Abstract
Desmoid tumor is a locally invasive, myofibroblastic, nonmetastatic tumor. Its pathogenesis remains unclear and it may involve genetic abnormalities, sex hormones and traumatic injury, including surgery. Postoperative intra-abdominal desmoid tumor is rare, especially in the retroperitoneum. We report a case of postoperative retroperitoneal desmoid tumor that developed 29 mo after the first excision of a gastrointestinal stromal tumor. Sporadic trauma-related intra-abdominal desmoid tumors reported in the English literature are also reviewed. Despite an extremely low incidence, postoperative desmoid tumor should be considered in the differential diagnosis when a recurrent neoplasm is found at least one year after operation. However, it is a clinical challenge to distinguish recurrent malignant neoplasms from desmoid tumors, and surgical resection is the treatment option depending on the anatomic location.
Keywords: Desmoid tumor, Gastrointestinal stromal tumor, Recurrence, Retroperitoneum, Surgery
INTRODUCTION
Desmoid tumor (also known as aggressive fibromatosis or fibromatosis) is an infrequently occurring, locally invasive, nonmetastatic tumor. Desmoid tumors account for 0.03% of all newly diagnosed neoplasms and 3% of all soft tissue neoplasms[1]. These tumors originate from musculoaponeurotic planes and are found intra- and extra-abdominally. The cause of desmoid tumor is unclear and it may be related to trauma, hormonal factors, or genetic associations[2]. The occurrence of a surgery-related retroperitoneal desmoid tumor after the excision of gastrointestinal stromal tumor (GIST) is rare. However, despite its scarcity, desmoid tumor should be differentiated from recurrent neoplasm in diagnosis, especially those occurring near the previous operative site.
CASE REPORT
A 56-year-old man complained of epigastralgia for three weeks without symptomatic improvement after receiving medical treatment at another hospital in 2009, where a deep-seated gastric ulcer had been found by panendoscope. Because there had been no symptomatic relief, he was referred to the in-patient department of our hospital. On physical examination, abdominal fullness with obvious rebounding pain was noticed. A perforated peptic ulcer was suspected and an abdominal computed tomography (CT) scan was performed. The abdominal CT scan revealed a 10-cm gastric mass with perforation (Figure 1A). In May 2009, the patient underwent an emergent explorative laparotomy with debulking of the intraperitoneal tumor and irrigation. A ruptured GIST, of intermediate risk category, with spreading intra-abdominal tumors was diagnosed pathologically (Figure 1B and C). He had no family history of familial adenomatous polyposis (FAP) or colorectal diseases among his close relatives. Because the abdominal CT scan had shown no signs of FAP, colonoscopy was not performed. His clinical course was uneventful and he was discharged on day 8 after admission. After the operation, imatinib (Glivec) 200 mg b.i.d. was prescribed. However, due to intractable diarrhea, the target therapy was discontinued. The follow-up abdominal CT scan showed no recurrent tumor two months after the operation. He was then lost to follow-up. The patient revisited our out-patient clinic 12 mo later after the previous operation. The abdominal CT scan showed a 4.7-cm hepatic tumor in segments 6 and 7 and an intra-abdominal 2.5-cm mass located in the upper greater curvature of the stomach (Figure 1D). The impression was metastatic GISTs. He received a second operation with a smooth course in June 2010 and metastatic GISTs were confirmed pathologically (Figure 1E). However, a 1.9-cm tumor between the pancreatic tail and splenic hilum was found in the follow-up abdominal CT scan 3 mo after the second operation. The tumor was located in the incision area of the first debulking operation. The patient refused another operation, and he was managed with target therapy with imatinib (Glivec) 200 mg b.i.d., which was well tolerated. A series of follow-up abdominal CT scans were performed. Fifteen months after the second operation, CT scan revealed progressive enlargement of the splenic hilar tumor from 1.9 cm to 3.2 cm (Figure 1F). The patient continued to take imatinib (Glivec) regularly. Under the impression of a second recurrent GIST, he underwent a third operation in October 2011. Intraoperatively, the tumor was found in the retroperitoneum adhering to the peri-tumor vessels, nerves and the pancreatic tail. This tumor was resected en bloc with sacrifice of adjacent vessels and nerves. Grossly, the tumor measured 3.6 cm × 2.5 cm × 1.1 cm and was elastic with grayish-brown cut surface. Histologically, the tumor demonstrated proliferative spindle cells in fibrotic background with keloid-like, glassy, hyalinized collagen fibers, nodular fasciitis-like erythrocyte extravasation and infiltrative growth pattern. Immunohistochemical staining of the tumor cells revealed positive nuclear stains for beta-catenin, but negative stains for CD117 and CD34 (Figure 1G). A desmoid tumor was diagnosed pathologically. The patient’s clinical course was uneventful and no recurrent tumor was found 6 mo after the third operation.
