Abstract
The relationship between the production of reactive oxygen species and the hypersensitive response (HR) of tobacco (Nicotiana tabacum L.) toward an incompatible race of the Oomycete Phytophthora parasitica var nicotianae has been investigated. A new assay for superoxide radical (O2−) production based on reduction of the tetrazolium dye sodium,3′-(1-[phenylamino-carbonyl]-3,4-tetrazolium)-bis(4-methoxy-6-nitro) benzene-sulfonic acid hydrate (XTT) has enabled the quantitative estimation of perhydroxyl/superoxide radical acid-base pair (HO2·/O2−) production during the resistant response. Tobacco suspension cells were inoculated with zoospores from compatible or incompatible races of the pathogen. Subsequent HO2·/O2− production was monitored by following the formation of XTT formazan. In the incompatible interaction only, HO2·/O2− was produced in a minor burst between 0 and 2 h and then in a major burst between 8 and 10 h postinoculation. During this second burst, rates of XTT reduction equivalent to a radical flux of 9.9 × 10−15 mol min−1 cell−1 were observed. The HO2·/O2− scavengers O2− dismutase and Mn(III)desferal each inhibited dye reduction. An HR was observed in challenged, resistant cells immediately following the second burst of radical production. Both scavengers inhibited the HR when added prior to the occurrence of either radical burst, indicating that O2− production is a necessary precursor to the HR.
The HR elicited in plant cells by incompatible pathogens has been widely studied in a variety of plant/pathogen systems (Goodman and Novacky, 1994). A decrease in passive membrane potential and depolarization (Pelissier et al., 1986; Davies, 1987), an increase in extracellular pH (Pavlovkin et al., 1986), a loss of electrolytes from the plant cell due to membrane damage (Atkinson et al., 1985; Keppler and Novacky, 1987; Keppler et al., 1989), an induction of mRNA for enzymes involved in the production of phytoalexins (Dixon and Lamb, 1990), and an accumulation of pathogenesis-related proteins (Linthorst, 1991) have all been linked to this response. Recently, the HR has been associated with an oxidative burst resulting from the production of ROS, particularly the perhydroxyl/superoxide radical acid-base pair (HO2·/O2−) and its stable dismutation product, H2O2 (for reviews, see Sutherland, 1991; Baker and Orlandi, 1995; Low and Merida, 1996; Mehdy et al., 1996; Wojtaszek, 1997). ROS may play a role in the induction of lignin production (Olson and Varner, 1993) and phytoalexin accumulation (Anderson et al., 1991; Degousee et al., 1994; see however, Davis et al., 1993). ROS-induced membrane lipid damage has been implicated in loss of membrane integrity and subsequent cell death (Adam et al., 1989).
Various methods have been used to detect and monitor O2− radicals during the HR. These include electron spin resonance (Von Goenner et al., 1993), chemiluminescence (Anderson et al., 1991), and reduction of the tetrazolium dye NBT (Doke, 1983a, 1983b) or of the redox protein Cyt c (Doke, 1983a, 1985). Each of these methods has potential shortcomings. Electron spin resonance requires expensive infrastructure and suffers from questions of signal specificity (Pou et al., 1989). Chemiluminescence does not directly detect HO2·/O2− but instead estimates production of H2O2, which may result from a variety of metabolic processes other than the dismutation of HO2·/O2− (Halliwell and Gutteridge, 1989). Whereas Cyt c reduction assays have been used routinely to quantify radical fluxes in animal cells (Babior et al., 1973), this 12.5-kD protein may not diffuse freely across plant cell walls from external solutions to the plasmalemma surface (the putative site of radical generation). Hence, a significant proportion of the radicals produced will escape reaction with the protein, because of their short half-life. In the case of the much smaller NBT molecule, the cell wall is unlikely to represent a barrier to diffusion. However, the insolubility of its reduced formazan, although rendering this agent useful for localization studies, does not assist kinetic studies or simple estimation of the yield of HO2·/O2− intercepted by the dye.
