Abstract
AIM: To identify the incidence and etiology of anemia after gastrectomy in patients with long-term follow-up after gastrectomy for early gastric cancer.
METHODS: The medical records of those patients with early gastric adenocarcinoma who underwent curative gastrectomy between January 2006 and October 2007 were reviewed. Patients with anemia in the preoperative workup, cancer recurrence, undergoing systemic chemotherapy, with other medical conditions that can cause anemia, or treated during follow up with red cell transfusions or supplements for anemia were excluded. Anemia was defined by World Health Organization criteria (Hb < 12 g/dL in women and < 13 g/dL in men). Iron deficiency was defined as serum ferritin < 20 μg/dL. Vitamin B12 deficiency was defined as serum vitamin B12 < 200 pg/mL. Iron deficiency anemia was defined as anemia with concomitant iron deficiency. Anemia from vitamin B12 deficiency was defined as megaloblastic anemia (mean cell volume > 100 fL) with vitamin B12 deficiency. The profile of anemia over 48 mo of follow-up was analyzed.
RESULTS: One hundred sixty-one patients with gastrectomy for early gastric cancer were analyzed. The incidence of anemia was 24.5% at 3 mo after surgery and increased up to 37.1% at 48 mo after surgery. The incidence of iron deficiency anemia increased during the follow up and became the major cause of anemia at 48 mo after surgery. Anemia of chronic disease and megaloblastic anemia were uncommon. The incidence of anemia in female patients was significantly higher than in male patients at 12 (40.0% vs 22.0%, P = 0.033), 24 (45.0% vs 25.0%, P = 0.023), 36 (55.0% vs 28.0%, P = 0.004), and 48 mo (52.0% vs 31.0%, P = 0.022) after surgery. Patients with total gastrectomy showed significantly higher incidence of anemia than patients with subtotal gastrectomy at 48 mo after surgery (60.7% vs 31.3%, P = 0.008). The incidence of iron deficiency was significantly higher in female patients than in male patients at 6 (35.4% vs 13.3%, P = 0.002), 12 (45.8% vs 16.8%, P < 0.001), 18 (52.1% vs 22.3%, P < 0.001), 24 (60.4% vs 20.9%, P < 0.001), 36 (62.5% vs 29.2%, P < 0.001), and 48 mo (66.7% vs 34.7%, P = 0.001) after surgery.
CONCLUSION: Anemia was frequent after gastrectomy for early gastric cancer, with iron deficiency being the major cause. Evaluation for anemia including iron status should be performed after gastrectomy and appropriate iron replacement should be considered.
Keywords: Gastrectomy, Stomach neoplasms, Anemia, Iron deficiency, Vitamin B12 deficiency
INTRODUCTION
Gastric cancer is the most common malignancy in Korea and the second most frequent cause of cancer-related death worldwide[1,2]. Curative resection has proven to be the only successful treatment modality for locally confined gastric cancer[3]. Anemia is a frequent complication after gastrectomy and deficiencies of iron, vitamin B12, or folate, either alone or in combination, have been reported after gastric surgery[4-6]. However, these studies included relatively small numbers of patients, follow-up visits were not systematically scheduled, and the definition and biochemical markers of anemia were ambiguous or insufficient. There are limited reports concerning anemia in patients who undergo gastrectomy for early gastric cancer who have systematically scheduled serial follow-up visits, but do not receive supplements for anemia.
The incidence of gastric bypass surgery for morbid obesity is increasing, and anemia after bypass surgery has been reported[7-11]. Dietary life after gastric surgery in patients with gastric cancer may differ from that in patients with morbid obesity: in contrast to patients with obesity, patients having gastrectomy for gastric cancer do not restrict their dietary intake for weight reduction. The aim of this study was to identify the natural history of anemia after gastrectomy in a cohort of patients undergoing gastrectomy for early gastric cancer who were systematically followed up in the long term.