Figure 1.
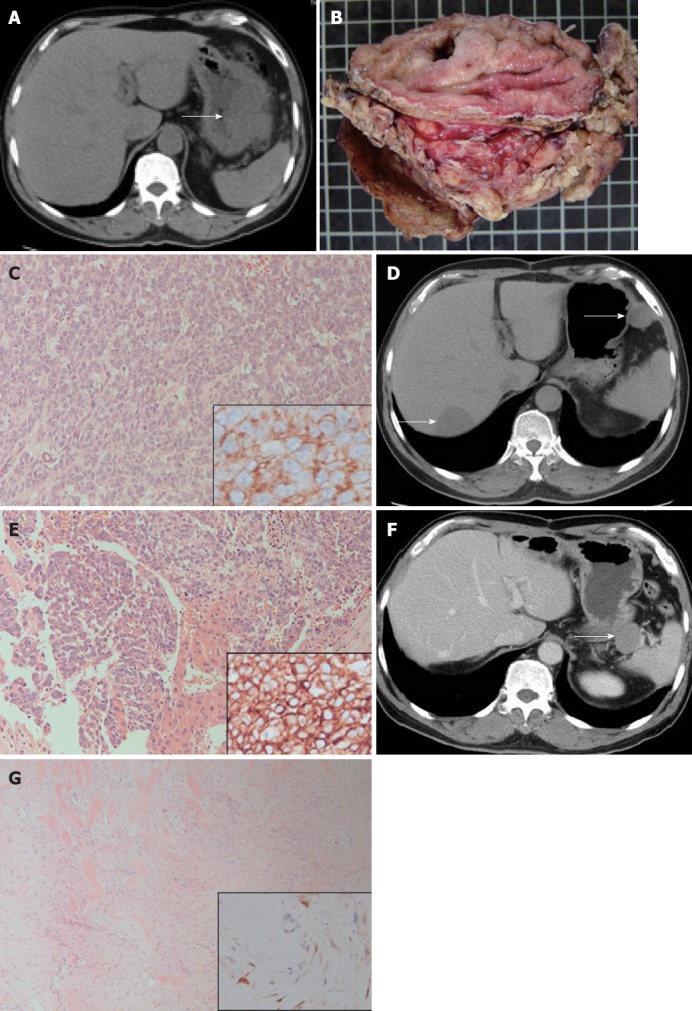
Abdominal computed tomography images and pathologic features of gastrointestinal stromal tumor, metastatic hepatic gastrointestinal stromal tumor and retroperitoneal desmoid tumor. A: Abdominal computed tomography (CT) scan reveals a 10-cm tumor (arrow) located at the greater curvature of the stomach; B: The excised specimen discloses a patch of gastric mucosa with a central deep-seated ulcer and the gastrointestinal stromal tumor adhering to the red yellow omental tissues; C: Histologically, the 10-cm gastric tumor demonstrated sheets of CD117-positive epithelioid cells after immunohistochemical (IHC) staining (right lower inset), hematoxylin and eosin (HE) stain, ×200; D: The follow-up abdominal CT scan shows metastatic tumors in the liver (left lower arrow) and upper greater curvature of the stomach (right upper arrow); E: The histology of metastatic liver tumor demonstrated nests and sheets of CD117-positive epithelioid tumor cells (right lower inset, IHC stain). Entrapped hepatocytes are seen in the middle lower portion, HE stain, ×200; F: Abdominal CT scan discloses a tumor neogrowth (arrow) located in the retroperitoneum between the pancreatic tail and splenic hilar region; G: Histologically, the retroperitoneal tumor demonstrated proliferative spindle cells with keloid-like bundles and erythrocyte extravasation, HE stain, ×200. IHC staining reveals a positive nuclear beta-catenin in spindle cells (right lower inset).