Recently, we developed a new assay for HO2·/O2− generation using the tetrazolium dye XTT, which is reduced by HO2·/O2− to a soluble formazan that can be readily quantified in solution (Sutherland and Learmonth, 1997). The synthesis of XTT was first reported in 1988 (Paull et al., 1988), and it has since been used primarily as an indicator dye in cell-proliferation studies in the presence of the redox intermediate phenazine methylsulfate (Scuderio et al., 1988). In this study, the XTT assay, performed in the absence of phenazine methylsulfate, has been applied to tobacco (Nicotiana tabacum L.) suspension cultures challenged with live zoospores from compatible and incompatible races of Ppn. This technique, coupled with a cell culture system that retains gene-for-gene specificity against Oomycete zoospores, provides a quantitative estimation of HO2·/O2− production during the HR of the host cells. Furthermore, in contrast to observations in other plant-pathogen interactions (Keppler et al., 1989; Glazener et al., 1991), both phases of the observed oxidative burst appear to be specific to an incompatible interaction.
MATERIALS AND METHODS
Chemicals
MS salts were obtained from ICN and XTT was purchased from Diagnostic Chemicals (Charlottetown, Prince Edward Island, Canada). All other reagents were obtained from Sigma.
Fungal Cultures
Pathogenicity of stock cultures of the tobacco (Nicotiana tabacum L.) black shank pathogen Ppn (Australian field isolates 4974 and 9201) was maintained by regular infection of susceptible tobacco cultivars and reisolation onto oatmeal agar (adapted from Hegelson and Haberlach, 1980). Subcultures were maintained on carrot agar in the dark at 26°C.
Zoospores were produced using a modification of the techniques of Gooding and Lucas (1959) and Hardham et al. (1991). After 2 to 3 weeks on oatmeal agar, mycelial mats were stripped from the agar surface and incubated in 15 mL of sterile water in the dark at 24°C for approximately 2 weeks. When numerous sporangia had developed, the plates were drained, flooded with chilled water, and refrigerated for 30 min at 4°C. Fungal mats were then returned to 24°C and placed on a light box for 20 to 30 min, during which time zoospores were released from sporangia. Zoospores (including both swimming and encysted spores) were counted and diluted such that approximately 2 × 104 zoospores were added to wells containing host cells and reagents to a final volume of 2 mL (see below).
Plant Cultures
Seed of tobacco was supplied by Peter Trevorrow (QDPI, Mareeba, Queensland, Australia). Tobacco callus was initiated from stem tissue of the near-isogenic cvs Hicks and North Carolina 2326 (Helgeson and Haberlach, 1980). Cultivar NC2326 contains a segmental substitution introgressed from Nicotiana plumbaginifolia (Chaplin, 1962) determining resistance to Ppn race 0 (Collins et al., 1971). Sterile stem pieces were transferred to MS agar medium (Murashige and Skoog, 1962) containing 2 μg mL−1 Gly, 0.5 μg mL−1 nicotinic acid, 0.5 μg mL−1 pyridoxine, 0.1 μg mL−1 thiamine hydrochloride, 3% (w/v) Suc, 2 μg mL−1 naphthalene acetic acid, and 0.25 μg mL−1 kinetin, and were incubated in the dark at 26°C for 2 to 4 weeks.
Suspension cultures were initiated from this callus into an identical liquid medium (pH 5.8) at 28°C, incubated at 100 rpm in a benchtop shaker incubator (model 4600C, Bioline, Edwards Instrument Co., Narellan, NSW, Australia), and subcultured every 7 to 8 d. When desired, large cell aggregates were removed by sequential passage of cultures through 500- and 200-μm mesh filters, and unclumped cells were retained on a 100-μm mesh filter.