MATERIALS AND METHODS
Study population
This study was a retrospective chart review of the registry of all patients who had undergone gastrectomy for early gastric cancer at Seoul St Mary’s Hospital, Seoul, Korea between January 2006 and October 2007. Patients with anemia in the preoperative workup, cancer recurrence, undergoing systemic chemotherapy, with other medical conditions that can cause anemia, or treated during follow up with red cell transfusions or supplements for anemia were excluded from the study.
Follow-up program
Patients with early gastric cancer were followed up at 3, 6, 9, 12, 18, 24, 48 and 60 mo after surgery. The follow-up program consisted of interim history taking, physical examination, imaging studies, endoscopic examination, hematology, and blood chemistry panels. Mean cell volume (MCV), serum iron, serum ferritin, total iron-binding capacity, serum vitamin B12, and serum folate level were included in the follow-up program.
Definition of anemia and related conditions
Anemia was defined by World Health Organization criteria (Hb <12 g/dL in women and <13 g/dL in men)[9]. Iron deficiency was defined as serum ferritin < 20 μg/dL. Vitamin B12 deficiency was defined as serum vitamin B12 < 200 pg/mL. Iron deficiency anemia was defined as anemia with concomitant iron deficiency. Anemia of chronic illness was defined as anemia with serum ferritin > 20 μg/dL. Anemia from vitamin B12 deficiency was defined as megaloblastic anemia (MCV >100 fL) with vitamin B12 deficiency.
Statistical analysis
Continuous data are presented as the mean ± SD and categorical data are presented as proportions. The data were analyzed using the paired t test to assess the differences in continuous data during follow up and a χ2 test or Fisher’s exact test to assess the differences according to sex and the type of gastrectomy. Differences were considered significant if the P value was less than 0.05. All statistical analysis were performed using SAS software (SAS Institute, Cary, NC, United States).
RESULTS
Four hundred fifty-eight patients with early gastric adenocarcinoma underwent curative gastrectomy in our institution during the study period. Of these, 297 were excluded from the analysis (anemia in preoperative work-up in 45 patients, systemic chemotherapy in 79, other medical conditions that can cause anemia in 13, follow-up loss in 86, insufficient biochemical profile for anemia in 73, red cell transfusion during the follow-up in one). Finally, 161 patients undergoing gastrectomy for early gastric cancer were analyzed. No patient in the study population received iron or vitamin B12 replacement therapy. Follow-up after surgery was possible at 3 mo in 159, 6 mo in 161, 12 mo in 161, 18 mo in 160, 24 mo in 158, 36 mo in 154, and 48 mo in 142.
The characteristics of the study population are shown in Table 1. The mean age was 56.2 ± 10.9 years (range, 23-78 years) and 113 patients were men (70.2%). Thirty-nine (24.2%) patients underwent Billroth I subtotal gastrectomy, 90 (55.9%) Billroth II subtotal gastrectomy, and 32 (19.9%) total gastrectomy.
Table 1.
Patients characteristics (n = 161)
| Variable | Value n (%) |
| Age (yr)1 | 56.2 ± 10.9 |
| Gender | |
| Male | 113 (70.2) |
| Female | 48 (29.8) |
| Preoperative hemoglobin (g/dL)1 | 14.2 ± 1.1 |
| Male | 14.6 ± 0.9 |
| Female | 13.2 ± 0.8 |
| Combined disease | |
| Hypertension | 20 (12.4) |
| Diabetes mellitus | 19 (11.8) |
| Pulmonary disease | 2 (1.2) |
| Coronary artery disease | 2 (1.2) |
| Chronic liver disease | 2 (1.2) |
| Type of operation | |
| Billroth I subtotal gastrectomy | 39 (24.2) |
| Billroth II subtotal gastrectomy | 90 (55.9) |
| Total gastrectomy | 32 (19.9) |
| Nodal Stage | |
| N0 | 143 (88.8) |
| N1 | 15 (9.4) |
| N2 | 2 (1.2) |
| N3 | 1 (0.6) |
Data are presented as mean ± SD.
