Abstract
Heat stress, whether passive (i.e. exposure to elevated environmental temperatures) or via exercise, results in pronounced cardiovascular adjustments that are necessary for adequate temperature regulation as well as perfusion of the exercising muscle, heart and brain. The available data suggest that generally during passive heat stress baroreflex control of heart rate and sympathetic nerve activity are unchanged, while baroreflex control of systemic vascular resistance may be impaired perhaps due to attenuated vasoconstrictor responsiveness of the cutaneous circulation. Heat stress improves left ventricular systolic function, evidenced by increased cardiac contractility, thereby maintaining stroke volume despite large reductions in ventricular filling pressures. Heat stress-induced reductions in cerebral perfusion likely contribute to the recognized effect of this thermal condition in reducing orthostatic tolerance, although the mechanism(s) by which this occurs is not completely understood. The combination of intense whole-body exercise and environmental heat stress or dehydration-induced hyperthermia results in significant cardiovascular strain prior to exhaustion, which is characterized by reductions in cardiac output, stroke volume, arterial pressure and blood flow to the brain, skin and exercising muscle. These alterations in cardiovascular function and regulation late in heat stress/dehydration exercise might involve the interplay of both local and central reflexes, the contribution of which is presently unresolved.
Keywords: baroreflexes, cerebral perfusion, dehydration, exercise, hyperthermia
Humans have the capability to withstand large variations in environmental temperatures, while relatively small increases in internal temperature (i.e. as little of ~3 °C) can lead to injury and even death. Elevations in skin blood flow and sweating are the primary heat exchange mechanisms in humans that protect against a heat-related injury. The importance of these mechanisms is exemplified in the calculation that if heat was not liberated from skin, internal temperature would reach the upper ‘safe’ limit within 10 min of moderate exercise (Kenney & Johnson 1992). These heat-dissipating responses are accompanied by important, even critical, cardiovascular adjustments, which, if they were not present, would compromise thermal regulation during exercise and/or exposure to elevated environmental temperatures. There is little doubt that Johannes Lindhard understood these important concepts. In fact, in 1910 he published an article titled ‘Investigations into the conditions governing the temperature of the body’ in which the relationship between rectal temperature and ‘heat-producing processes of the body’ was investigated (Lindhard 1910). Later he reported on the temperature of human muscles at rest and during exercise (Buchthal et al. 1944). A student who studied under Lindhard’s and Krogh’s guidance, Marius Nielsen, continued to investigate human temperature regulation (Nielsen 1938, Christensen et al. 1942, Asmussen & Nielsen 1947, Nielsen & Nielsen 1962, 1965a,b).
The objective of this article is to present findings pertaining to cardiovascular responses associated with human temperature regulation. The review is divided into two parts with part one focusing on cardiovascular responses to passive (i.e. non-exercising) heat stress, while the second part focuses on cardiovascular responses associated with exercise heat stress. Given numerous outstanding review articles on these topics (Rowell 1974, 1983, 1986a,b,c, Johnson 1992, Johnson & Proppe 1996, González-Alonso et al. 2008), this review will focus primarily on relatively new findings (i.e. within the prior ~15 years), while salient work done prior to this period will be only briefly addressed.
Key cardiovascular responses to passive heat stress
Clearly the primary stimulus by which internal temperature is elevated in humans is through exercise. However, heat-related injuries and deaths can occur in non-exercising humans, especially those with prior medical conditions such as cardiovascular disease (Semenza et al. 1996, 1999). Particularly prevalent was the large number of ‘excess’ deaths during the 1995 Chicago heat wave of individuals with a prior ‘heart condition’ (Semenza et al. 1996). Investigations into cardiovascular responses to passive heat stress are valuable towards the identification of potential mechanisms responsible for heat-induced injuries and deaths in patient populations. Moreover, cardiovascular responses to passive heat stress provide a benchmark from which the effects of combined exercise and heat stress can be compared.
Studies investigating thermal and cardiovascular responses to passive heat stress typically heat the subject via water-perfused suits, in which hot water is perfused through a tube-lined suit worn by each subject; by water immersion of the entire body or just limbs (such as the legs) in warm water; or by exposure of the individual to warm environmental conditions using a climatic chamber. In all cases an elevation in skin temperature is the primary mode by which internal temperature is elevated; although depending on the approach the magnitude of the increase in skin temperature can greatly differ. A limitation of the interpretation of these studies is the lack of consistency of the magnitude of the heat stress, primarily defined by the magnitude of the increase in internal temperature; with some studies reporting heat stress being as little as a 0.5 °C increase in internal temperature whereas other studies report increasing internal temperature upwards to 2 °C.
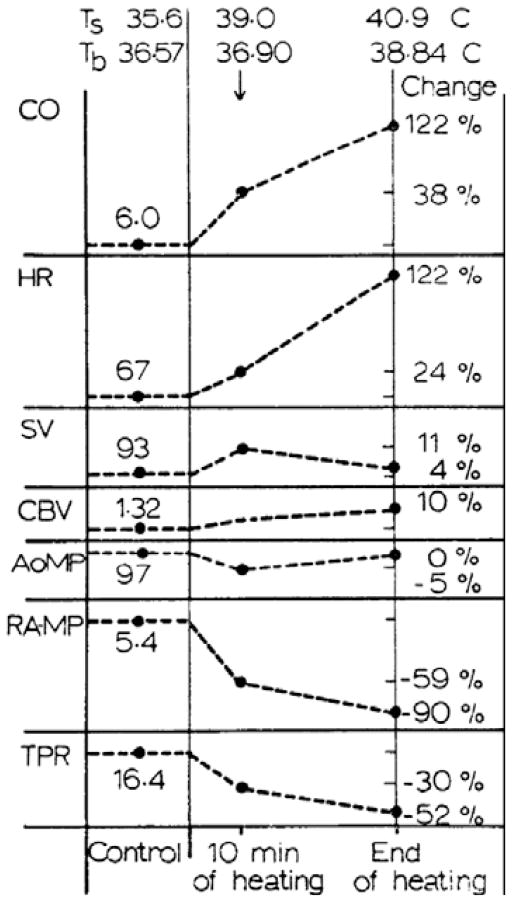
During pronounced passive heat stress, skin blood flow of humans is estimated to increase from ~300 mL min−1 upwards to 7500 mL min−1 (Rowell et al. 1969a, Rowell 1986c). In order to prevent large decreases in arterial blood pressure due to pronounced increases in total vascular conductance associated with cutaneous vasodilation, cardiac output must increase along with decreases in vascular conductance of non-cutaneous beds. The combination of these responses results in either no change, or only minimal reductions in arterial blood pressure. Rowell et al. (1969a) and Rowell (1986c) showed that during a pronounced passive heat stress, cardiac output can be as high as 13 L min−1, with ~50% of that value estimated to be directed towards skin. This elevation in cardiac output is primarily mediated through increases in heart rate as stroke volume either does not change or is marginally increased in young healthy heat-stressed subjects (Damato et al. 1968, Rowell et al. 1969a, Minson et al. 1998). Redistribution of blood flow from non-cutaneous tissues is evident from passive heat stress studies showing that post-ganglionic muscle sympathetic nerve activity increases (Niimi et al. 1997, Crandall et al. 1999a, 2003, Cui et al. 2002b,c, 2004a, Keller et al. 2006) while vascular conductance in other beds (i.e. splanchnic and renal) decreases (Rowell et al. 1971, Rowell 1986c, Minson et al. 1998). Thus, pronounced heat stress in humans has been termed a ‘hyperadrenergic state’ as it is accompanied with the aforementioned responses (Rowell 1990). These primary cardiovascular responses to passive heat stress are depicted in Figure 1. Recent studies provide further insight into the effects of heat stress on cardiovascular responses, specifically the effects of heat stress on baroreflex responsiveness, central blood volume, cardiac function, and control of the cerebral vasculature. These areas are the primary focus of the first part of this review.
Figure 1.
Classic cardiovascular responses to increases in skin temperature (Ts) resulting in a large increases in body temperature (Tb) reported as a per cent change in the indicated value relative to pre-heat stress baseline. CO, cardiac output; HR, heart rate; SV, stroke volume; CBV, central blood volume; AoMP, aortic mean arterial blood pressure; RAMP, right atrial mean blood pressure; TPR, total peripheral resistance. Note that the effects of heat stress on central blood volume have recently been shown to decrease as opposed to slight increases observed by these investigators (see Fig. 2). Figure from Rowell et al. (1969a); republished with permission from The American Physiological Society.
Heat stress and baroreflex responsiveness
The mechanisms responsible for reduced orthostatic tolerance in heat-stressed humans have not been fully elucidated, although Lind et al. (1968) showed this response was not due to enhanced pooling of blood in the leg during the orthostatic challenge. The central components governing thermoregulation are located in the hypothalamus (Strom 1960), and electrical stimulation of the hypothalamus modifies the baroreceptor reflex (Reis & Cuénod 1965, Gebber & Snyder 1970). Thus it seemed feasible that heat stress may impair baroreflex function, which could compromise blood pressure control while individuals are in this thermal condition.
Based upon numerous studies evaluating the effects of heat stress on baroreflex responses, four key observations can be summarized. (1) Generally, heat stress does not alter baroreflex control of heart rate (Crandall 2000, Yamazaki & Sone 2000, Wilson et al. 2001, Yamazaki et al. 2001, Cui et al. 2002b). The exceptions are from a few studies in which the change in heart rate during relatively small spontaneous oscillations in blood pressure is attenuated during heat stress (Crandall et al. 2000, Lee et al. 2003). It is possible that these latter observations are due to reduced cardiac vagal activity associated with heating (Crandall et al. 2000) given that when greater changes in baroreceptor loading were caused either mechanically (Crandall 2000, Yamazaki & Sone 2000) or pharmacologically (Wilson et al. 2001, Cui et al. 2002b), the baroreflex gain of the blood pressure to heart rate relationship was unchanged due to whole-body heating. (2) Baroreflex control of muscle sympathetic nerve activity is typically not attenuated by heat stress, but rather is unchanged or even elevated (Cui et al. 2002b, 2004a, Keller et al. 2006). The exception is a study showing attenuated changes in muscle sympathetic nerve activity relative to blood pressure changes induced by the valsalva manoeuvre in heat-stressed subjects (Yamazaki et al. 2003). Apart from the acute nature of the blood pressure change during the valsalva manoeuvre (i.e. typically less than 10 cardiac cycles), relative to the longer duration of the hypotensive challenge to systemic nitric oxide administration and lower body negative pressure, reasoning for these differing responses is not forthcoming. (3) Cutaneous vasoconstrictor responses are attenuated by local and whole-body heat stress (Wilson et al. 2002a), whereas muscle vasoconstrictor responses are not impaired when muscle temperature is elevated ~4 °C (Keller et al. 2007). Attenuated cutaneous adrenergic vasoconstrictor responsiveness (Wilson et al. 2002a), coupled with upwards to 50% of cardiac output being directed towards the skin in the heat-stressed subject, may be the mechanism responsible for attenuated blood pressure changes upon changing carotid sinus transmural pressure (Crandall 2000), as well as during systemic phenylephrine administration (Cui et al. 2002c). (4) Despite somewhat varied baroreflex responses to heat stress, this exposure consistently shifts the baroreflex curve to the prevailing heart rate, muscle sympathetic nerve activity and blood pressure (Crandall 2000, Yamazaki et al. 2001, 2003, Cui et al. 2002b, Crandall et al. 2003, Keller et al. 2006). In summary, the bulk of the data do not support the hypothesis that heat stress attenuates baroreceptor control of heart rate or muscle sympathetic nerve activity to relatively pronounced changes in arterial blood pressure. However, heat-induced alterations in post-synaptic cutaneous vasoconstrictor responsiveness may attenuate baroreflex control modulation of systemic vascular conductance.