Log-phase cell cultures (for zoospore challenge) were prepared by subculturing approximately 25 mL of cells into approximately 75 mL of fresh medium and growing for 4 d at 24 or 28°C as above. Cells were then washed twice in 10 mm potassium phosphate buffer (pH 7.8) and resuspended into 10 mm potassium phosphate buffer (pH 7.5) plus 1% (w/v) Suc. One milliliter of cell suspension with a wet cell mass of 0.1 g mL−1 was added to wells in 24-well tissue-culture plates (catalog no. 3820–024, Iwaki, Tokyo, Japan) and incubated at 24°C for 2 h to allow for adjustment to altered conditions (Devlin and Gustine, 1992) before addition of zoospores and reagents. The viability of tobacco cell suspensions was determined in 20-μL aliquots using an assay based on the active uptake of hypertonic neutral red by viable cells (O'Connell et al., 1985). Cell protein content was determined by the method of Bradford (1976).
Protoplasts were prepared using log-phase cultures by the method of Razdan (1993), and viability was monitored using an assay based on the uptake of Evans blue by nonviable cells (Baker and Mock, 1994).
O2− Anion Generation
XTT was added to the Ppn-tobacco system to indicate HO2·/O2− generation by the cells. Stock solutions (10−2 m) were prepared and stored for no longer than 1 week at 4°C. Before use, the XTT stock was warmed to 45°C to ensure full dissolution. A final concentration of 5 × 10−4 m XTT was added to culture wells at 0 h. Multiple wells of each treatment type were prepared to permit replication of measurements at each sampling time. Two to four replicate wells were harvested at desired intervals, and the supernatants were collected for spectrophotometric analysis of XTT formazan production at the peak A470 (Sutherland and Learmonth, 1997). Alternatively, 20 μm Cyt c was added to the wells at 0 h and reduced Cyt c production was monitored at 550 nm (McCord and Fridovich, 1969). The quantity of HO2·/O2− produced was determined using the molar extinction coefficients 2.1 × 104 m−1 s−1 for Cyt c at 550 nm (Massey, 1959) and 2.16 × 104 m−1 s−1 for XTT at 470 nm (Sutherland and Learmonth, 1997).
To confirm the role of HO2·/O2− in these reductions, 100 units of active SOD in 10 mm potassium phosphate buffer (pH 7.5) was added to each well. Inactive SOD was prepared by boiling an aliquot of stock solution for 10 min. Alternatively, Mn(III)desferal prepared according to the method of Rabinowitch et al. (1987) was added at concentrations (40–280 μm) equivalent to 100 units of SOD (as determined by Faulkner et al., 1994).
Statistical Analysis
Data were analyzed by appropriate Student's t tests or other analyses of variance. Significant differences between individual treatments were determined by lsd or Newman-Kuhl tests.
RESULTS
Cell Viability and Mycelium Development Postinoculation
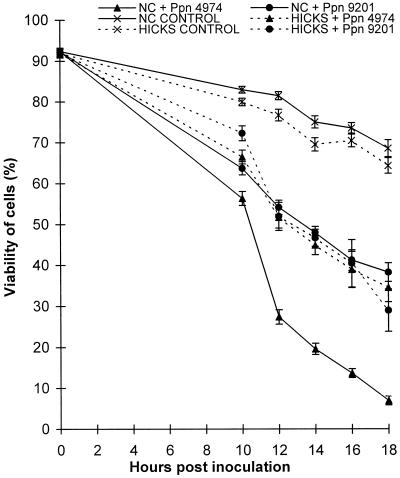
Uninoculated cells incubated in wells containing 10 mm potassium phosphate buffer and 1% Suc at pH 7.5 showed a slow loss of viability over the course of experiments (Fig. 1). Omission of Suc significantly reduced cell survival. Higher levels of Suc were avoided because the resulting increase in medium viscosity affected the swimming behavior of the zoospores.
Figure 1.
The viability of tobacco suspension-cultured cells inoculated with compatible and incompatible zoospores from Ppn. Data are means ± se of 20 replicates from six independent experiments.