DISCUSSION
Although desmoid tumor is locally aggressive with infiltrative growth behavior, it is considered to be a benign neoplasm for its bland cellular appearance, scant mitosis and lack of metastasis. The term “desmoid” originates from the Greek word “demos”, meaning band or tendon like, and was first named by Müller[3] in 1838. Desmoid tumor accounts for 0.03% of all neoplasms and 3.0% of all soft tissue tumors[1].
Although the actual cause of desmoid tumor is still being debated, its likely pathogenesis includes genetic abnormalities, sex hormones and trauma, including surgical trauma. Kulaylat et al[2] found that 10%-30% of all sporadic abdominal wall desmoid tumors occurred following surgical intervention and half of these tumors developed within 4 years after the surgery. Warren[4] described the criteria for post-traumatic neoplasm, including prior integrity of the tumor site, injury severe enough to initiate reparative cell proliferation, reasonable latent period, and tumor compatible with the scar tissue and anatomic location of the injury. In the present case, the splenic hilar region was incised during the debulking operation for the ruptured GIST of the stomach, and the desmoid tumor occurred 29 mo after the surgery. The present case of postoperative retroperitoneal desmoid tumor was compatible with Warren’s criteria[4] and occurred within an appropriate latent period.
The English literature includes 12 cases, including the present case, of sporadic postoperative intra-abdominal desmoid tumor[5-15] (Table 1). The male-to-female ratio was 8:3 (one case showed no gender datum) and the mean age was 49.6 years (range, 27-79 years). Five of the 12 cases were located on the mesentery, which is the most frequent site of this type of desmoid tumor to date. Tumor sizes ranged from 2.8 cm to 18 cm and the mean duration from previous operative insult to excision of desmoid tumor was 2.3 years (range, 11 mo to 7 years). It indicates that a reasonable latent period for this type of desmoid tumor is at least one year. None of the desmoid tumors was diagnosed preoperatively. This denotes the diagnostic challenge of this type of desmoid tumor. Ten of the 12 desmoid tumors were widely excised under the impression of recurrent malignant neoplasm. No recurrence was found in 8 cases followed up between 6 mo to 2 years. Up till now, only 3 postoperative desmoid tumors after resection of gastric GIST have been reported in the literature. Whether GIST is a risk factor for the development of desmoid tumors or just a coincidence should be further elucidated.
Table 1.