The incidence of anemia was 24.5% at 3 mo after surgery and increased to 37.1% at 48 mo after surgery. The incidence of iron deficiency anemia increased during the follow-up and became the major cause of anemia at 48 mo after surgery (Figure 1). Anemia of chronic disease and megaloblastic anemia were uncommon. Only one patient had a picture consistent with megaloblastic anemia with vitamin B12 deficiency at 48 mo after surgery. The incidence of anemia, iron deficiency, and vitamin B12 deficiency increased during follow-up after surgery (Figure 2). The incidence during follow-up of iron deficiency without anemia was considerable. Most vitamin B12 deficiency occurred in patients who had total gastrectomy. At 48 mo after gastrectomy, the incidence of vitamin B12 deficiency was 3.2% in patients with Billroth Isubtotal gastrectomy, 7.5% in Billroth II subtotal gastrectomy, and 76.9% in total gastrectomy. The incidence of vitamin B12 deficiency in patients with total gastrectomy was 0%, 16.1%, 50.0% and 76.9% at 12, 24, 36 and 48 mo after gastrectomy, respectively.
Figure 1.
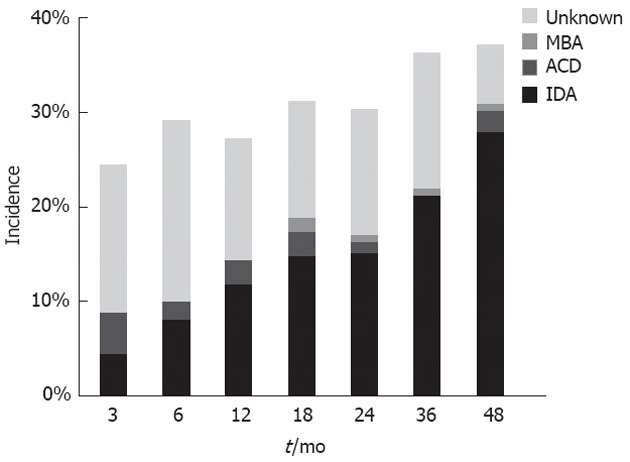
Incidence and distribution of anemia after gastrectomy. IDA: Iron deficiency anemia; ACD: Anemia of chronic disease; MBA: Megaloblastic anemia.
Figure 2.
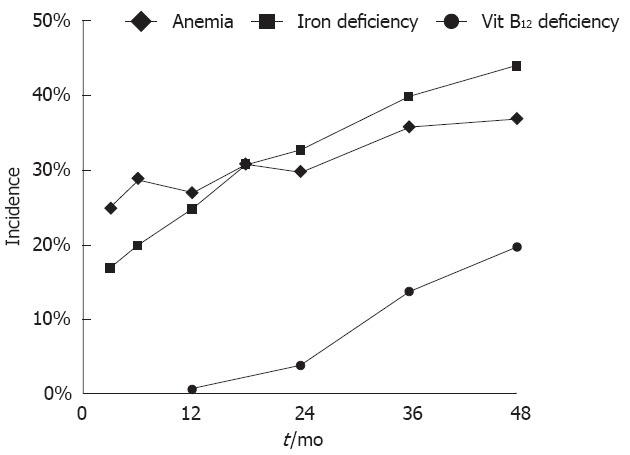
Incidence of anemia, iron deficiency state, and vitamin B12 deficiency state after gastrectomy.
Serial follow-up hematology and blood chemistry data are shown in Table 2. Hemoglobin level, serum ferritin level, and vitamin B12 level decreased during the follow-up. The average serum albumin level was 3.3 ± 0.4 g/dL before surgery and recovered to 4.2 ± 0.3 g/dL at 3 mo after surgery.
Table 2.