At 24°C, cv NC2326 cells inoculated with Ppn 9201 and cv Hicks cells inoculated with Ppn 4974 or Ppn 9201 (compatible interactions) gradually lost viability compared with the controls (Fig. 1). Although cv NC2326 suspension cells inoculated with Ppn 4974 (incompatible interaction) initially showed a loss of viability similar to the compatible interactions, the rate of cell death significantly increased 10 h after inoculation, indicating that the cv NC2326 cells were undergoing an HR to infection by Ppn 4974.
In compatible interactions, encystment of zoospores occurred within 2 h, formation of an appressorial peg occurred by 4 h, followed by penetration of cells leading to prolific growth of hyphae by 12 h. In contrast, whereas appressorial pegs were also formed during the incompatible interaction, postpenetration growth of hyphae was severely restricted. At 28°C, cv NC2326 cells inoculated with Ppn 4974 did not exhibit a resistant response. Both the subsequent viability of these cells and the extent of fungal mycelium growth were similar to those observed in susceptible cells.
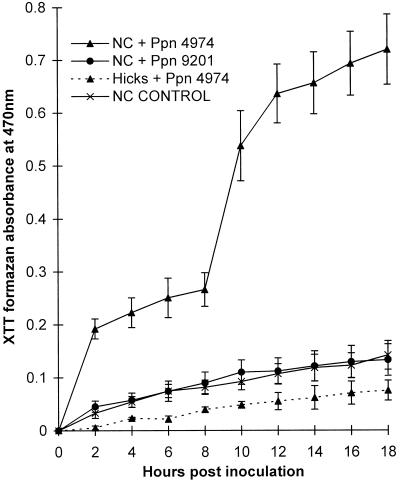
XTT Reduction by Inoculated Tobacco Cells
When XTT was added at 0 h postinoculation and XTT formazan was allowed to accumulate for more than 18 h at 24°C, there was a very low background reduction by uninoculated control cells. Inoculated susceptible cells did not reduce the dye significantly more than control cells during this period (Fig. 2). In contrast, cv NC2326 cells inoculated with Ppn 4974 (incompatible interaction) significantly reduced the dye in two steps. The first burst of reducing activity occurred in the 2 h immediately following inoculation. A more substantial second burst of reduction took place 8 to 10 h after inoculation. The timing of the second burst varied between repeats of the same experiment by up to 1 h. Observation of infection sites indicated that this variation in timing correlated with similar variations in the timing of zoospore encystment on the tobacco cell walls. At 28°C, there was no significant reduction of XTT above the rate for controls during any interaction. When the accumulation of formazan was followed using 2 mm XTT at pH 5.8 and at 24°C in 10 mm Mes buffer or MS medium plus 1% Suc, trends similar to those described above were observed.
Figure 2.
Cumulative reduction of XTT by tobacco suspension-cultured cells inoculated with Ppn zoospores. cv NC2326 cells were inoculated with Ppn 4974 and Ppn 9201 zoospores, representing the incompatible and compatible interactions, respectively. cv Hicks cells were inoculated with Ppn 4974, also representing the compatible interaction. XTT (5 × 10−4 m) was added at 0 h and its formazan was allowed to accumulate. Data are means ± se of 20 replicates from six independent experiments.