Heat stress and blood volume distribution
Passive heat stress causes pronounced reductions in central venous pressure which, depending on the magnitude of the heat stress, can approach 0 mmHg (Rowell et al. 1969a, Johnson & Proppe 1996, Minson et al. 1998, Crandall et al. 1999b, Peters et al. 2000, Wilson et al. 2007, Keller et al. 2009). The mechanism responsible for this reduction in central venous pressure has been proposed to be due to a redistribution of blood from the central circulation to cutaneous vascular beds (Johnson & Proppe 1996). Despite heat stress-induced reductions in central blood volume being hypothesized (Müller 1905, Glaser et al. 1950, Eisalo 1956, Koroxenidis et al. 1961, Frayser et al. 1966), Rowell et al. (1969a) reported a slight increase in central blood volume during whole-body heat stress when indexed from mean transit time of a dye injected into the right atrium and sampled at the aortic arch (see Fig. 1). Such a finding is perplexing given the discord between reductions in central venous pressure and Rowell’s observation of a slight increase in central blood volume.
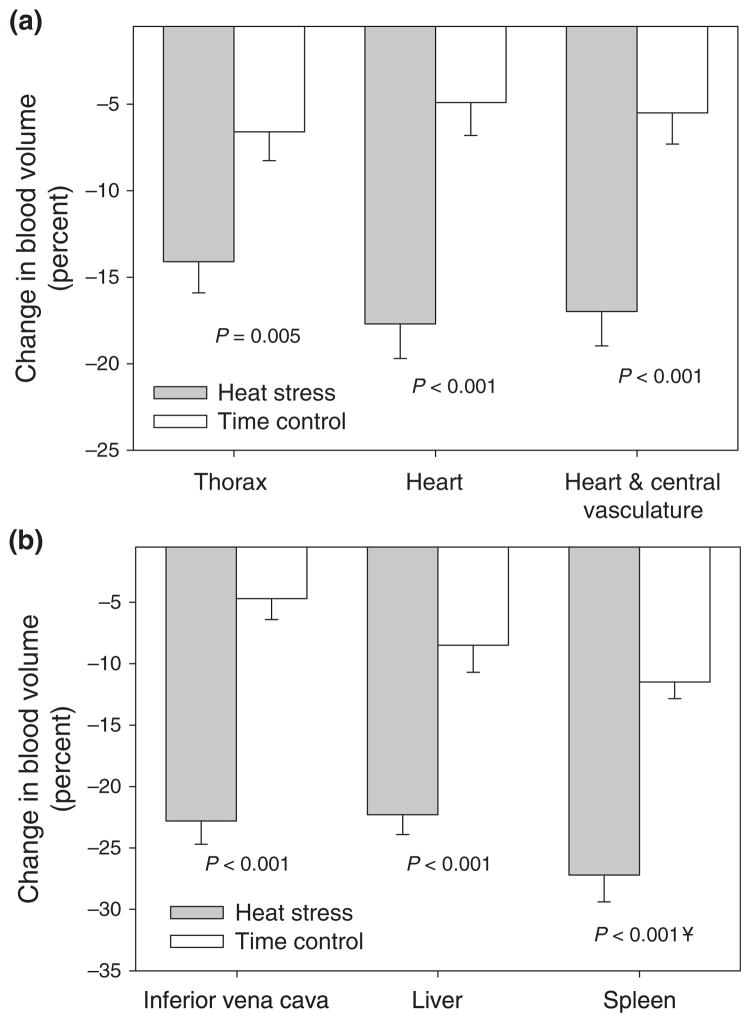
To clarify this question, Crandall et al. (2008) evaluated changes in regional blood volume during passive heat stress using technetium-99m labelled autologous red blood cells, coupled with gamma camera imaging. Although with this approach absolute measures of central blood volume were not obtained, relative changes in regional blood volumes were quantified. The heat stress caused typical haemodynamic responses, similar to that outlined in Figure 1, including a reduction in central venous pressure from 5.5 ± 0.7 to 0.2 ± 0.6 mmHg (P < 0.001). Accompanying this central venous pressure response were greater reductions in thoracic blood volume (14 ± 2%), heart blood volume (18 ± 2%) and the volumes of the heart plus the central vascular structures (17 ± 2%), relative to responses in subjects who were not heat stressed but were supine for a similar duration following technetium-99m administration as the heat-stressed subjects (Fig. 2). In addition, heat stress decreased liver (23 ± 2%) and spleen (27 ± 2%) blood volume, which is consistent with previously reported reductions in splanchnic blood flow by heat stress (Rowell et al. 1969a, 1971, Minson et al. 1998). These data provide clear evidence that heat stress decreases central blood volume, which is likely due to a redistribution of blood from the central to the cutaneous vascular beds, coupled with increases in cardiac output (Johnson & Proppe 1996).
Figure 2.
Percent change in blood volume from the indicated regions between experimental (i.e. heat stressed) and time control subjects. In each of the indicated regions heat stress significantly reduced blood volume relative to the time control trials. Figure from Crandall et al. (2008); republished with permission from Wiley-Blackwell.
Heat stress and cardiac function
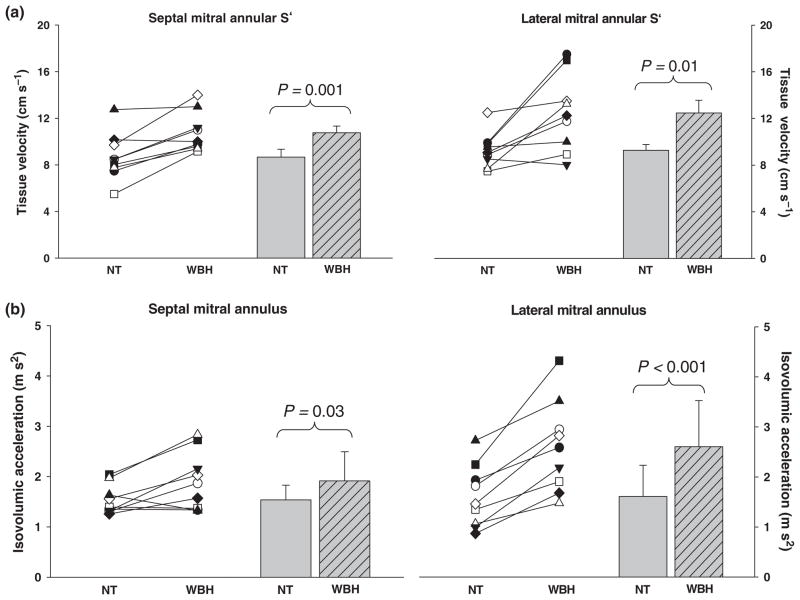
Consistent with a reduction in central venous pressure, passive heat stress results in parallel decreases in left ventricular filling pressure as indexed from pulmonary capillary wedge pressure (Wilson et al. 2007, 2009). Reduced central blood volume and ventricular filling pressures, accompanied with preserved or slightly elevated stroke volumes has led to the suggestion that heat stress increases the inotropic state of the heart (Rowell 1986c, Johnson & Proppe 1996). Consistent with that hypothesis, Crandall et al. (2008) showed that whole-body heat stress increases ejection fraction. However, these findings must be interpreted with the recognition of the indirect nature of this measurement and the load dependency of ejection fraction, resulting in an imprecise measure of systolic function, particularly given changes in ventricular loading status during a heat stress. For this reason, Brothers et al. (2009a) identified the effects of heat stress on echocardiographic indices of cardiac systolic and diastolic function. The primary observations from that study were: (1) Heat stress has no effect on indices of diastolic function as indicated by an unchanged left ventricular filling velocity, an unchanged early diastolic mitral annular velocity and an unchanged ratio of blood velocity/mitral annular velocity during the early phase of diastole. However, the preservation of these indices of diastolic function, despite heat stress-induced decreases in ventricular filling pressures (Rowell et al. 1969a, Minson et al. 1998, Crandall et al. 1999b, Wilson et al. 2007, 2009) and central blood volume (Crandall et al. 2008), leaves room for speculation that diastolic function is perhaps improved during heat stress. (2) Heat stress increased left ventricular systolic function as evidenced by an increase in peak septal and lateral mitral annular systolic velocities and isovolumic acceleration (Fig. 3). (3) Heat stress significantly increased left atrial systolic function as evidenced by an increased velocity of blood during the atrial contraction phase of left ventricular diastolic filling and an increased velocity of the septal and lateral mitral annulus during the late phase of diastolic relaxation relative to normothermia.
Figure 3.
Peak septal and lateral mitral annular systolic velocities (S′; panel a) and isovolumic acceleration of the septal and lateral mitral annulus (panel b). Individual (left-hand side of each panel) and group averaged (right-hand side of each panel) echocardiographic measurements of the indicated data during normothermic (NT) and whole-body heat-stress (WBH) conditions. Increases in the indicated parameters by heat stress are indicative of an increase in cardiac systolic function. Figure from Brothers et al. (2009a); used with permission from The American Physiological Society.
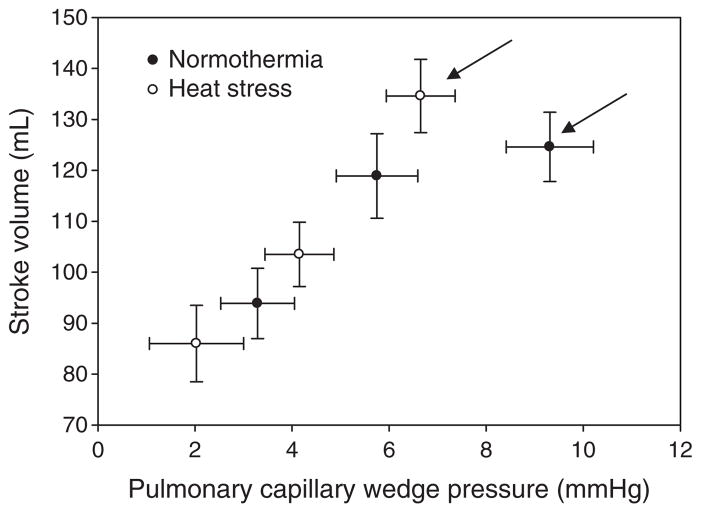
Increases in left ventricular end-diastolic cross-sectional diameter, often indicated by its filling pressure, increase the ability of the left ventricle to produce force and thereby stroke volume (Sarnoff & Mitchell 1961, Katz 2006). This concept, termed Frank–Starling mechanism (Frank 1895, Patterson & Starling 1914, Patterson et al. 1914), is represented in vivo by a series of hyperbolic curves relating changes in pulmonary capillary wedge pressure (as an index of left ventricular end-diastolic volume) relative to stroke volume. Given the hyperbolic shape of these curves, the magnitude of change in stroke volume for a given change in filling pressure is largely affected by the shape of the curve and the location of the operating point on that curve. A reduction in left ventricular filling pressure caused by passive heat stress, coupled with an absence of a reduction in stroke volume, suggests heat stress causes a leftward shift of the Frank–Starling curve. Wilson et al. (2009) experimentally validated this hypothesis upon examining the relationship between pulmonary capillary wedge pressure and stroke volume during 15 and 30 mmHg lower body negative pressure while subjects were normothermic and heat stressed (Fig. 4). Heat stress shifted the operating point to a steeper portion of the Frank–Starling curve such that for a given reduction in ventricular filling pressure there was a greater reduction in stroke volume. This larger reduction in stroke volume was not compensated for by a proportionally greater increase in heart rate, resulting in a larger reduction in cardiac output for a given reduction in left ventricular filling pressure or level of lower body negative pressure when the individual was heat stressed (Wilson et al. 2009), which likely is a primary mechanism for heat stress-induced reductions in orthostatic tolerance (Keller et al. 2009).