Biochemical marker after gastrectomy
| Before surgery | 3 mo | 6 mo | 12 mo | 18 mo | 24 mo | 36 mo | 48 mo | |
| Hemoglobin (g/dL) | 14.2 ± 1.1 | 13.4 ± 1.2 | 13.3 ± 1.2a | 13.4 ± 1.3 | 13.2 ± 1.3a | 13.2 ± 1.4a | 13.1 ± 1.5a | 13.1 ± 1.6a |
| Serum ferritin (μg/L) | 70.8 ± 59.1 | 63.9 ± 52.4a | 55.2 ± 43.4a | 49.3 ± 51.3a | 43.3 ± 46.0a | 34.7 ± 32.2a | 33.7 ± 30.7a | |
| Serum vitamin B12 (pg/mL) | 1167.7 ± 597.6 | 1113.9 ± 629.4 | 773.7 ± 999.9c | 560.3 ± 472.9c | ||||
| Serum albumin (g/dL) | 3.3 ± 0.4 | 4.2 ± 0.3 | 4.2 ± 0.4 | 4.3 ± 0.3 | 4.3 ± 0.3 | 4.3 ± 0.3 | 4.3 ± 0.3 | 4.3 ± 0.3 |
P < 0.05 vs 3 mo;
P < 0.05 vs 12 mo.
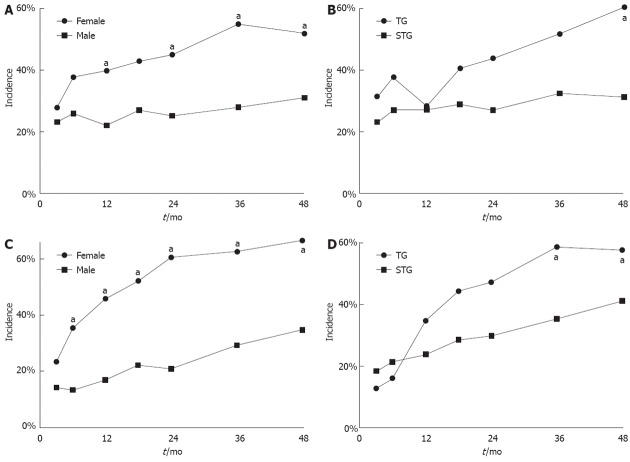
The incidence of anemia in female patients was significantly higher than in male patients at 12 mo, 24 mo, 36 mo, and 48 mo after surgery (Figure 3A). Patients with total gastrectomy showed significantly higher incidence of anemia at 48 mo after surgery than patients with subtotal gastrectomy (60.7% vs 31.3%, P = 0.008) (Figure 3B). There was no significant difference in the incidence of anemia 48 mo after surgery between patients having Billroth I and Billroth II subtotal gastrectomy (18.8% vs 36.1%, P = 0.078). The incidence of iron deficiency was also significantly higher in female patients than in male patients at all times in the follow-up period except at 3 mo after surgery (Figure 3C). The incidence of iron deficiency was significantly higher in patients with total gastrectomy than those with in subtotal gastrectomy only at 36 mo after surgery (58.1% vs 35.0%, P = 0.024) (Figure 3D). There was no significant difference in the incidence of anemia and iron deficiency between patients with Billroth I and II subtotal gastrectomy at 48 mo after surgery (28.1% vs 45.8%, P = 0.095).
Figure 3.
Incidence of anemia and deficiency state after gastrectomy. A: Significantly higher incidence of anemia in female patients than in male patients at 12, 24, 36 and 48 mo after surgery, aP < 0.05 vs female group; B: Significantly higher incidence of anemia in patients with total gastrectomy (TG) than in patients with subtotal gastrectomy (STG) at 48 mo after surgery, aP < 0.05 vs STG group; C: Significantly higher incidence of iron deficiency state in female patients than in male patients at all times in the follow-up period except at 3 mo after surgery, aP < 0.05 vs male group; D: Significantly higher incidence of iron deficiency in patients with TG than in patients with STG at 36 mo after surgery, aP < 0.05 vs STG group.
DISCUSSION
In this study, we identified the incidence and etiology of anemia after gastrectomy in a cohort of patients having gastrectomy for gastric cancer who were systematically followed up over the long term. We found that the incidence of anemia in the patients with gastrectomy increased during follow-up. Iron deficiency anemia became the major cause of anemia after gastrectomy, and megaloblastic anemia with vitamin B12 deficiency was rare.