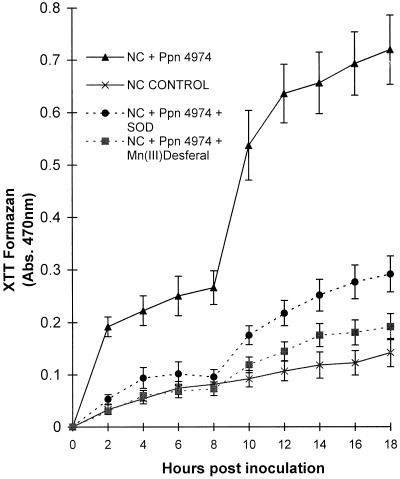
Effects of O2− Scavengers
The addition of active SOD significantly inhibited the production of XTT formazan by cv NC2326 cells inoculated with Ppn 4974 (Fig. 3). Inactivated enzyme had no effect. Active SOD had no effect on the low levels of dye reduction seen in susceptible interactions or control cells. In the incompatible interaction, Mn(III)desferal was a significantly more effective inhibitor of dye reduction from 10 h postinoculation than SOD, and the rate of formazan formation in the presence of Mn(III)desferal was not significantly different from the levels seen in uninoculated control cells (Fig. 3). Mn(III)desferal had no effect on the dye reduction in susceptible interactions or uninoculated cells. Neither MnO2 nor free desferal (desferrioxamine) inhibited XTT reduction in the resistant interaction, although MnO2 slightly increased the background rate of formazan production in controls.
Figure 3.
The effect of O2− scavengers on the cumulative reduction of XTT by tobacco suspension cells inoculated with Ppn. Assays were performed as described in Figure 2 except that 100 units of SOD or 100 equivalent SOD units of Mn(III)desferal was added when required at 0 h. Data are means ± se of 10 replicates from three independent experiments.
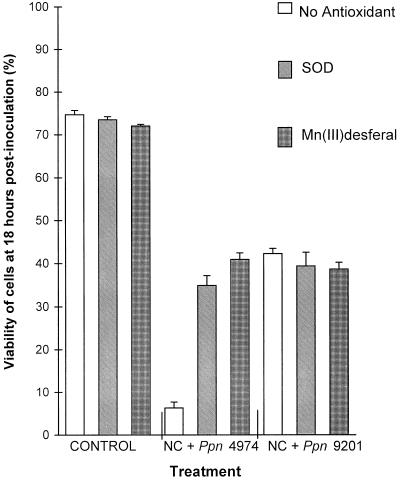
The two O2− scavengers had no effect on the viability of control cells or compatibly challenged cells. However, their addition at the time of inoculation increased the viability of cv NC2326 cells challenged with Ppn 4974 such that cell viabilities after 18 h approached those of the cells infected by a compatible pathogen race (Fig. 4). In the presence of the radical scavengers, development of fungal hyphae in the incompatible interaction was similar to that observed in compatible interactions. When the scavengers were added at 8 h postinoculation, immediately prior to the HR, levels of inhibition of both the HR and XTT reduction were similar to those that occurred when scavengers were added at 0 h (results not shown).
Figure 4.
Effect of radical scavengers on viability of inoculated tobacco suspension cells at 18 h postinoculation. The assay was as described in Figure 1. Data are means ± se of 12 replicates from three independent experiments.
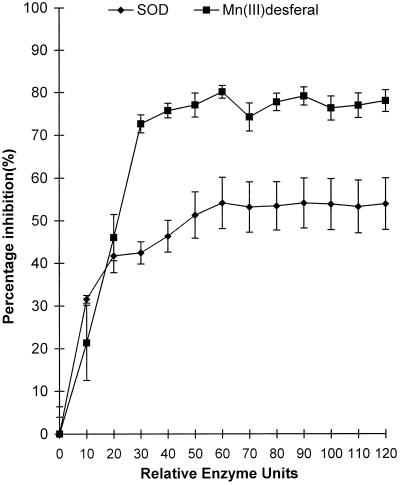
The effectiveness of Mn(III)desferal as an inhibitor of XTT formazan formation in the incompatible interaction was further investigated by comparing the percentage inhibition of formazan formation by Mn(III)desferal with inhibition by SOD, over a range of comparable concentrations (Faulkner et al., 1994; Fig. 5). Whereas addition of SOD gave a maximum inhibition of 55% above a level of 50 units per well, Mn(III)desferal gave a maximum inhibition of 80% at 30 unit equivalents per well.
Figure 5.