Figure 4.
Effect of thermal stress on the Frank–Starling relation via plotting the relation between pulmonary capillary wedge pressure and stroke volume. Data points were generated via lower body negative pressures (LBNP) of 0, 15 and 30 mmHg. The arrows indicate the operating point for the respective thermal conditions. The operating point is defined as the prevailing pulmonary capillary wedge pressure and stroke volume prior to the onset of LBNP. Figure from Wilson, Brothers, Tollund, Dawson, Nissen, Yoshiga, Jons, Secher and Crandall. J Physiol 587, 3383–3392, 2009; republished with permission from Wiley-Blackwell.
Heat stress and the cerebral vasculature
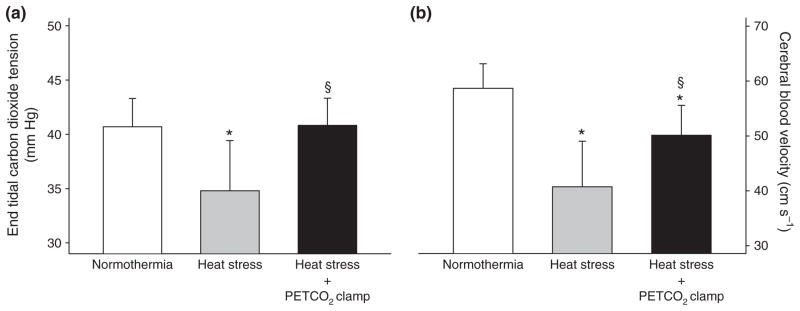
Whole-body heat stress decreases cerebral perfusion in the supine resting human (Wilson et al. 2002b, 2006, Fan et al. 2008, Fujii et al. 2008, Low et al. 2008, 2009, Brothers et al. 2009b), which is not entirely accounted for by concurrent reductions in arterial carbon dioxide tension (Wilson et al. 2006, Brothers et al. 2009b). The latter point was recently confirmed given that cerebral perfusion and cerebral vascular conductance remained well below pre-heat stress levels after end-tidal carbon dioxide partial pressures, which can be reduced by 15+ mmHg during heating (Wilson et al. 2006, Crandall et al. 2008, Fan et al. 2008, Fujii et al. 2008, Brothers et al. 2009b), were returned to pre-heat stress levels (Brothers et al. 2009b) (Fig. 5). The mechanism by which heat stress decreases cerebral vascular conductance and perfusion independent of reduced arterial carbon dioxide partial pressures is not clear. One possibility could be increased cerebral sympathetic activity. Sympathetic nerve activity increases by approximately 90% to the muscle vasculature and 300–600% to the skin vasculature during passive heat stress (Bini et al. 1980, Niimi et al. 1997, Cui et al. 2002a, 2004b,c, Keller et al. 2006). Furthermore, blood flow to the renal and splanchnic regions is reduced presumably as a result of increased sympathetically mediated vasoconstriction (Rowell et al. 1970, 1971, Johnson & Proppe 1996, Minson et al. 1998). While the role of sympathetic control of the cerebral vasculature remains controversial (van Lieshout & Secher 2008, Strandgaard & Sigurdsson 2008), animal studies have shown that the cerebral arteries are richly innervated with sympathetic nerve fibres (Nelson & Rennels 1970, Edvinsson 1975). Evidence for cerebral sympathetic activity has also been demonstrated in human studies that have identified a reduction in cerebral perfusion during unilateral trigeminal ganglion stimulation (Visocchi et al. 1996) and an increase in cerebral perfusion after stellate ganglionic blockade (Umeyama et al. 1995, Ide et al. 2000). Furthermore, cerebral autoregulation is impaired following removal of autonomic neural activity with trimethaphan (Zhang et al. 2002) and systemic blockade of α1-adrenergic receptors with prazosin (Ogoh et al. 2008). Therefore, it is plausible to speculate that heat stress-induced increases in cerebral sympathetic activity may contribute to reductions in cerebral perfusion in the heat-stressed human.
Figure 5.
End-tidal carbon dioxide tension and middle cerebral artery blood velocity (MCA Vmean) during normothermia, heat stress, and heat stress after end-tidal carbon dioxide (PETCO2) concentration was returned to pre-heat stress levels. The reduction in PETCO2 concentration during heat stress was completely abolished by the PETCO2 clamping procedure (panel a). Heat stress reduced MCA Vmean relative to normothermia. Restoration of PETCO2 to the normothermic level while subjects were heat stressed (heat stress + clamp) attenuated the decrease in MCA Vmean relative to control heat stress without the clamp; however MCA Vmean remained reduced when compared with normothermia (panel b). These data indicate that mechanisms other than reduced PETCO2 contribute to the reduced cerebral perfusion that occurs in heat-stressed individuals. *Significantly different relative to normothermia; §significantly different relative to control heat stress. Figure from Brothers et al. (2009b); republished with permission from Wiley-Blackwell.
From the aforementioned findings, it is clear that even prior to the onset of an orthostatic event cerebral perfusion is compromised by whole-body heat stress. Reduced cerebral perfusion in the heat-stressed human will attenuate the reserve by which cerebral blood flow can further decrease prior to the onset of syncope. Thus, heat-stress induced decreases in cerebral perfusion likely contribute to reductions in orthostatic tolerance.
Maintenance of cerebral perfusion over a wide range of systemic blood pressures (e.g. from ~60 to ~150 mmHg) is carried out by a variety of mechanisms including cerebral autoregulation which acts to offset changes in perfusion pressure by adjusting the resistance of the cerebral vasculature (Heistad & Kontos 1983, Paulson et al. 1990). Should heat stress attenuate cerebral autoregulation, this would result in a greater reduction in cerebral perfusion for a given reduction in perfusion pressure, ultimately leading to reduced orthostatic tolerance. Consistent with this hypothesis, Wilson et al. (2006) identified that for a given level of orthostatic stress (via lower body negative pressure) when subjects were heat stressed the reduction in cerebral vascular conductance and perfusion was significantly greater, relative to when subjects were normothermic. Doering et al. (1999) were the first to seek whether heat stress alters cerebral autoregulation. Counter to an expected reduction, in mildly heat-stressed subjects (~0.4 °C increase in internal temperature) they reported an increase in an index of cerebral autoregulation. Given the relatively minor heat stress in that study, Brothers et al. (2009c) and Low et al. (2009) further investigated this question, using varied techniques in more profoundly heat-stressed subjects relative to the level of heating in the Doering et al. study. Regardless of whether the blood pressure oscillations were relatively small (Low et al. 2009) or quite large (Brothers et al. 2009c), cerebral autoregulation was not compromised by heat stress. In contrast, and depending on the frequency of blood pressure oscillation analysed, these investigators found either no change or enhanced cerebral autoregulation by heat stress; the latter being consistent with the original findings of Doering et al. (1999). Taken together, whole-body heat stress either does not change or perhaps improves cerebral vascular autoregulation.
Cardiovascular responses to combined exercise and heat stress
The combination of exercise and heat stress can pose one of the most severe challenges to the regulation of the cardiovascular system in humans. The interplay between the magnitude of thermal stress, the intensity and duration of exercise and the individual training, heat acclimatization and hydration status will dictate the extent of the cardiovascular challenge. These factors should therefore be thoroughly considered in predicting whether heat stress will promote compensatory adjustments or severe circulatory strain that could lead to accelerated fatigue or collapse in exercising people. Conceivably, the greatest cardiovascular strain and alterations in cardiovascular regulation are expected to occur in untrained, unacclimated and hypohydrated subjects who perform intense, prolonged exercise in a hot and humid environment. World-class, heat-acclimated and euhydrated athletes might be on the other end of a continuum and show minimal alterations in cardiovascular function under comparable extreme exercise and environmental conditions. This second component of the review will primarily discuss evidence and ideas concerning the responses and regulation of the human cardiovascular system when confronted with the combined stresses of exercise and environmental heat stress or dehydration-induced hyperthermia. The impact of alterations in limb blood flow on convective heat transfer and internal temperature regulation will also be briefly discussed in the context of the classic observations of Nielsen (1938).
Over the past 45 years our understanding of human cardiovascular function during thermal stress and exercise has been influenced very profoundly by the work of Loring Rowell and colleagues at the University of Washington. In a landmark study, they (Rowell et al. 1966) were the first to demonstrate in a group of untrained individuals performing graded exercise in a hot and a thermoneutral environment that severe thermal stress readily perturbs cardiovascular function when the intensity of exercise is moderate to intense. The key features indicative of cardiovascular strain during exercise were the significantly lower stroke volume, central blood volume, aortic pressure and cardiac output and the markedly elevated heart rate reaching near-maximal values. The decreased central blood volume with heat stress across all four submaximal exercise intensities examined supported the idea that a lowering in venous return and filling of the heart might be an important factor involved in the depressed stroke volume and ultimately cardiac output with combined heat stress and exercise. In line with this view, they showed in a subsequent study using a water-perfused suit that the marked alterations in central blood volume, central venous pressure, stroke volume, heart rate and cardiac output evoked by superimposing severe heat stress upon light and high intensity cycling were almost restored to normothermic control values by perfusing the suit with cold water (Rowell et al. 1969b). During walking in a very hot environment, however, cardiac output increased over time due solely to increases in heart rate (Rowell et al. 1967). Thus this pioneer research demonstrated that the repercussions of combined heat stress and exercise on cardiovascular function are dependent upon the intensity of exercise and implied that cardiac output and perfusion pressure are compromised in untrained subjects when both the metabolic and thermoregulatory demands are high.
