ueda N., Akiyama M. Difference in sero-diagnostic values among KL-6-associated mucins classified as cluster 9. Int. J. Cancer. 1994;Suppl 8:81–83. doi: 10.1002/ijc.2910570717. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan B., Truong L. D. Renal epithelial neoplasms: the diagnostic implications of electron microscopic study in 55 cases. Hum. Pathol. 2002;33:68–79. doi: 10.1053/hupa.2002.30210. [DOI] [PubMed] [Google Scholar]
- 9.Langner C., Ratschek M., Rehak P., Schips L., Zigeuner R. Expression of MUC1 (EMA) and E-cadherin in renal cell carcinoma: a systematic immunohistochemical analysis of 188 cases. Mod. Pathol. 2004;17:180–188. doi: 10.1038/modpathol.3800032. [DOI] [PubMed] [Google Scholar]
- 10.Latham B., Dickersin G. R., Oliva E. Subtypes of chromophobe cell renal carcinoma: an ultrastructural and histochemical study of 13 cases. Am. J. Surg. Pathol. 1999;23:530–535. doi: 10.1097/00000478-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Matsukita S., Nomoto M., Kitajima S., Tanaka S., Goto M., Irimura T., Kim Y. S., Sato E., Yonezawa S. Expression of mucins (MUC1, MUC2, MUC5AC and MUC6) in mucinous carcinoma of the breast: comparison with invasive ductal carcinoma. Histopathology. 2003;42:26–36. doi: 10.1046/j.1365-2559.2003.01530.x. [DOI] [PubMed] [Google Scholar]
- 12.Ortmann M., Vierbuchen M., Fischer R. Sialylated glycoconjugates in chromophobe cell renal carcinoma compared with other renal cell tumors. Indication of its development from the collecting duct epithelium. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1991;61:123–132. doi: 10.1007/BF02890414. [DOI] [PubMed] [Google Scholar]
- 13.Picken M. M. The evolving concept of renal neoplasia: impact of emerging molecular and electron microscopic studies. Ultrastruct. Pathol. 2005;29:277–282. doi: 10.1080/01913120590951266. [DOI] [PubMed] [Google Scholar]
- 14.Seko A., Ohkura T., Ideo H., Yamashita K. Novel O-linked glycans containing 6'-sulfo-Gal/GalNAc of MUC1 secreted from human breast cancer YMB-S cells: possible carbohydrate epitopes of KL-6 (MUC1) monoclonal antibody. Glycobiology. 2012;22:181–195. doi: 10.1093/glycob/cwr118. [DOI] [PubMed] [Google Scholar]
- 15.Skinnider B. F., Jones E. C. Renal oncocytoma and chromophobe renal cell carcinoma. A comparison of colloidal iron staining and electron microscopy. Am. J. Clin. Pathol. 1999;111:796–803. doi: 10.1093/ajcp/111.6.796. [DOI] [PubMed] [Google Scholar]
- 16.Storkel S., Martignoni G., van den Berg E. In “WHO Classification Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs”, ed. by J. Eble, G. Sauter, J. Epstein, I. Sesterhenn. IARC Press; Lyon: 2004. Chromophobe renal cell carcinoma; pp. 30–32. [Google Scholar]
- 17.Storkel S., Steart P. V., Drenckhahn D., Thoenes W. The human chromophobe cell renal carcinoma: its probable relation to intercalated cells of the collecting duct. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1989;56:237–245. doi: 10.1007/BF02890022. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi T., Takamura K., Sakaue S., Ishii J., Yokouchi H., Nasuhara Y. Elevated serum KL-6 concentrations in patients with diabetes mellitus. J. Diabetes Complications. 2002;16:352–358. doi: 10.1016/s1056-8727(01)00220-3. [DOI] [PubMed] [Google Scholar]
- 19.Tan M. H., Wong C. F., Tan H. L., Yang X. J., Ditlev J., Matsuda D., Khoo S. K., Sugimura J., Fujioka T., Furge K. A., Kort E., Giraud S., Ferlicot S., Vielh P., Amsellem-Ouazana D., Debre B., Flam T., Thiounn N., Zerbib M., Benoit G., Droupy S., Molinie V., Vieillefond A., Tan P. H., Richard S., Teh B. T. Genomic expression and single-nucleotide polymorphism profiling discriminates chromophobe renal cell carcinoma and oncocytoma. BMC Cancer. 2010;10:196. doi: 10.1186/1471-2407-10-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thoenes W., Storkel S., Rumpelt H. J. Human chromophobe cell renal carcinoma. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1985;48:207–217. doi: 10.1007/BF02890129. [DOI] [PubMed] [Google Scholar]
- 21.Thoenes W., Storkel S., Rumpelt H. J., Moll R., Baum H. P., Werner S. Chromophobe cell renal carcinoma and its variants—a report on 32 cases. J. Pathol. 1988;155:277–287. doi: 10.1002/path.1711550402. [DOI] [PubMed] [Google Scholar]
- 22.Toma V., Zuber C., Sata T., Komminoth P., Hailemariam S., Eble J. N., Heitz P. U., Roth J. Thomsen-Friedenreich glycotope is expressed in developing and normal kidney but not in renal neoplasms. Hum. Pathol. 2000;31:647–655. doi: 10.1053/hp.2000.6689. [DOI] [PubMed] [Google Scholar]
- 23.Truong L. D., Shen S. S. Immunohistochemical diagnosis of renal neoplasms. Arch. Pathol. Lab. Med. 2011;135:92–109. doi: 10.5858/2010-0478-RAR.1. [DOI] [PubMed] [Google Scholar]
- 24.Yasui M., Kwon T. H., Knepper M. A., Nielsen S., Agre P. Aquaporin-6: An intracellular vesicle water channel protein in renal epithelia. Proc. Natl. Acad. Sci. U S A. 1999;96:5808–5813. doi: 10.1073/pnas.96.10.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yusenko M. V. Molecular pathology of renal oncocytoma: a review. Int. J. Urol. 2010;17:602–612. doi: 10.1111/j.1442-2042.2010.02574.x. [DOI] [PubMed] [Google Scholar]