In this study, anemia was relatively frequent after gastrectomy for early gastric cancer. Anemia was detected shortly after surgery and increased during the follow-up period. The incidence of anemia was 24.5% at 3 mo after surgery and increased to 37.1% at 48 mo after surgery. Anemia has been reported to occur in up to 60% of patients after partial gastrectomy[12,13]. The incidence of anemia after bypass surgery has been reported as from 5% to 64%[7-9,14-17]. A recent study of postsurgical anemia after gastrectomy reported that the incidence of anemia was 37.9%, 33.5% and 34.7% at 3, 6 and 12 mo after surgery, respectively[18].
Our study showed that iron deficiency anemia increased during follow-up and was the major cause of anemia at 48 mo after surgery. Serial follow-up showed that serum ferritin gradually decreased from 70.8 μg/L to 30.1 μg/L during follow up and the incidence of iron deficiency gradually increased to 44% at 48 mo after gastrectomy. Our results are similar to those of a previous study of patients after Roux-en-Y gastric bypass[19]. Iron deficiency anemia was reported to be very common in post-gastrectomy patients at twenty five to thirty years after surgery[20]. Iron deficiency after gastrectomy or bypass results from malabsorption of iron because of a surgically altered gastrointestinal tract. Inadequate oral intake or occult blood loss may also contribute to this condition. Iron is absorbed in the duodenum and proximal jejunum and its absorption is enhanced by gastric acid secretion. A lack of acidity results in impaired conversion of ingested ferric iron to absorbable ferrous iron[21]. Duodenal bypass also contributes to iron deficiency in patients with gastrectomy or bypass surgery. Atrophic gastritis and Helicobacter pylori infection were reported to play an important role in iron deficiency after gastrectomy for cancer of the stomach[22]. In our study, the patients with Billroth I subtotal gastrectomy showed the lowest incidence of iron deficiency compared with all groups of patients with duodenal bypass (total gastrectomy and Billroth II subtotal gastrectomy) at 48 mo after surgery (28.1% vs 48.6%, P = 0.045). Our study showed a higher incidence of anemia and iron deficiency in patients with total gastrectomy than in those with subtotal gastrectomy. This difference may originate from the more depleted nutritional status of patients with total gastrectomy[15,23,24]. In contrast to the subjects of other studies of severe obesity, our study population was patients with early gastric cancer and did not need weight reduction. They were recommended an adequate oral intake and serum albumin level was maintained at an adequate level. Therefore, our results show an etiology and natural course of postgastrectomy anemia that is close to reality.
The incidence of vitamin B12 deficiency gradually increased to 20% of patients in our study at 48 mo after gastrectomy. The majority of the patients with vitamin B12 deficiency at 48 mo were those with total gastrectomy. Vitamin B12 deficiency causes megaloblastic anemia and neurological symptoms, and is known to develop within 5 or 6 years after total gastrectomy, a delay that reflects the time needed to exhaust cobalamin stores after cobalamin absorption ceases[25]. Our study showed that 16.1%, 50.0% and 76.9% of the patients with total gastrectomy presented with vitamin B12 deficiency at 24, 36 and 48 mo after gastrectomy, respectively. Although the incidence of vitamin B12 deficiency at 48 mo after gastrectomy was high in our study, only one patient with vitamin B12 deficiency presented with megaloblastic anemia. Because 70% of patients with vitamin B12 deficiency at 48 mo after gastrectomy showed anemia and the majority of these were iron-deficient, the generation of macrocytosis by concomitant iron deficiency should be considered[26]. Vitamin B12 deficiency can develop within 2 years after total gastrectomy and vitamin B12 replacement may be considered for postgastrectomy patients with persistent anemia despite iron replacement or with neurological symptoms combined with vitamin B12 deficiency.
Our study has some limitations. This study was a single-center retrospective study. The symptoms of anemia and other nutritional parameters such as body weight or body mass were not investigated. However, a strength of our study is the tightly controlled long-term follow-up of the patient cohort after gastrectomy for early gastric cancer, with systematically and prospectively scheduled serial follow-up visits and without any supplement for anemia. Our results enabled us to understand the development of post-gastrectomy anemia.