Limb muscle and skin perfusion during combined exercise and heat stress
These early observations of a compromised cardiovascular function during heat stress exercise and the latter finding that forearm blood flow (index of skin blood flow in the resting forearm) increases progressively during leg exercise (Johnson & Rowell 1975) form the basis for the most influential hypothesis in this field postulating that the combination of heat stress and exercise results in a competition between the exercising skeletal muscles and the skin compartments for the available cardiac output such that blood flow to the active muscles would be reduced at the expense of an elevated skin circulation (Rowell 1974, 1983). According to this hypothesis, the pumping capacity of the heart cannot meet the joint demands for blood flow of the exercising muscle and the skin during exercise and heat stress. Experimental evidence in animals and humans, however, shows that blood flow to active limb muscles and tissues is either maintained or increased when heat stress is superimposed upon light to moderate intensity prolonged exercise (Laughlin & Armstrong 1983, Armstrong et al. 1987, Savard et al. 1988, McKirnan et al. 1989, Nielsen et al. 1990, 1993, 1997). In the human studies, the cardiovascular system appeared to respond adequately to the additional thermoregulatory demand for an elevated skin perfusion by increasing cardiac output (Savard et al. 1988, Nielsen et al. 1990, 1993, 1997) and possibly reducing visceral blood flow beyond the control exercise levels (Rowell et al. 1965, Ho et al. 1997). To understand this response, it is important to bear in mind that cutaneous vasodilatation is noticeably restrained during combined heat stress and exercise compared with the levels observed during resting hyperthermic conditions (Johnson 1992, Kenney & Johnson 1992). During upright exercise, skin perfusion in the resting forearm reaches a plateau at a core temperature of ~38 °C despite further increases in skin and internal temperature (Brengelmann et al. 1977, González-Alonso et al. 1999c). Although knowledge of the skin perfusion across all body segments including the exercising limbs is very limited, the magnitude of the cardiac output increase and the reduction in visceral blood flow during heat stress exercise indirectly suggest that whole-body skin blood flow might be elevated by 1–2 L min−1 above control exercise (Nielsen et al. 1993, 1997, Ho et al. 1997, González-Alonso et al. 2000a). This estimate is based on the still unresolved concept that hyperthermia-induced muscle vasodilatation does not contribute to heat stress-mediated hyperaemia. Regardless of this possibility, evidence from submaximal exercise studies does not seem to support the hypothesis of a regulatory priority of the skin circulation over the skeletal muscle circulation whereby flow from active muscles is redistributed to the skin.
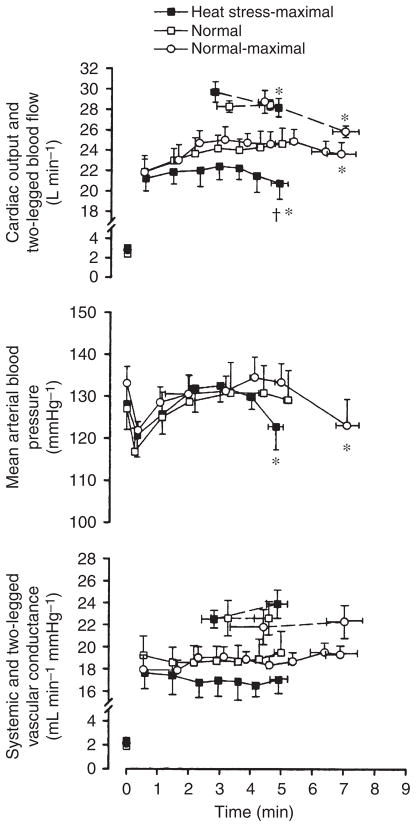
It is universally accepted that severe heat stress suppresses VO2max and work capacity during exhaustive incremental exercise (Rowell 1974, Hales et al. 1996, Sawka & Coyle 1999). The question then arises as to whether blood flow to active muscles is indeed reduced at the expense of an elevated skin perfusion when heat stress is superimposed upon exercise requiring maximal cardiovascular function and aerobic capacity. Surprisingly, direct data are still lacking on the blood flow responses of the active limb muscles and skin and the systemic circulation during incremental whole-body exercise in heat-stressed individuals. Notwithstanding, an integrative view of the exercising limb and systemic circulatory responses to maximal exercise was recently reported in trained individuals performing constant load cycling under heat stress and normal conditions (González-Alonso & Calbet 2003, González-Alonso et al. 2004). An advantage of this exercise model is that it allows investigation of the functional and regulatory capacity of the cardiovascular system in conditions where workload-related changes in metabolic demand and active muscle mass are minimal in comparison with that occurring during exhaustive incremental exercise or self-selective pacing trials. The potentially confounding influence of altered workload-related metabolic and mechanical reflexes on cardiovascular control is therefore minimized. In using this model, González-Alonso& Calbet (2003) showed that blood flow to the exercising legs increases similarly with and without heat stress during the first minute of maximal cycling but thereafter is attenuated and drops faster with heat stress accompanying a quicker decline in cardiac output and arterial blood pressure (Fig. 6). Strikingly, vascular conductance in the active legs and systemic circulation do not decline despite the concomitant increases in circulating catecholamines indicative of enhanced sympathetic vasoconstrictor activity. Importantly, systemic blood flow and arterial pressure responses are elevated or unchanged with heat stress compared with control conditions during the early stages of constant maximal exercise (González-Alonso & Calbet 2003). This contrasts with the diminished cardiac output and arterial pressure seen during moderate and intense exercise in untrained individuals (Rowell et al. 1966). It therefore seems that during the early stages of maximal heat stress exercise in trained individuals the higher thermoregulatory demand for skin blood flow is met at least in part by a 1–2 L min−1 higher cardiac output. However, hyperthermia more quickly pushes the cardiovascular system to its regulatory limit, where cardiac output and blood flow to exercising limb muscles and skin cannot be maintained for a longer duration.
Figure 6.
Haemodynamics during maximal whole-body exercise in heat-stressed humans. Systemic and exercising limb blood flow and vascular conductance during constant maximal exercise with heat stress and control conditions. Note the significant reductions in cardiac output and leg blood flow and arterial blood pressure leading to unchanged vascular conductance. *Significantly lower than corresponding peak exercise values, P < 0.05. †Significantly lower than control (normal) trials, P < 0.05. Figure from González-Alonso & Calbet (2003); republished with permission from the American Heart Association.
The mechanisms underlying the restrictions in locomotor limb blood flow during maximal exercise have not been systematically investigated, but they plausibly involve the interplay of local and central reflexes signalling alterations in thermal, metabolic, mechanical, barosensitive and vascular events in different regions of the body including the skeletal muscle, brain and heart (Rowell 2004, Mortensen et al. 2008). The reductions in locomotor limb blood flow and cardiac output during constant maximal exercise and the levelling off in these variables during incremental maximal exercise are temporally related (González-Alonso & Calbet 2003, Mortensen et al. 2005, 2008, Calbet et al. 2007, Vogiatzis et al. 2009). During constant load maximal exercise with and without heat stress, arterial and central venous pressures decline such that the vascular conductance of the active legs and systemic circulation remain unchanged. This suggests that active limb blood flow is reduced secondary to the decline in perfusion pressure, rather than an actual vasoconstriction of the vasculature perfusing the active muscle and skin. In this construct, the reduction in cardiac output might be indirectly involved in this process via its effect on perfusion pressure. Locally, the unaltered leg vascular conductance takes place in the presence of an everincreasing plasma noradrenaline concentration suggesting that neurally mediated vasoconstriction does not occur in the vessels perfusing the exercising limb muscles and skin, even though muscle and skin sympathetic vasoconstrictor activity are likely to be augmented (Ray & Gracey 1997, Ichinose et al. 2008). An enhanced metabolic vasodilatation evoked by accumulation of vasodilator substances in the blood (including ATP and other adenine nucleotides) and the muscle interstitium might offset the increased sympathetic vasoconstrictor drive at least in skeletal muscle thereby maintaining limb muscle vascular conductance (Vanhoutte et al. 1981). This phenomenon resembles the functional sympatholysis occurring in the skeletal muscle vasculature in conditions of increased sympathetic nerve drive during exercise and hypoxia (Remensnyder et al. 1962, Hanada et al. 2003, Rosenmeier et al. 2004), which underlies the maintenance of resting limb perfusion and the increase in exercising limb blood flow during hypoxic exercise.
Cardiac function during combined exercise and heat stress
Our knowledge and understanding of cardiac function during intense exercise and of the mechanisms underpinning the fall in stroke volume prior to exhaustion are still inadequate. From a cardiac perspective, the fall in stroke volume and cardiac output during constant maximal exercise with and without exogenous heat stress might be the result of a number of factors that negatively affect cardiac pre-load, ventricular afterload and/or myocardial contractility (Rowell 1974, Poliner et al. 1980, Higginbotham et al. 1986). Neither blood pooling in the compliant cutaneous vasculature (which could potentially diminish venous return) nor augmented ventricular afterload appear likely possibilities as stroke volume, central venous pressure and arterial pressure fall similarly during heat stress and control conditions (González-Alonso et al. 2000a, González-Alonso & Calbet 2003). The decline in central venous pressure could be interpreted to mean that a reduction in venous return contributes to the fall in stroke volume (Rowell et al. 1966, Rowell 1974). However, a reduced venous return and a concomitant diminution in left ventricular pre-load do not seem to exert an independent effect because stroke volume only declines during the last minute of exercise, but right atrial pressure declines from the start of exercise. This suggests that several factors interact to transiently depress diastolic and/or systolic cardiac function during maximal exercise.
Seeing the declines in cardiac output, exercising limb blood flow and brain circulation, it seems possible that the coronary circulation and left ventricular function are transiently suppressed during maximal exercise, thereby contributing to the reduction in stroke volume. A small attenuation in the myocardial perfusion-to-work relationship could lead to myocardial dysfunction, as oxygen (extraction) reserve is very small to compensate for a significant blunting in oxygen supply. Severe tachycardia might be another factor. Studies in humans and dogs manipulating heart rate by pacing the heart demonstrate that severe tachycardia leads to disproportional reductions in diastolic filling time and left ventricular enddiastolic volume which compromise stroke volume and cardiac output (Templeton et al. 1972, Parker & Case 1979). Human studies demonstrate that hyperthermiainduced tachycardia reduces stroke volume during exercise (González-Alonso et al. 1997, Fritzsche et al. 1999) and that blunting the increase in internal temperature and heart rate restores most of the fall in VO2max evoked by marked hyperthermia and dehydration (Nybo et al. 2001). Taken together, the decline in stroke volume during maximal exercise with and without exogenous heat stress is associated with reduced venous return, severe tachycardia and possibly a blunting in myocardial oxygen supply in relation to actual cardiac work.