Sporadic postoperative intra-abdominal desmoid tumors reported in the literature
| No. | Authors | Sex/age | Location | Tumor size(cm) | Diagnosis of previous operation | Type of primary trauma | Duration | Treatment | R/F |
| 1 | Mizuno et al[5] | M/61 | Mesentery near anastomosis | 2.8 | Ascending colon cancer (pT3N1M0) | Right hemicolectomy | 16 mo | Excision with ileum and colon | N/2 yr |
| 2 | Liao et al[6] | F/79 | Left lower abdomen | 17 × 14× 13 | Encapsulated lipoma | Resection | 7 yr | Excision | N/6 mo |
| 3 | Khan et al[7] | -/37 | Mesentery | 6 × 4.5 × 4 | Gastric GIST | Total gastrectomy | 11 mo | Excision | N/8 mo |
| 4 | Vendrell et al[8] | M/58 | Posterior side of stomach | 8 × 6 × 4 | Gastric GIST | Laparoscopic tumorectomy | 2 yr | Wide excision with splenectomy and total gastrectomy | - |
| 5 | Komatsu et al[9] | M/50 | Mesentery in left upper abdominal cavity | 10 × 6 | Gastric adenocarcinoma (pT3N1M0) | Total gastrectomy | 1 yr | Excision with jejunum | N/9 mo |
| 6 | Tamura et al[10] | F/73 | Mesentery of jejunal pouch | 6.3 × 5 × 5 | Gastric cancer (pT1N1M0) | Total gastrectomy | 1 yr | Excision and reconstruction | N/4 yr |
| 7 | Lawatsch et al[11] | M/27 | Retroperitoneum | 17 × 13.5 × 8.5 | Mixed germ cell tumor of right testis | Retroperitoneal lymph node dissection | 2 yr | Excision with ileum and colon | N/10 mo |
| 8 | Lai et al[12] | M/45 | Anterior lower abdomen | - | Mesenteric injury and bowel gangrene | Abdominal surgery | 1 yr | Excision with ileum | - |
| 9 | Firoozmand et al[13] | F/27 | Pelvic | 17 × 14 × 10 | - | Colectomy with ileoanal J pouch anastomosis | 4 yr | En bloc resection | - |
| 10 | Little et al[14] | M/31 | Left upper abdomen | 12 to 14 | Mixed germ cell tumor of right testis | Retroperitoneal lymph node dissection | 2 yr | Excision with jejunum | N/18 mo |
| 11 | Pasciak et al[15] | M/51 | Mesentery | 18 × 12 × 11 | Transitional cell carcinoma of urinary bladder | Radical cystectomy | 3 yr | Excision with small bowel | - |
| 12 | Shih et al, present | M/56 | Retroperitoneum | 3.6 × 2.5 × 1.1 | Gastric GIST | Exploratory debulking | 29 mo | En bloc excision | N/6 mo |
R: Recurrency; F: Follow-up; N: No; -: Not mentioned; GIST: Gastrointestinal stromal tumor.
Recently, the Wingless/Wnt signaling pathway was hypothesized to be involved in the tumorigenesis of desmoid tumors, especially the two key proteins, adenomatous polyposis coli (APC) and beta-catenin[16]. APC is considered to be a tumor suppressor gene and beta-catenin an oncogene. Sporadic desmoid tumors typically present oncogenic mutation in beta-catenin. However, FAP-associated desmoid tumors are associated with germline APC mutation followed by somatic inactivation of the wild-type APC allele[17,18]. Beta-catenin protein level is upregulated in desmoid tumors, due to either APC mutations and subsequent ineffective regulation of beta-catenin activation, or beta-catenin gene mutations that led to stabilization and constitutive activation of the beta-catenin. These pathways indicated that the expression of nuclear beta-catenin may play a role in the differential diagnosis of desmoid tumors from fibroblastic or smooth muscle neoplasms[16].
Primary wide surgical resection with tumor-free margins is the treatment of choice for desmoid tumor. This surgical strategy made it essential to sometimes sacrifice the adhered normal vital tissues such as vessels and nerves. Resections with tumor-positive margins indicate a high risk of recurrence, and secondary resection, chemotherapy or radiotherapy may be performed consequently according to the patient’s condition[19]. Garbay et al[20] treated 62 patients with cytotoxic chemotherapy for progressive or recurrent desmoid tumors, and 80% of the patients had a clinical benefit (objective response plus stable disease) from the cytotoxic chemotherapy. Anthracycline-containing regimens appeared to be associated with a higher response rate. Radiotherapy for unresectable cases or local tumor control after surgery has been empirically applied in some instances[21]. However, the efficacy of the adjuvant treatment still needs to be further elucidated. Recently, three discrete mutations (ACC41GCC, TCT45TTT and TCT45CCT) in two codons of CTNNB1 exon 3 were reported[22]. Target therapy for desmoid tumor may be feasible and become another treatment option in the future.