Comparison of the scavenging abilities of SOD and Mn(III)desferal during the HR. The y axis is the percentage inhibition of XTT reduction relative to the absence of the scavenger 18 h postinoculation of cv NC2326 with Ppn 4974. Relative enzyme units were determined by the xanthine/xanthine oxidase assay of Faulkner et al. (1994). Data are means ± se of eight replicates from two independent experiments.
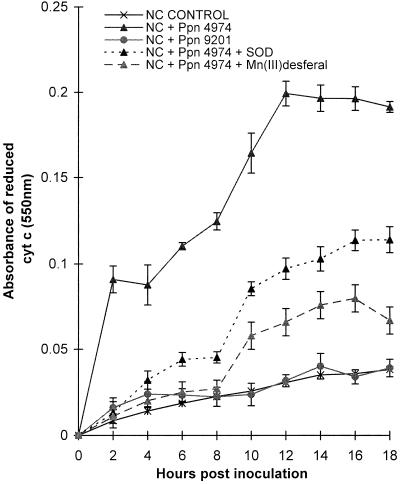
Reduction of Cyt c during the HR
When Cyt c was used in place of XTT to detect O2− production, the incompatible interaction (cv NC2326/Ppn 4974) showed a significant reduction of Cyt c between 0 and 2 h and then again between 8 and 12 h (Fig. 6). In contrast, control cells and susceptible interactions reduced the protein at very low rates. Neither SOD nor Mn(III)desferal had any effect on the reduction of Cyt c during compatible interactions or in control cells. However, both scavengers significantly inhibited the reduction of Cyt c during the incompatible interaction, with Mn(III)desferal being significantly more effective than SOD at inhibiting the second burst.
Figure 6.
The reduction of Cyt c and the effect of SOD and Mn(III)desferal on the reduction of Cyt c by tobacco suspension cells inoculated with zoospores from Ppn. Cyt c (20 μm) was added at 0 h and its reduced product was allowed to accumulate. When required, 100 units of SOD or 100 equivalent SOD units of Mn(III)desferal was added at 0 h. Data are means ± se of 12 replicates from four independent experiments.
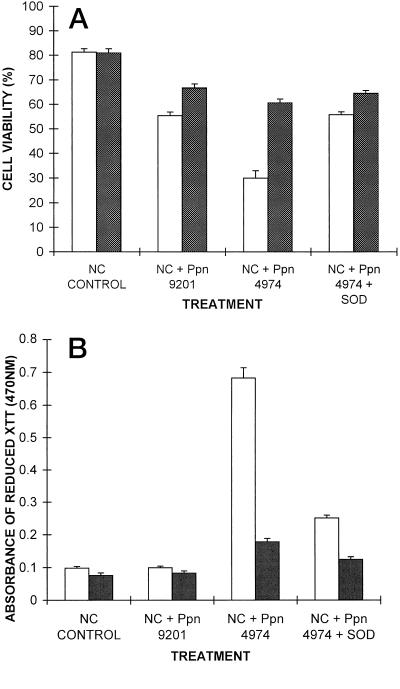
Effects of Cell Aggregation
When cells were filtered before inoculation so that all major aggregates (usually approximately 15–50 cells) were removed, the response of inoculated resistant cells was diminished in terms of both the reduction of XTT and the loss of cell viability. Their response more closely resembled that of inoculated susceptible cells (Fig. 7). Filtered cells showed higher viabilities than unfiltered cells in all treatments except controls, in which they were similar. The observed extent of mycelial growth was less in inoculated, susceptible, filtered cells than in equivalent unfiltered cultures.
Figure 7.
Effect of cell aggregation on viability of inoculated cells (A) and on reduction of XTT (B) at 18 h postinoculation. Data are means ± se of nine replicates from three independent experiments. White bars, Unfiltered; black bars, filtered.
Effect of Cell Wall Removal
When protoplasts were challenged with Ppn zoospores, little or no infection occurred in any treatment. In the incompatible interaction, little or no reduction of XTT and no HR was observed. When the cell wall had not been entirely removed, zoospore attachment (via an appressorium) was occasionally observed. When the cell wall was allowed to regenerate prior to challenge, the numbers of appressoria formed increased, as did subsequent reduction of XTT and evidence of an HR during the incompatible interaction.