Cerebral perfusion during combined exercise and heat stress
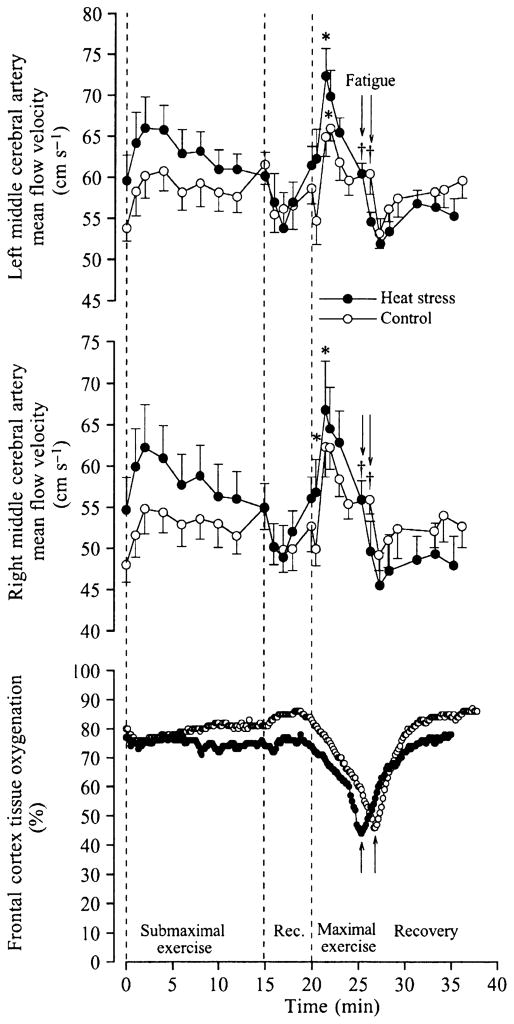
The active limb muscles are not the only tissues that might experience a reduction in perfusion during exercise and heat stress. The human brain circulation might also be compromised (Nybo & Nielsen 2001, Nybo et al. 2002, González-Alonso et al. 2004). In this regard, middle cerebral blood velocity declines significantly during prolonged exercise in the heat while it is kept constant during exercise in a thermoneutral environment (Nybo & Nielsen 2001, Nybo et al. 2002). During constant maximal exercise, however, cerebral perfusion declines after ~90 s, regardless of the presence or absence of heat stress, as indicated by a progressive drop in both middle cerebral artery mean blood velocity and frontal cortex tissue oxygenation and the concomitant increases in brain oxygen extraction (González-Alonso et al. 2004) (Fig. 7). These responses are in sharp contrast to that happening during the first ~90 s of exercise where middle cerebral artery mean blood velocity increases and cerebral oxygen extraction and frontal cortex tissue oxygenation remain stable. This suggests that global brain aerobic metabolism increases early in exercise possibly in response to enhanced neural activation in regions of the brain related to locomotion, the maintenance of equilibrium, vision and cardiovascular control (Delp et al. 2001). The large increases in oxygen extraction that occur after ~90 s of maximal exercise are accompanied by smaller reductions in cerebral perfusion, signifying that global brain metabolism and neural drive is further enhanced when approaching exhaustion. Therefore, the physiological repercussions of reductions in perfusion to the brain are apparently less severe than in contracting skeletal and cardiac muscle because, in contrast to the muscles’ exhausted oxygen reserve, the human brain maintains a large oxygen reverse on exhaustion, which appears to protect this vital organ against the small declines in oxygen delivery occurring during exercise.
Figure 7.
Cerebral circulation and oxygenation during maximal whole-body exercise in heat-stressed humans. Left and right middle cerebral artery blood velocity and near-infrared spectroscopy-determined cerebral tissue oxygenation at rest, during submaximal and maximal cycling and during 10 min of recovery in heat stress and control conditions. Note the marked reductions in blood velocity accompanying the declines in tissue oxygenation. *Higher than value at start of exercise, P < 0.05. †Lower than peak value during maximal exercise, P < 0.05. From González-Alonso et al. (2004); republished with permission from Wiley-Blackwell.
The suppression of brain blood flow early in upright exercise is related to factors other than cardiac output because middle cerebral blood velocity declines when cardiac output is increasing during exercise (Nybo et al. 2002, González-Alonso et al. 2004). The blunted perfusion pressure is a more likely factor because the decreases in left and right middle cerebral artery blood velocity are temporally associated with reductions in arterial and central venous pressures (González-Alonso et al. 2004). A role of perfusion pressure on brain circulation is demonstrated during an orthostatic challenge where middle cerebral artery blood velocity declines drastically when arterial and central venous pressures are compromised (Van Lieshout et al. 2003). The decline in PaCO2 associated with hyperthermia-induced hyperventilation may also be a factor accounting for a portion of the decline in cerebral blood flow during hyperthermic exercise (Rasmussen et al. 2006). Another all-encompassing possibility is that local factors reducing the vasodilator and/or increasing the vasoconstrictor activities suppress brain perfusion. In this regard, the plasma concentration of the potent vasodilator adenosine triphosphate is elevated in the jugular vein (accompanying decreases in venous oxygen saturation), while the brain is apparently taking up large amounts of catecholamines on exhaustion in both conditions and the arterial and jugular venous carbon dioxide partial pressure is declining, suggesting that both vasodilator and vasoconstrictor activities are elevated. Clearly the elucidation of the local mechanisms involved in the decline in cerebral perfusion during prolonged and maximal heat stress exercise warrants further studies quantifying the contribution of the vasodilator and vasoconstrictor systems.
Cardiovascular strain during exercise with dehydration and hyperthermia
Cardiovascular responses to exercise in the heat with and without dehydration have been extensively studied (Saltin & Stenberg 1964, Sawka et al. 1979, Nadel et al. 1980, Montain & Coyle 1992a,b, González-Alonso et al. 1995, 1997, 1998, 1999c, 2000a). Different experimental approaches that reduce body water either prior to exercise (e.g. diuretics, sauna, exercise, water restriction) or during exercise (fluid restriction) have been used to investigate the effects of reduced body fluids on cardiovascular function. The cardiovascular strain produced by different methods of body water deficits is essentially similar (Sawka & Coyle 1999). However, factors such as environmental conditions, intensity, position and type of exercise all influence the extent of the dehydration-mediated cardiovascular alterations (Montain et al. 1998, González-Alonso et al. 1999c, 2000a). The cardiovascular strain inflicted by environmental heat stress and that evoked by dehydration bear several similarities probably because internal body hyperthermia and reduced body fluids are common elements accompanying both stresses. A major difference, however, is that skin temperature in the exercise-induced dehydration studies (usually performed in compensable hot environments with fan cooling) is normally maintained at ~34–35 °C (Montain & Coyle 1992b, González-Alonso et al. 1999a), while in the heat stress studies using a water-perfused suit or no fan cooling it might gradually increase up to ~38–39 °C (Rowell et al. 1969b, González-Alonso et al. 1999c).
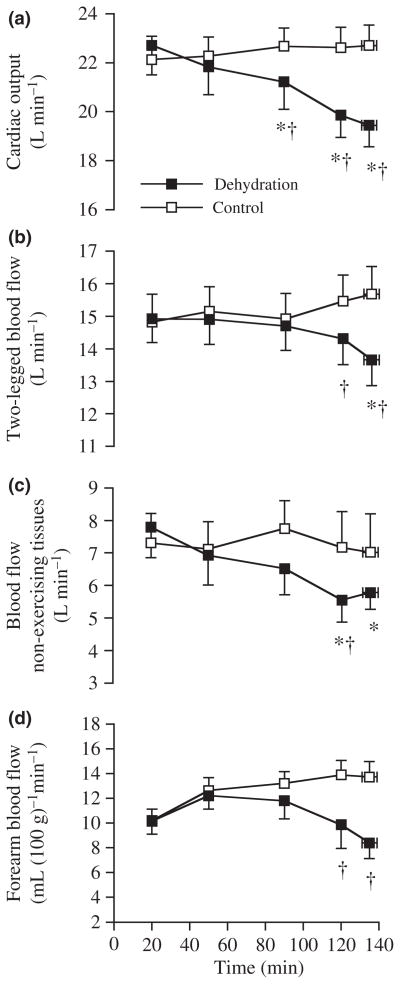
The progressive dehydration incurred during prolonged moderate intensity cycling in compensable heat conditions is associated with gradual reductions in perfusion pressure and blood flow to the skin and locomotor limb tissues accompanying increases in plasma noradrenaline levels and core temperature (Sawka et al. 1979, Montain & Coyle 1992a,b, González-Alonso et al. 1995, 1998, 2000a) (Fig. 8). Cardiac output also declines with marked dehydration and hyperthermia because the larger declines in stroke volume compared with the parallel increases in heart rate (Sawka et al. 1979, Montain & Coyle 1992a,b, González-Alonso et al. 1997). Brain and visceral perfusion are likely to also decline as the lowering in exercising leg blood flow and skin blood flow account for two-thirds of the decline in cardiac output (González-Alonso et al. 1998) and similar levels of hyperthermia and global cardiovascular instability have been associated with reductions in the middle cerebral artery blood velocity and renal and splanchnic blood flow (Rowell et al. 1965, Nybo & Nielsen 2001). Remarkably, these alterations in cardiovascular function are completely prevented when people fully maintain hydration status by fluid ingestion during exercise in the heat (González-Alonso et al. 1995, 1998) or when dehydrated subjects exercise in the cold and intravascular fluid losses are restored with a plasma volume expander (González-Alonso et al. 1997). The diminished intravascular fluids (or related haematological changes) and hyperthermia are therefore important factors underpinning the cardiovascular strain produced by dehydration during exercise in the heat.
Figure 8.
Haemodynamics with dehydration during prolonged exercise in the heat. Systemic and peripheral blood flow during prolonged cycling in the heat with and without dehydration and hyperthermia. Note that the declines in cardiac output are accompanied by reductions in blood flow to the exercising legs, the skin and possible visceral blood flow. *Significantly lower than 20 min value, P < 0.05. †Significantly lower than control, P < 0.05. From González-Alonso et al. (1998); republished with permission from Wiley-Blackwell.
The reduction in stroke volume underlies the fall in cardiac output with dehydration and hyperthermia (Montain & Coyle 1992a, González-Alonso et al. 1997, 1999b, 2000a). Interestingly, when thermoregulatory demands of exercise are minimized in a cold environment and the rise in core temperature is blunted, the decline in stroke volume and the increase in heart rate are significantly attenuated. In these conditions the significant drop in cardiac output normally observed with dehydration and hyperthermia in the heat is prevented (González-Alonso et al. 2000a). Plasma volume expansion in dehydrated and normothermic subjects completely reverses the otherwise small reduction in stroke volume during exercise in the cold, despite the persistent 3–4 L extravascular dehydration (González-Alonso et al. 1997). Similarly, hyperthermia without dehydration induces a small decline in stroke volume during exercise in the heat, which is associated with an increased heart rate; whereas preventing dehydration and hyperthermia via fluid ingestion fully ameliorates these alterations in both stroke volume and heart rate (González-Alonso et al. 1995, 1997, 1998). Hence the reductions in cardiac stroke volume underlying the decline in cardiac output in dehydrated and hyperthermic individuals might be largely related to diminished intravascular volume and the hyperthermia-induced tachycardia.
In 1938, Marius Nielsen (who worked closely with Lindhard and Krogh in The Zoophysiological Laboratory at the University of Copenhagen) demonstrated that core temperature during prolonged moderate exercise was similar across a wide range of ambient temperatures (i.e. 5–35 °C) (Nielsen 1938). Because heat exchange pathways are very different in cold and hot environments, Nielsen’s seminal observation provided the foundations for the idea that core temperature and thus the underlying heat fluxes between the exercising limb muscles and the environment surrounding the limbs, the exercising limbs and the body core, and the body core and the environment surrounding the trunk are well-regulated responses. In this context, the aforementioned dehydration-induced reductions in exercising limb blood flow might impair convective heat transport from active muscle to the surrounding environment and the body core and thus contribute to hyperthermia, independently of the influences of concurrent reductions in sweating rate and skin blood flow (Nadel et al. 1980, Montain&Coyle 1992a). This idea was put to test by quantifying the convective heat transfer from the exercising leg to the body core (i.e. leg blood flow × arterial–venous (a–v) temperature difference, according to the Fick principle) and total heat production from the heat equivalent of leg VO2 (González-Alonso et al. 1999a, 2000b). The results suggested that dehydration impairs heat transfer from the leg to core. More strikingly, they indicated that more than onehalf of the metabolic heat liberated in the contracting leg muscles is dissipated directly to the environment surrounding the leg. These findings highlight the importance of investigating heat-dissipating mechanisms in the exercising limbs and how the stresses of dehydration and heat stress impact upon thermoregulatory function.