Postoperative intra-abdominal desmoid tumor is exceptionally rare and is often overlooked by clinicians. Most clinicians believe that a tumor with locally infiltrative growth behavior in patients with a history of malignancy is a recurrent malignant tumor, and a wide excision with sacrifice of adjacent organs is usually done. However, despite an extremely low incidence, a postoperative desmoid tumor should be included in the differential diagnoses.
In conclusion, postoperative intra-abdominal desmoid tumor is rare and a correct preoperative diagnosis is a clinical challenge. Physicians should keep in mind the possibility that a postoperative desmoid tumor may appear as a so-called “recurrent” neoplasm, especially when the tumor presents at the previous surgical site.
Footnotes
Supported by The Buddhist Dalin Tzu-Chi General Hospital
Peer reviewers: Cesare Ruffolo, MD, PhD, IV Unit of Surgery, Regional Hospital Cà Foncello, Piazza Ospedale 1, 31100 Treviso, Italy; Jai Dev Wig, MS, FRCS, Former Professor and Head, Department of General Surgery, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India; Roderick M Quiros, MD, FACS, Surgical Oncologist, Cancer Care Associates, 801 Ostrum Street, Bethlehem, PA 18015, United States
S- Editor Shi ZF L- Editor A E- Editor Li JY
References
- 1.Nuyttens JJ, Rust PF, Thomas CR, Turrisi AT. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: A comparative review of 22 articles. Cancer. 2000;88:1517–1523. [PubMed] [Google Scholar]
- 2.Kulaylat MN, Karakousis CP, Keaney CM, McCorvey D, Bem J, Ambrus Sr JL. Desmoid tumour: a pleomorphic lesion. Eur J Surg Oncol. 1999;25:487–497. doi: 10.1053/ejso.1999.0684. [DOI] [PubMed] [Google Scholar]
- 3.Müller J. Ueber den feinern Bau und die Formen der krankhaften Geschwülste. Berlin: G Reimer; 1838. 80 pp. [Google Scholar]
- 4.Warren S. Minimal criteria required to prove causation of traumatic or occupational neoplasms. Ann Surg. 1943;117:585–595. doi: 10.1097/00000658-194304000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizuno R, Akiyoshi T, Kuroyanagi H, Fujimoto Y, Ueno M, Oya M, Kanda H, Yamaguchi T. Intra-abdominal desmoid tumor mimicking locoregional recurrence after colectomy in a patient with sporadic colon cancer: report of a case. Surg Today. 2011;41:730–732. doi: 10.1007/s00595-010-4340-y. [DOI] [PubMed] [Google Scholar]
- 6.Liao CM, Chang WC, Ko KH, Kao HW, Cheng MF, Huang GS, Wu CJ. Desmoid tumor arising in the site of previous surgery in the left lower quadrant of the abdomen. South Med J. 2010;103:162–164. doi: 10.1097/smj.0b013e3181bf2d61. [DOI] [PubMed] [Google Scholar]
- 7.Khan M, Bozas G, Cooke J, Wedgwood K, Maraveyas A. Mesenteric desmoid tumor developing on the site of an excised gastrointestinal stromal tumor. Rare Tumors. 2010;2:e33. doi: 10.4081/rt.2010.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vendrell JF, Mazars R, Funakoshi N, Ramos J, Gallix B, Navarro F, Blanc P. Desmoid tumor subsequent to resection of a gastrointestinal stromal tumor. European Journal of Radiology Extra. 2008;65:9–11. [Google Scholar]
- 9.Komatsu S, Ichikawa D, Kurioka H, Koide K, Ueshima Y, Shioaki Y, Lee CJ, Mutoh F, Hosokawa Y, Oka T, et al. Intra-abdominal desmoid tumor mimicking lymph node recurrence after gastrectomy for gastric cancer. J Gastroenterol Hepatol. 2006;21:1224–1226. doi: 10.1111/j.1440-1746.2006.04210.x. [DOI] [PubMed] [Google Scholar]