In conclusion, anemia was frequent after gastrectomy for early gastric cancer and iron deficiency was the major cause. Evaluation for anemia including iron status should be performed after gastrectomy and appropriate iron replacement should be considered.
COMMENTS
Background
Curative resection has proven to be the only successful treatment modality for locally confined gastric cancer. Anemia is a frequent complication after gastrectomy. The present study identified the natural history of anemia after gastrectomy in a cohort of patients undergoing gastrectomy for early gastric cancer who were systematically followed up in the long term.
Research frontiers
Anemia was frequent after gastrectomy for early gastric cancer, with iron deficiency being the major cause.
Innovations and breakthroughs
Anemia is a common complication of gastrectomy, particularly in female patients and it worsens as follow-up lengthens in a cohort with long-term follow-up for 48 mo. The incidence of iron deficiency gradually increased during follow up and became the major cause of anemia. Megaloblastic anemia is uncommon although the incidence of vitamin B12 deficiency gradually increased after gastrectomy, particularly particularly after total gastrectomy.
Applications
The results of this study suggest that evaluation for anemia including iron status should be performed after gastrectomy and appropriate iron replacement should be considered.
Peer review
This study examined anemia in a large cohort of patients undergoing gastrectomy for early gastric cancer. The results of this study can serve as a benchmark in the follow up of patients undergoing partial or total gastrectomy for gastric carcinoma.
Footnotes
Peer reviewer: Luigi Bonavina, Professor, Department of Surgery, IRCCS Policlinico San Donato, Piazza Malan, 20097 Milano, Italy
S- Editor Gou SX L- Editor A E- Editor Lu YJ
References
- 1.Shin HR, Jung KW, Won YJ, Park JG. 2002 annual report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004;36:103–114. doi: 10.4143/crt.2004.36.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Song KY, Park SM, Kim SN, Park CH. The role of surgery in the treatment of recurrent gastric cancer. Am J Surg. 2008;196:19–22. doi: 10.1016/j.amjsurg.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 4.Wastell C. Malabsorptive states after gastrointestinal surgery. Br Med J. 1968;3:661–664. doi: 10.1136/bmj.3.5619.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harju E. Metabolic problems after gastric surgery. Int Surg. 1990;75:27–35. [PubMed] [Google Scholar]
- 6.Beyan C, Beyan E, Kaptan K, Ifran A, Uzar AI. Post-gastrectomy anemia: evaluation of 72 cases with post-gastrectomy anemia. Hematology. 2007;12:81–84. doi: 10.1080/10245330600938554. [DOI] [PubMed] [Google Scholar]
- 7.Skroubis G, Sakellaropoulos G, Pouggouras K, Mead N, Nikiforidis G, Kalfarentzos F. Comparison of nutritional deficiencies after Roux-en-Y gastric bypass and after biliopancreatic diversion with Roux-en-Y gastric bypass. Obes Surg. 2002;12:551–558. doi: 10.1381/096089202762252334. [DOI] [PubMed] [Google Scholar]
- 8.Toh SY, Zarshenas N, Jorgensen J. Prevalence of nutrient deficiencies in bariatric patients. Nutrition. 2009;25:1150–1156. doi: 10.1016/j.nut.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Cable CT, Colbert CY, Showalter T, Ahluwalia R, Song J, Whitfield P, Rodriguez J. Prevalence of anemia after Roux-en-Y gastric bypass surgery: what is the right number? Surg Obes Relat Dis. 2011;7:134–139. doi: 10.1016/j.soard.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Amaral JF, Thompson WR, Caldwell MD, Martin HF, Randall HT. Prospective hematologic evaluation of gastric exclusion surgery for morbid obesity. Ann Surg. 1985;201:186–193. doi: 10.1097/00000658-198502000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747–1750. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hines JD, Hoffbrand AV, Mollin DL. The hematologic complications following partial gastrectomy. A study of 292 patients. Am J Med. 1967;43:555–569. doi: 10.1016/0002-9343(67)90179-9. [DOI] [PubMed] [Google Scholar]