Conclusions and future directions
Passive heat stress has the capacity of causing pronounced strain on the cardiovascular system, evidenced by large increases in sympathetic neural activity, heart rate and left ventricular contractility, coupled with reductions in central blood volume, left ventricular filling pressures and cerebral perfusion. The mechanisms resulting in compromised blood pressure control that accompanies such exposure are not entirely clear, although the prevailing data do not support the hypothesis of heat stress-induced impairment in baroreflex responsiveness.
Compelling evidence indicates that the combination of intense whole-body exercise and environmental heat stress or dehydration induced-hyperthermia results in significant cardiovascular strain prior to exhaustion, which is characterized by reductions in cardiac output, stroke volume, arterial pressure and blood flow to the brain, skin and exercising muscle. The local and central mechanisms underpinning these responses remain unresolved. Reductions in skeletal muscle blood flow might not only affect muscle metabolism during exercise but also convective heat transfer to the environment surrounding the exercising limbs and the body core. The study of the muscle and skin circulations in the exercising and non-exercising limbs and the heat fluxes within the body could provide novel insight into how core temperature and its effector responses are regulated in exercising humans.
Acknowledgments
This work was supported by the National Institutes of Health HL61388 and HL84072 (C.G.C.), Marie Curie Training program, Gatorade Sports Science Institute, Team Denmark, Novo Nordisk and the Danish National Research Foundation (J.G.-A.).
Footnotes
Conflict of interest
There is no conflict of interest.
References
- Armstrong RB, Delp MD, Goljan EF, Laughlin MH. Progressive elevations in muscle blood flow during prolonged exercise in swine. J Appl Physiol. 1987;63:285–291. doi: 10.1152/jappl.1987.63.1.285. [DOI] [PubMed] [Google Scholar]
- Asmussen E, Nielsen M. The regulation of the body-temperature during work performed with the arms and with the legs. Acta Physiol Scand. 1947;14:373–382. doi: 10.1111/j.1748-1716.1947.tb00473.x. [DOI] [PubMed] [Google Scholar]
- Bini G, Hagbarth KE, Hynninen P, Wallin BG. Thermoregulatory and rhythm-generating mechanisms governing the sudomotor and vasoconstrictor outflow in human cutaneous nerves. J Physiol. 1980;306:537–552. doi: 10.1113/jphysiol.1980.sp013413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brengelmann GL, Johnson JM, Hermansen L, Rowell LB. Altered control of skin blood flow during exercise at high internal temperatures. J Appl Physiol. 1977;43:790–794. doi: 10.1152/jappl.1977.43.5.790. [DOI] [PubMed] [Google Scholar]
- Brothers RM, Bhella PS, Shibata S, Wingo JE, Levine BD, Crandall CG. Cardiac systolic and diastolic function during whole body heat stress. Am J Physiol Heart Circ Physiol. 2009a;296:H1150–H1156. doi: 10.1152/ajpheart.01069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers RM, Wingo JE, Hubing KA, Crandall CG. The effects of reduced end-tidal carbon dioxide tension on cerebral blood flow during heat stress. J Physiol. 2009b;587:3921–3927. doi: 10.1113/jphysiol.2009.172023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers RM, Zhang R, Wingo JE, Hubing KA, Crandall CG. Effects of heat stress on dynamic cerebral autoregulation during large fluctuations in arterial blood pressure. J Appl Physiol. 2009c;107:1722–1729. doi: 10.1152/japplphysiol.00475.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchthal F, Honcke P, Lindhard J. Temperature measurements in human muscle in situ at rest and during muscular work. Acta Physiol Scand. 1944;8:230–258. [Google Scholar]
- Calbet JA, Gonzalez-Alonso J, Helge JW, Sondergaard H, Munch-Andersen T, Boushel R, Saltin B. Cardiac output and leg and arm blood flow during incremental exercise to exhaustion on the cycle ergometer. J Appl Physiol. 2007;103:969–978. doi: 10.1152/japplphysiol.01281.2006. [DOI] [PubMed] [Google Scholar]
- Christensen EH, Nielsen M, Hannisdahl B. Investigations of the circulation in the skin at the beginning of muscular work. Acta Physiol Scand. 1942;4:171–174. [Google Scholar]
- Crandall CG. Carotid baroreflex responsiveness in heat-stressed humans. Am J Physiol Heart Circ Physiol. 2000;279:H1955–H1962. doi: 10.1152/ajpheart.2000.279.4.H1955. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Farr D, Etzel RA. Cardiopulmonary baroreceptor control of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol. 1999a;277(6 Pt 2):H2348–H2352. doi: 10.1152/ajpheart.1999.277.6.h2348. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Levine BD, Etzel RA. Effect of increasing central venous pressure during passive heating on skin blood flow. J Appl Physiol. 1999b;86:605–610. doi: 10.1152/jappl.1999.86.2.605. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Zhang R, Levine BD. Effects of whole body heating on dynamic baroreflex regulation of heart rate in humans. Am J Physiol Heart Circ Physiol. 2000;279:H2486–H2492. doi: 10.1152/ajpheart.2000.279.5.H2486. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Cui J, Wilson TE. Effects of heat stress on baroreflex function in humans. Acta Physiol Scand. 2003;177:321–328. doi: 10.1046/j.1365-201X.2003.01076.x. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Wilson TE, Marving J, Vogelsang TW, Kjaer A, Hesse B, Secher NH. Effects of passive heating on central blood volume and ventricular dimensions in humans. J Physiol. 2008;586:293–301. doi: 10.1113/jphysiol.2007.143057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during cold pressor test in humans. Am J Physiol Heart Circ Physiol. 2002a;282:H1717–H1723. doi: 10.1152/ajpheart.00899.2001. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Baroreflex modulation of sympathetic nerve activity to muscle in heat-stressed humans. Am J Physiol Regul Integr Comp Physiol. 2002b;282:R252–R258. doi: 10.1152/ajpregu.00337.2001. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Phenylephrine-induced elevation in arterial blood pressure is attenuated in heat-stressed humans. Am J Physiol Regul Integr Comp Physiol. 2002c;283:R1221–R1226. doi: 10.1152/ajpregu.00195.2002. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Muscle sympathetic nerve activity during lower body negative pressure is accentuated in heat-stressed humans. J Appl Physiol. 2004a;96:2103–2108. doi: 10.1152/japplphysiol.00717.2003. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Orthostatic challenge does not alter skin sympathetic nerve activity in heat-stressed humans. Auton Neurosci. 2004b;116:54–61. doi: 10.1016/j.autneu.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Cui J, Zhang R, Wilson TE, Crandall CG. Spectral analysis of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol. 2004c;286:H1101–H1106. doi: 10.1152/ajpheart.00790.2003. [DOI] [PubMed] [Google Scholar]
- Damato AM, Lau SH, Stein E, Haft JI, Kosowsky B, Cohen SI. Cardiovascular response to acute thermal stress (hot dry environment) in unacclimatized normal subjects. Am Heart J. 1968;76:769–774. doi: 10.1016/0002-8703(68)90262-7. [DOI] [PubMed] [Google Scholar]
- Delp MD, Armstrong RB, Godfrey DA, Laughlin MH, Ross CD, Wilkerson MK. Exercise increases blood flow to locomotor, vestibular, cardiorespiratory and visual regions of the brain in miniature swine. J Physiol. 2001;533:849–859. doi: 10.1111/j.1469-7793.2001.t01-1-00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering TJ, Aaslid R, Steuernagel B, Brix J, Niederstadt C, Breull A, Schneider B, Fischer GC. Cerebral autoregulation during whole-body hypothermia and hyperthermia stimulus. Am J Phys Med Rehabil. 1999;78:33–38. doi: 10.1097/00002060-199901000-00009. [DOI] [PubMed] [Google Scholar]
- Edvinsson L. Neurogenic mechanisms in the cerebrovascular bed. Autonomic nerves, amine receptors and their effects on cerebral blood flow. Acta Physiol Scand Suppl. 1975;427:1–35. [PubMed] [Google Scholar]
- Eisalo A. Effects of the Finnish sauna on circulation. Ann Med Exptl Biol Fenniae. 1956;4(Suppl):7–96. [Google Scholar]
- Fan JL, Cotter JD, Lucas RA, Thomas K, Wilson L, Ainslie PN. Human cardiorespiratory and cerebrovascular function during severe passive hyperthermia: effects of mild hypohydration. J Appl Physiol. 2008;105:433–445. doi: 10.1152/japplphysiol.00010.2008. [DOI] [PubMed] [Google Scholar]
- Frank O. Zur dynamik des hermuskels. Z Biol. 1895;32:370–437. [Google Scholar]
- Frayser R, Ross JC, Levin HS, Messer JV, Pines J. Effects of increased environmental temperature on pulmonary diffusing capacity. J Appl Physiol. 1966;21:147–150. doi: 10.1152/jappl.1966.21.1.147. [DOI] [PubMed] [Google Scholar]
- Fritzsche RG, Switzer TW, Hodgkinson BJ, Coyle EF. Stroke volume decline during prolonged exercise is influenced by the increase in heart rate. J Appl Physiol. 1999;86:799–805. doi: 10.1152/jappl.1999.86.3.799. [DOI] [PubMed] [Google Scholar]
- Fujii N, Honda Y, Hayashi K, Soya H, Kondo N, Nishiyasu T. Comparison of hyperthermic hyperpnea elicited during rest and submaximal, moderate-intensity exercise. J Appl Physiol. 2008;104:998–1005. doi: 10.1152/japplphysiol.00146.2007. [DOI] [PubMed] [Google Scholar]
- Gebber GL, Snyder DW. Hypothalamic control of baroreceptor reflexes. Am J Physiol. 1970;218:124–131. doi: 10.1152/ajplegacy.1970.218.1.124. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Berridge FR, Prior KM. Effects of heat and cold on the distribution of blood within the human body. Clin Sci. 1950;9:181–187. [PubMed] [Google Scholar]