- 10.Tamura K, Tani M, Kinoshita H, Yamaue H. Mesenteric desmoid tumor of the interposed jejunal pouch after total gastrectomy. World J Surg Oncol. 2006;4:27. doi: 10.1186/1477-7819-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawatsch EJ, Datta MW, Van Tuinen P, Sudakoff GS, Davis NB, Langenstroer P. Intra-abdominal desmoid tumor following retroperitoneal lymph node dissection for testicular germ cell tumor. Int J Urol. 2006;13:84–86. doi: 10.1111/j.1442-2042.2006.01231.x. [DOI] [PubMed] [Google Scholar]
- 12.Lai KKT, Chan YYR, Chan HCE, Chin CWA. Intra-abdominal desmoid tumour. J HK Coll Radiol. 2003;6:97–99. [Google Scholar]
- 13.Firoozmand E, Prager E. Pelvic desmoid tumor: threat to mother and fetus. Am Surg. 2001;67:1213–1215. [PubMed] [Google Scholar]
- 14.Little JS, Foster RS. Intra-abdominal desmoid tumor: an unusual case of recurrent tumor in a testis cancer patient. J Urol. 1992;147:1619–1621. doi: 10.1016/s0022-5347(17)37648-6. [DOI] [PubMed] [Google Scholar]
- 15.Pasciak RM, Kozlowski JM. Mesenteric desmoid tumor presenting as an abdominal mass following salvage cystectomy for invasive bladder cancer. J Urol. 1987;138:145–146. doi: 10.1016/s0022-5347(17)43026-6. [DOI] [PubMed] [Google Scholar]
- 16.Lips DJ, Barker N, Clevers H, Hennipman A. The role of APC and beta-catenin in the aetiology of aggressive fibromatosis (desmoid tumors) Eur J Surg Oncol. 2009;35:3–10. doi: 10.1016/j.ejso.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Gebert C, Hardes J, Kersting C, August C, Supper H, Winkelmann W, Buerger H, Gosheger G. Expression of beta-catenin and p53 are prognostic factors in deep aggressive fibromatosis. Histopathology. 2007;50:491–497. doi: 10.1111/j.1365-2559.2007.02619.x. [DOI] [PubMed] [Google Scholar]
- 18.Tejpar S, Michils G, Denys H, Van Dam K, Nik SA, Jadidizadeh A, Cassiman JJ. Analysis of Wnt/Beta catenin signalling in desmoid tumors. Acta Gastroenterol Belg. 2005;68:5–9. [PubMed] [Google Scholar]
- 19.Buitendijk S, van de Ven CP, Dumans TG, den Hollander JC, Nowak PJ, Tissing WJ, Pieters R, van den Heuvel-Eibrink MM. Pediatric aggressive fibromatosis: a retrospective analysis of 13 patients and review of the literature. Cancer. 2005;104:1090–1099. doi: 10.1002/cncr.21275. [DOI] [PubMed] [Google Scholar]
- 20.Garbay D, Le Cesne A, Penel N, Chevreau C, Marec-Berard P, Blay JY, Debled M, Isambert N, Thyss A, Bompas E, et al. Chemotherapy in patients with desmoid tumors: a study from the French Sarcoma Group (FSG) Ann Oncol. 2012;23:182–186. doi: 10.1093/annonc/mdr051. [DOI] [PubMed] [Google Scholar]
- 21.El-Haddad M, El-Sebaie M, Ahmad R, Khalil E, Shahin M, Pant R, Memon M, Al-Hebshi A, Khafaga Y, Al-Shabanah M, et al. Treatment of aggressive fibromatosis: the experience of a single institution. Clin Oncol (R Coll Radiol) 2009;21:775–780. doi: 10.1016/j.clon.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Lazar AJ, Tuvin D, Hajibashi S, Habeeb S, Bolshakov S, Mayordomo-Aranda E, Warneke CL, Lopez-Terrada D, Pollock RE, Lev D. Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol. 2008;173:1518–1527. doi: 10.2353/ajpath.2008.080475. [DOI] [PMC free article] [PubMed] [Google Scholar]