Measurement of O2− Production
The concentration of HO2·/O2− generated in reaction wells during an incompatible interaction over 18 h was determined for each combination of scavenger and dye (Table I). The radical yield generated, based on the inhibition of XTT reduction by Mn(III)desferal, was equivalent to 48.9 μm HO2·/O2− in the well. This yield was significantly higher than that based on SOD inhibition. Irrespective of which inhibitor was used, calculated yields were higher when XTT was used as a radical detector in place of Cyt c. From the cell viability counts, the number of cells per 2 mL of well volume can be estimated at approximately 35,000 cells; therefore, during the course of the experiment 2.79 × 10−12 mol HO2·/O2− cell−1 were generated, assuming complete stability of the XTT formazan. From the protein estimates, 2.57 × 10−6 mol HO2·/O2− mg−1 protein were generated over 18 h.
Table I.
Yield of HO2·/O2− generated over 18 h during an incompatible interaction based on inhibition of reaction of the radical with XTT and Cyt c
| Inhibitor | XTT | Cyt c |
|---|---|---|
| μm | ||
| SOD | 39.62 ± 6.16 | 3.68 ± 0.36 |
| Mn(III)desferal | 48.90 ± 6.16 | 4.86 ± 0.40 |
DISCUSSION
To our knowledge, this is the first report of the use of Oomycete zoospores to elicit hypersensitive cell death in plant cells maintained in a liquid medium. Several cytological studies have previously examined the interaction of germinated hyphae with immobilized protoplasts or whole cells (Odermatt et al., 1988; Gross et al., 1993). In resistant tobacco cells challenged with zoospores from an incompatible race, an accelerated loss of viability 10 to 12 h postinoculation is observed, when compared with viability curves obtained from compatible infections. We equate this accelerated viability loss with the HR, recognized widely in resistant host-pathogen interactions (Goodman and Novacky, 1994). We have conducted a careful study of the relationship between ROS generation and the onset of the HR in a cell culture system that closely models the gene-for-gene specificity observed in planta (Guest et al., 1989; Nemestothy and Guest, 1990). In particular, both ROS production and the HR are observed in culture at 24°C but not at 28°C in the cv NC2326-Ppn 4974 interaction, a result that parallels the temperature sensitivity of responses in planta against race 0 of the black shank pathogen (Robin and Guest, 1994). The few previous studies of resistance responses to Oomycete zoospores have been in whole-plant tissues (Doke 1983a, 1985; Doke and Chai, 1985; Jahnen and Hahlbrock, 1988), in which it is difficult to study physiological responses in fine detail because of uncertainties concerning effective penetration and diffusion of exogenous reporter molecules and inhibitors.
The majority of studies of ROS production and the HR (Sutherland, 1991; Goodman and Novacky, 1994; Mehdy et al., 1996) have used nonspecific fungal pathogen extracts (elicitors) that elicit HR on both resistant and susceptible cultivars of host species. When race- and cultivar-specific elicitors have been used, the responses generated in resistant cultivars frequently differ from those observed in response to nonspecific elicitors (Friend, 1993; Vera-Estrella et al., 1992, 1993).
The first direct evidence for the involvement of HO2·/O2− in hypersensitive cell death came from studies of potato tissue slices infected with Phytophthora infestans, in which the presence of the radical was detected by either NBT or Cyt c (Doke, 1983a). Other tissue-culture-based studies have also implicated ROS generation as an early resistance response (Keppler and Baker, 1989; Keppler et al., 1989; Vera-Estrella et al., 1993), but these studies have largely monitored ROS production by chemiluminescence detection of H2O2 and used either nonspecific elicitors or live bacteria to challenge the host cells. In contrast, the involvement of ROS in the resistance responses of suspension-cultured host cells to specific elicitors from fungi has been well documented in the tomato-Cladosporium fulvum interaction (Vera-Estrella et al., 1992, 1994).