- González-Alonso J, Calbet JA. Reductions in systemic and skeletal muscle blood flow and oxygen delivery limit maximal aerobic capacity in humans. Circulation. 2003;107:824–830. doi: 10.1161/01.cir.0000049746.29175.3f. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Mora-Rodríguez R, Bellow PR, Coyle EF. Dehydration reduces cardiac output and increases systemic and cutaneous vascular resistance during exercise. J Appl Physiol. 1995;79:1487–1496. doi: 10.1152/jappl.1995.79.5.1487. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Mora-Rodriguez R, Below PR, Coyle EF. Dehydration markedly impairs cardiovascular function in hyperthermic endurance athletes during exercise. J Appl Physiol. 1997;82:1229–1236. doi: 10.1152/jappl.1997.82.4.1229. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Calbet JA, Nielsen B. Muscle blood flow is reduced with dehydration during prolonged exercise in humans. J Physiol. 1998;513(Pt 3):895–905. doi: 10.1111/j.1469-7793.1998.895ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Calbet JA, Nielsen B. Metabolic and thermodynamic responses to dehydration-induced reductions in muscle blood flow in exercising humans. J Physiol. 1999a;520(Pt 2):577–589. doi: 10.1111/j.1469-7793.1999.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Mora-Rodriguez R, Coyle EF. Supine exercise restores arterial blood pressure and skin blood flow despite dehydration and hyperthermia. Am J Physiol. 1999b;277:H576–H583. doi: 10.1152/ajpheart.1999.277.2.H576. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol. 1999c;86:1032–1039. doi: 10.1152/jappl.1999.86.3.1032. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Mora-Rodriguez R, Coyle EF. Stroke volume during exercise: interaction of environment and hydration. Am J Physiol Heart Circ Physiol. 2000a;278:H321–H330. doi: 10.1152/ajpheart.2000.278.2.H321. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Quistorff B, Krustrup P, Bangsbo J, Saltin B. Heat production in human skeletal muscle at the onset of intense dynamic exercise. J Physiol. 2000b;524(Pt 2):603–615. doi: 10.1111/j.1469-7793.2000.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Dalsgaard MK, Osada T, Volianitis S, Dawson EA, Yoshiga CC, Secher NH. Brain and central haemodynamics and oxygenation during maximal exercise in humans. J Physiol. 2004;557:331–342. doi: 10.1113/jphysiol.2004.060574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Crandall CG, Johnson JM. The cardiovascular challenge of exercising in the heat. J Physiol. 2008;586:45–53. doi: 10.1113/jphysiol.2007.142158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales JRS, Hubbard RW, Gaffin SL. Limitation of heat tolerance. In: Blatteis C, Fregly M, editors. Handbook of Physiology Environmental Physiology. American Physiological Society; Bethesda, MD: 1996. pp. 285–355. [Google Scholar]
- Hanada A, Sander M, Gonzalez-Alonso J. Human skeletal muscle sympathetic nerve activity, heart rate and limb haemodynamics with reduced blood oxygenation and exercise. J Physiol. 2003;551:635–647. doi: 10.1113/jphysiol.2003.044024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistad D, Kontos H. Cerebral circulation. In: Shepherd J, Abboud F, editors. Handbook of Physiology, Section 2: The Cardiovascular System. American Physiological Society; Bethesda, MD: 1983. pp. 137–182. [Google Scholar]
- Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–291. doi: 10.1161/01.res.58.2.281. [DOI] [PubMed] [Google Scholar]
- Ho CW, Beard JL, Farrell PA, Minson CT, Kenney WL. Age, fitness, and regional blood flow during exercise in the heat. J Appl Physiol. 1997;82:1126–1135. doi: 10.1152/jappl.1997.82.4.1126. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Fujii N, Ogawa T, Hayashi K, Kondo N, Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during incremental leg cycling. J Physiol. 2008;586:2753–2766. doi: 10.1113/jphysiol.2007.150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide K, Boushel R, Sorensen HM, Fernandes A, Cai Y, Pott F, Secher NH. Middle cerebral artery blood velocity during exercise with beta-1 adrenergic and unilateral stellate ganglion blockade in humans. Acta Physiol Scand. 2000;170:33–38. doi: 10.1046/j.1365-201x.2000.00757.x. [DOI] [PubMed] [Google Scholar]
- Johnson JM. Exercise and the cutaneous circulation. Exer Sports Sci Rev. 1992;20:59–97. [PubMed] [Google Scholar]
- Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Blatteis C, Fregly M, editors. Handbook of Physiology: Adaptations to the Environment. American Physiological Society; Bethesda, MD: 1996. pp. 215–243. [Google Scholar]
- Johnson JM, Rowell LB. Forearm skin and muscle vascular responses to prolonged leg exercise in man. J Appl Physiol. 1975;39:920–924. doi: 10.1152/jappl.1975.39.6.920. [DOI] [PubMed] [Google Scholar]
- Katz AM. Physiology of the Heart. Lippincott William & Wilkins; Philadelphia: 2006. [Google Scholar]
- Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol. 2006;573:445–451. doi: 10.1113/jphysiol.2006.108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Sander M, Crandall CG. The effect of passive leg heating on alpha-adrenergic mediated vasoconstriction in humans. Faseb J. 2007;21:Abstract 612.613. [Google Scholar]
- Keller D, Low DA, Wingto J, Brothers RM, Hastings J, Davis SL, Crandall CG. Acute volume expansion preserves orthostatic tolerance during whole-body heat stress in humans. J Physiol. 2009;587:1131–1139. doi: 10.1113/jphysiol.2008.165118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney WL, Johnson JM. Control of skin blood flow during exercise. Med Sci Sports Exerc. 1992;24:303–312. [PubMed] [Google Scholar]
- Koroxenidis GT, Shepherd JT, Marshall RJ. Cardiovascular response to acute heat stress. J Appl Physiol. 1961;16:869–872. doi: 10.1152/jappl.1961.16.5.869. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Armstrong RB. Rat muscle blood flows as a function of time during prolonged slow treadmill exercise. Am J Physiol. 1983;244:H814–H824. doi: 10.1152/ajpheart.1983.244.6.H814. [DOI] [PubMed] [Google Scholar]
- Lee K, Jackson DN, Cordero DL, Nishiyasu T, Peters JK, Mack GW. Change in spontaneous baroreflex control of pulse interval during heat stress in humans. J Appl Physiol. 2003;95:1789–1798. doi: 10.1152/japplphysiol.01019.2001. [DOI] [PubMed] [Google Scholar]
- van Lieshout JJ, Secher NH. Point:Counterpoint: Sympathetic activity does/does not influence cerebral blood flow. Point: Sympathetic activity does influence cerebral blood flow. J Appl Physiol. 2008;105:1364–1366. doi: 10.1152/japplphysiol.90597.2008. [DOI] [PubMed] [Google Scholar]
- Lind AR, Leithead CS, McNicol GW. Cardiovascular changes during syncope induced by tilting men in the heat. J Appl Physiol. 1968;25:268–276. doi: 10.1152/jappl.1968.25.3.268. [DOI] [PubMed] [Google Scholar]
- Lindhard J. Investigations into the conditions governing the temperature of the body. In: Mylius-Erichsen L, editor. Danmark-ekspeditionen til Gronlands Nordostkyst 1906–1908. Bianco Lunos Bogtrykkeri; Kobenhavn: 1910. pp. 3–53. [Google Scholar]
- Low DA, Wingo JE, Keller DM, Davis SL, Zhang R, Crandall CG. Cerebrovascular responsiveness to steady-state changes in end-tidal CO2 during passive heat stress. J Appl Physiol. 2008;104:976–981. doi: 10.1152/japplphysiol.01040.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low DA, Wingo JE, Keller DM, Davis SL, Cui J, Zhang R, Crandall CG. Dynamic cerebral autoregulation during passive heat stress in humans. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1598–R1605. doi: 10.1152/ajpregu.90900.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKirnan MD, Gray CG, White FC. Plateau in muscle blood flow during prolonged exercise in miniature swine. J Appl Physiol. 1989;66:2101–2108. doi: 10.1152/jappl.1989.66.5.2101. [DOI] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters cardiovascular response to direct passive heating. J Appl Physiol. 1998;84:1323–1332. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- Montain SJ, Coyle EF. Fluid ingestion during exercise increases skin blood flow independent of increases in blood volume. J Appl Physiol. 1992a;73:903–910. doi: 10.1152/jappl.1992.73.3.903. [DOI] [PubMed] [Google Scholar]
- Montain SJ, Coyle EF. Influence of graded dehydration on hyperthermia and cardiovascular drift during exercise. J Appl Physiol. 1992b;73:1340–1350. doi: 10.1152/jappl.1992.73.4.1340. [DOI] [PubMed] [Google Scholar]
- Montain SJ, Sawka MN, Latzka WA, Valeri CR. Thermal and cardiovascular strain from hypohydration: influence of exercise intensity. Int J Sports Med. 1998;19:87–91. doi: 10.1055/s-2007-971887. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Dawson EA, Yoshiga CC, Dalsgaard MK, Damsgaard R, Secher NH, Gonzalez-Alonso J. Limitations to systemic and locomotor limb muscle oxygen delivery and uptake during maximal exercise in humans. J Physiol. 2005;566:273–285. doi: 10.1113/jphysiol.2005.086025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Damsgaard R, Dawson EA, Secher NH, Gonzalez-Alonso J. Restrictions in systemic and locomotor skeletal muscle perfusion, oxygen supply and VO2 during high-intensity whole-body exercise in humans. J Physiol. 2008;586:2621–2635. doi: 10.1113/jphysiol.2007.149401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller O. Uber die Blutverteilung im menschichen Korper unter dem Einfluss thermischer Reize. Arch Klin Med. 1905;82:547–585. [Google Scholar]
- Nadel ER, Fortney SM, Wenger CB. Effect of hydration state on circulatory and thermal regulations. J Appl Physiol. 1980;49:715–721. doi: 10.1152/jappl.1980.49.4.715. [DOI] [PubMed] [Google Scholar]
- Nelson E, Rennels M. Innervation of intracranial arteries. Brain. 1970;93:475–490. doi: 10.1093/brain/93.3.475. [DOI] [PubMed] [Google Scholar]
- Nielsen M. Die Regulation der Korpertemperature bei Muskelarbeit. Skand Arch Physiol. 1938;79:193–230. [Google Scholar]
- Nielsen B, Nielsen M. Body temperature during work at different environmental temperatures. Acta Physiol Scand. 1962;56:120–129. doi: 10.1111/j.1748-1716.1962.tb02489.x. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Nielsen M. Influence of passive and active heating on the temperature regulation of man. Acta Physiol Scand. 1965a;64:323–331. doi: 10.1111/j.1748-1716.1965.tb04186.x. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Nielsen M. On the regulation of sweat secretion in exercise. Acta Physiol Scand. 1965b;64:314–322. doi: 10.1111/j.1748-1716.1965.tb04185.x. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Savard G, Richter EA, Hargreaves M, Saltin B. Muscle blood flow and muscle metabolism during exercise and heat stress. J Appl Physiol. 1990;69:1040–1046. doi: 10.1152/jappl.1990.69.3.1040. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Hales JR, Strange S, Christensen NJ, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol. 1993;460:467–485. doi: 10.1113/jphysiol.1993.sp019482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen B, Strange S, Christensen NJ, Warberg J, Saltin B. Acute and adaptive responses in humans to exercise in a warm, humid environment. Pflugers Arch. 1997;434:49–56. doi: 10.1007/s004240050361. [DOI] [PubMed] [Google Scholar]
- Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman ASM, Ito H, Sobue G, Mano T. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst. 1997;63:61–67. doi: 10.1016/s0165-1838(96)00134-8. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B. Middle cerebral artery blood velocity is reduced with hyperthermia during prolonged exercise in humans. J Physiol. 2001;534:279–286. doi: 10.1111/j.1469-7793.2001.t01-1-00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybo L, Jensen T, Nielsen B, Gonzalez-Alonso J. Effects of marked hyperthermia with and without dehydration on VO2 kinetics during intense exercise. J Appl Physiol. 2001;90:1057–1064. doi: 10.1152/jappl.2001.90.3.1057. [DOI] [PubMed] [Google Scholar]