- 13.Tovey FI, Clark CG. Anaemia after partial gastrectomy: a neglected curable condition. Lancet. 1980;1:956–958. doi: 10.1016/s0140-6736(80)91406-3. [DOI] [PubMed] [Google Scholar]
- 14.Brolin RE, Gorman JH, Gorman RC, Petschenik AJ, Bradley LJ, Kenler HA, Cody RP. Are vitamin B12 and folate deficiency clinically important after roux-en-Y gastric bypass? J Gastrointest Surg. 1998;2:436–442. doi: 10.1016/s1091-255x(98)80034-6. [DOI] [PubMed] [Google Scholar]
- 15.Braga M, Molinari M, Zuliani W, Foppa L, Gianotti L, Radaelli G, Cristallo M, Di Carlo V. Surgical treatment of gastric adenocarcinoma: impact on survival and quality of life. A prospective ten year study. Hepatogastroenterology. 1996;43:187–193. [PubMed] [Google Scholar]
- 16.Vargas-Ruiz AG, Hernández-Rivera G, Herrera MF. Prevalence of iron, folate, and vitamin B12 deficiency anemia after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2008;18:288–293. doi: 10.1007/s11695-007-9310-0. [DOI] [PubMed] [Google Scholar]
- 17.Coupaye M, Puchaux K, Bogard C, Msika S, Jouet P, Clerici C, Larger E, Ledoux S. Nutritional consequences of adjustable gastric banding and gastric bypass: a 1-year prospective study. Obes Surg. 2009;19:56–65. doi: 10.1007/s11695-008-9571-2. [DOI] [PubMed] [Google Scholar]
- 18.Jeong O, Park YK, Ryu SY. Prevalence, severity, and evolution of postsurgical anemia after gastrectomy, and clinicopathological factors affecting its recovery. J Korean Surg Soc. 2012;82:79–86. doi: 10.4174/jkss.2012.82.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruz M, Carrasco F, Rojas P, Codoceo J, Inostroza J, Rebolledo A, Basfi-fer K, Csendes A, Papapietro K, Pizarro F, et al. Iron absorption and iron status are reduced after Roux-en-Y gastric bypass. Am J Clin Nutr. 2009;90:527–532. doi: 10.3945/ajcn.2009.27699. [DOI] [PubMed] [Google Scholar]
- 20.Tovey FI, Godfrey JE, Lewin MR. A gastrectomy population: 25-30 years on. Postgrad Med J. 1990;66:450–456. doi: 10.1136/pgmj.66.776.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs A, Miles PM. Role of gastric secretion in iron absorption. Gut. 1969;10:226–229. doi: 10.1136/gut.10.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roviello F, Fotia G, Marrelli D, De Stefano A, Macchiarelli R, Pinto E. Iron deficiency anemia after subtotal gastrectomy for gastric cancer. Hepatogastroenterology. 2004;51:1510–1514. [PubMed] [Google Scholar]
- 23.Bae JM, Park JW, Yang HK, Kim JP. Nutritional status of gastric cancer patients after total gastrectomy. World J Surg. 1998;22:254–260; discussion 260-261. doi: 10.1007/s002689900379. [DOI] [PubMed] [Google Scholar]
- 24.Bozzetti F, Ravera E, Cozzaglio L, Dossena G, Agradi E, Bonfanti G, Koukouras D, Gennari L. Comparison of nutritional status after total or subtotal gastrectomy. Nutrition. 1990;6:371–375. [PubMed] [Google Scholar]
- 25.Maclean LD, Sundberg RD. Incidence of megaloblastic anemia after total gastrectomy. N Engl J Med. 1956;254:885–893. doi: 10.1056/NEJM195605102541902. [DOI] [PubMed] [Google Scholar]
- 26.Mahmud K, Ripley D, Swaim WR, Doscherholmen A. Hematologic complications of partial gastrectomy. Ann Surg. 1973;177:432–435. doi: 10.1097/00000658-197304000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]