- Nybo L, Moller K, Volianitis S, Nielsen B, Secher NH. Effects of hyperthermia on cerebral blood flow and metabolism during prolonged exercise in humans. J Appl Physiol. 2002;93:58–64. doi: 10.1152/japplphysiol.00049.2002. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Brothers RM, Eubank WL, Raven PB. Autonomic neural control of the cerebral vasculature: acute hypotension. Stroke. 2008;39:1979–1987. doi: 10.1161/STROKEAHA.107.510008. [DOI] [PubMed] [Google Scholar]
- Parker JO, Case RB. Normal left ventricular function. Circulation. 1979;60:4–12. doi: 10.1161/01.cir.60.1.4. [DOI] [PubMed] [Google Scholar]
- Patterson SW, Starling EH. On the mechanical factors which determine the output of the ventricles. J Physiol (Lond) 1914;48:357–379. doi: 10.1113/jphysiol.1914.sp001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SW, Piper H, Starling EH. The regulation of the ventricles. J Physiol (Lond) 1914;48:465–513. doi: 10.1113/jphysiol.1914.sp001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Peters JK, Nishiyasu T, Mack GW. Reflex control of the cutaneous circulation during passive body core heating in humans. J Appl Physiol. 2000;88:1756–1764. doi: 10.1152/jappl.2000.88.5.1756. [DOI] [PubMed] [Google Scholar]
- Poliner LR, Dehmer GJ, Lewis SE, Parkey RW, Blomqvist CG, Willerson JT. Left ventricular performance in normal subjects: a comparison of the responses to exercise in the upright and supine positions. Circulation. 1980;62:528–534. doi: 10.1161/01.cir.62.3.528. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Stie H, Nielsen B, Nybo L. Enhanced cerebral CO2 reactivity during strenuous exercise in man. Eur J Appl Physiol. 2006;96:299–304. doi: 10.1007/s00421-005-0079-3. [DOI] [PubMed] [Google Scholar]
- Ray CA, Gracey KH. Augmentation of exerciseinduced muscle sympathetic nerve activity during muscle heating. J Appl Physiol. 1997;82:1719–1725. doi: 10.1152/jappl.1997.82.6.1719. [DOI] [PubMed] [Google Scholar]
- Reis DJ, Cuénod M. Central neural regulation of carotid baroreceptor reflexes in the cat. Am J Physiol. 1965;209:1267–1279. doi: 10.1152/ajplegacy.1965.209.6.1267. [DOI] [PubMed] [Google Scholar]
- Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, Gonzalez-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54:75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Cardiovascular adjustments to thermal stress. In: Rowell LB, editor. Handbook of Physiology, The Cardiovascular System. American Physiological Society; Bethesda, MD: 1983. pp. 967–1024. [Google Scholar]
- Rowell LB. Circulatory adjustments to dynamic exercise. In: Rowell LB, editor. Human Circulation Regulation During Physical Stress. Oxford University Press; New York: 1986a. pp. 213–256. [Google Scholar]
- Rowell LB. Circulatory adjustments to dynamic exercise and heat stress: competing controls. In: Rowell LB, editor. Human Circulation Regulation During Physical Stress. Oxford; New York: 1986b. pp. 363–406. [Google Scholar]
- Rowell LB. Thermal stress. In: Rowell LB, editor. Human Circulation Regulation During Physical Stress. Oxford University Press; New York: 1986c. pp. 174–212. [Google Scholar]
- Rowell LB. Hyperthermia: a hyperadrenergic state. Hypertension. 1990;15:505–507. doi: 10.1161/01.hyp.15.5.505. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Ideas about control of skeletal and cardiac muscle blood flow (1876–2003): cycles of revision and new vision. J Appl Physiol. 2004;97:384–392. doi: 10.1152/japplphysiol.01220.2003. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Blackmon JR, Martin RH, Mazzarella JA, Bruce RA. Hepatic clearance of indocyanine green in man under thermal and exercise stresses. J Appl Physiol. 1965;20:384–394. doi: 10.1152/jappl.1965.20.3.384. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Marx HJ, Bruce RA, Conn RD, Kusumi F. Reductions in cardiac output, central blood volume, and stroke volume with thermal stress in normal men during exercise. J Clin Invest. 1966;45:1801–1816. doi: 10.1172/JCI105484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB, Kraning KK, 2nd, Kennedy JW, Evans TO. Central circulatory responses to work in dry heat before and after acclimatization. J Appl Physiol. 1967;22:509–518. doi: 10.1152/jappl.1967.22.3.509. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol. 1969a;27:673–680. doi: 10.1152/jappl.1969.27.5.673. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Murray JA, Brengelmann GL, Kraning KK., 2nd Human cardiovascular adjustments to rapid changes in skin temperature during exercise. Circ Res. 1969b;24:711–724. doi: 10.1161/01.res.24.5.711. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Brengelmann GL, Blackmon JR, Murray JA. Redistribution of blood flow during sustained high skin temperature in resting man. J Appl Physiol. 1970;28:415–420. doi: 10.1152/jappl.1970.28.4.415. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Detry JR, Profant GR, Wyss C. Spanchnic vasoconstriction in hyperthermic man – role of falling blood pressure. J Appl Physiol. 1971;31:864–869. doi: 10.1152/jappl.1971.31.6.864. [DOI] [PubMed] [Google Scholar]
- Saltin B, Stenberg J. Circulatory response to prolonged severe exercise. J Appl Physiol. 1964;19:833–838. doi: 10.1152/jappl.1964.19.5.833. [DOI] [PubMed] [Google Scholar]
- Sarnoff SJ, Mitchell JH. The regulation of the performance of the heart. Am J Med. 1961;30:747–771. doi: 10.1016/0002-9343(61)90211-x. [DOI] [PubMed] [Google Scholar]
- Savard GK, Nielsen B, Laszczynska J, Larsen BE, Saltin B. Muscle blood flow is not reduced in humans during moderate exercise and heat stress. J Appl Physiol. 1988;64:649–657. doi: 10.1152/jappl.1988.64.2.649. [DOI] [PubMed] [Google Scholar]
- Sawka MN, Coyle EF. Influence of body water and blood volume on thermoregulation and exercise performance in the heat. Exerc Sport Sci Rev. 1999;27:167–218. [PubMed] [Google Scholar]
- Sawka MN, Knowlton RG, Critz JB. Thermal and circulatory responses to repeated bouts of prolonged running. Med Sci Sports. 1979;11:177–180. [PubMed] [Google Scholar]
- Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, Howe HL, Wilhelm JL. Heatrelated deaths during the July 1995 heat wave in Chicago. N Engl J Med. 1996;335:84–90. doi: 10.1056/NEJM199607113350203. [DOI] [PubMed] [Google Scholar]
- Semenza JC, McCullough JE, Flanders WD, McGeehin MA, Lumpkin JR. Excess hospital admissions during the July 1995 heat wave in Chicago [see comments] Am J Prev Med. 1999;16:269–277. doi: 10.1016/s0749-3797(99)00025-2. [DOI] [PubMed] [Google Scholar]
- Strandgaard S, Sigurdsson ST. Point:Counterpoint: Sympathetic activity does/does not influence cerebral blood flow. Counterpoint: Sympathetic nerve activity does not influence cerebral blood flow. J Appl Physiol. 2008;105:1366–1367. doi: 10.1152/japplphysiol.90597.2008a. discussion 1367–1368. [DOI] [PubMed] [Google Scholar]
- Strom G. Central nervous regulation of body temperature. In: Field J, Magoun HW, Hall VE, editors. Handbook of Physiology, Section 1: Neurophysiology. II. Waverly Press; Washington, DC: 1960. pp. 1173–1196. [Google Scholar]
- Templeton GH, Ecker RR, Mitchell JH. Left ventricular stiffness during diastole and systole: the influence of changes in volume and inotropic state. Cardiovasc Res. 1972;6:95–100. doi: 10.1093/cvr/6.1.95. [DOI] [PubMed] [Google Scholar]
- Umeyama T, Kugimiya T, Ogawa T, Kandori Y, Ishizuka A, Hanaoka K. Changes in cerebral blood flow estimated after stellate ganglion block by single photon emission computed tomography. J Auton Nerv Syst. 1995;50:339–346. doi: 10.1016/0165-1838(94)00105-s. [DOI] [PubMed] [Google Scholar]
- Van Lieshout JJ, Wieling W, Karemaker JM, Secher NH. Syncope, cerebral perfusion, and oxygenation. J Appl Physiol. 2003;94:833–848. doi: 10.1152/japplphysiol.00260.2002. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Verbeuren TJ, Webb RC. Local modulation of adrenergic neuroeffector interaction in the blood vessel wall. Physiol Rev. 1981;61:151–247. doi: 10.1152/physrev.1981.61.1.151. [DOI] [PubMed] [Google Scholar]
- Visocchi M, Chiappini F, Cioni B, Meglio M. Cerebral blood flow velocities and trigeminal ganglion stimulation. A transcranial Doppler study. Stereotact Funct Neurosurg. 1996;66:184–192. doi: 10.1159/000099687. [DOI] [PubMed] [Google Scholar]
- Vogiatzis I, Athanasopoulos D, Habazettl H, Kuebler W, Wagner H, Roussos C, Wagner P, Zakynthinos S. Intercostal muscle blood flow limitation in athletes during maximal exercise. J Physiol. 2009;587:3665–3677. doi: 10.1113/jphysiol.2009.171694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Crandall CG. Absence of arterial baroreflex modulation of skin sympathetic activity and sweat rate during whole-body heating in humans. J Physiol. 2001;536:615–623. doi: 10.1111/j.1469-7793.2001.0615c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Crandall CG. Effect of whole-body and local heating on cutaneous vasoconstrictor responses in humans. Auton Neurosci. 2002a;97:122–128. doi: 10.1016/s1566-0702(02)00046-2. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J Appl Physiol. 2002b;93:85–91. doi: 10.1152/japplphysiol.01043.2001. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Zhang R, Crandall CG. Heat stress reduces cerebral blood velocity and markedly impairs orthostatic tolerance in humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1443–R1448. doi: 10.1152/ajpregu.00712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Tollund C, Yoshiga CC, Dawson EA, Nissen P, Secher NH, Crandall CG. Effects of heat and cold stress on central vascular pressure relationships during orthostasis in humans. J Physiol. 2007;585:279–285. doi: 10.1113/jphysiol.2007.137901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Brothers RM, Tollund C, Dawson E, Nissen P, Yoshiga C, Jons C, Secher N, Crandall CG. Effect of thermal stress on Frank–Starling relations in humans. J Physiol. 2009;587:3383–3392. doi: 10.1113/jphysiol.2009.170381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki F, Sone R. Modulation of arterial baroreflex control of heart rate by skin cooling and heating in humans. J Appl Physiol. 2000;88:393–400. doi: 10.1152/jappl.2000.88.2.393. [DOI] [PubMed] [Google Scholar]
- Yamazaki F, Matsumura N, Nagata J, Ando A, Imura T. Spontaneous arterial baroreflex control of the heart rate during head-down tilt in heat-stressed humans. Eur J Appl Physiol. 2001;85:208–213. doi: 10.1007/s004210100482. [DOI] [PubMed] [Google Scholar]
- Yamazaki F, Yamauchi K, Tsutsui Y, Endo Y, Sagawa S, Shiraki K. Whole body heating reduces the baroreflex response of sympathetic nerve activity during Valsalva straining. Auton Neurosci. 2003;103:93–99. doi: 10.1016/s1566-0702(02)00140-6. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation. 2002;106:1814–1820. doi: 10.1161/01.cir.0000031798.07790.fe. [DOI] [PubMed] [Google Scholar]