The use of the tetrazolium dye XTT as an assay for HO2·/O2− production (Sutherland and Learmonth, 1997) indicates that two bursts of ROS generation take place during the response of resistant host cells following pathogen challenge, and that the second and larger burst immediately precedes the onset of hypersensitive cell death. Neither burst is observed in compatible interactions, in which the HR is absent.
These findings differ from published observations of bacterial host-pathogen interactions in which the first burst is common to both compatible and incompatible interactions. In tobacco cell suspensions inoculated with Pseudomonas syringae, all bacterial treatments resulted in an initial, rapid oxidative burst between 0 and 1 h. A second burst specific to cells treated with incompatible bacteria occurred between 3 and 6 h, just prior to the HR (Keppler et al., 1989). A nonspecific first burst was also observed by Glazener et al. (1991) in a soybean-P. syringae system. It has been suggested that this first burst may be a transducing signal in both compatible and incompatible interactions (Mehdy et al., 1996; Wojtaszek, 1997). Alternatively, it is possible that this nonspecific early response is due to the use of suspension cultures that have not been allowed time to adjust to altered conditions before addition of elicitors (Devlin and Gustine, 1992; Qian et al., 1993).
Although host cells were routinely maintained in culture medium at pH 5.8, we transferred these cells to potassium phosphate buffer containing 1% Suc at pH 7.5 before challenge with zoospores to avoid potential interactions between media components and the radical detection system and to assist with ease of measurement of HO2·/O2−, which spontaneously dismutates to H2O2 at a rate that is pH dependent. As pH increases, there are lower relative concentrations of HO2· and hence the rate of second-order dismutation decreases (Bielski et al., 1985). The resulting increase in half-life of the radical means that lower concentrations of reactive molecules such as XTT are required to quantitatively scavenge the radicals produced.
In the tobacco-Ppn interaction, both SOD and Mn(III)desferal significantly inhibit reduction of XTT and the frequency of hypersensitive cell death. Both scavengers reduce cell loss significantly whether added at 0 h or just before the onset of the HR, indicating that the production of O2− plays a significant role in promoting hypersensitivity. Recent experiments using P. syringae with mutations in the hrp/hrm region (Glazener et al., 1996) suggest that ROS generation is not a sufficient condition for the onset of the HR. Nevertheless, our results indicate that in tobacco ROS generation is a necessary condition for the HR to occur and that the inhibition of both events leads to mycelial proliferation in resistant cultures.
Mn(III)desferal appears to be a more effective inhibitor of XTT reduction by zoospore-challenged resistant cell cultures than SOD (Fig. 5; Table I). HO2·/O2− is generally assumed to be produced at the plasma membrane (Doke, 1983a, 1983b). Since SOD is too large to cross the cell wall, it can only compete with XTT for those radicals that have diffused beyond the outer cell wall matrix. The greater effectiveness of Mn(III)desferal is probably the result of its smaller size, which enables it to diffuse to the plasmalemma surface and compete with XTT for reaction with all HO2·/O2− radicals produced. Free desferrioxamine had no effect on XTT reduction during the incompatible interaction. This indicates that inhibition by Mn(III)desferal is not due to an inhibition of transition-metal-catalyzed OH− production resulting from the chelation of transition metals by traces of uncomplexed desferrioxamine.
The magnitude of both HO2·/O2− production and the HR depends on inoculum density, degree of host cell aggregation, and the presence of a cell wall. When cells are filtered so that all major aggregates are removed, the response of resistant cells in terms of O2− production and the HR is lessened, suggesting that a degree of cell aggregation is required for resistance to be fully expressed. This is consistent with observations by Kitazawa and Tomiyama (1973) that, in resistant potato tuber tissue infected with P. infestans, the intensity of the HR is higher in challenged cells associated with multiple layers of surrounding tissue. The requirement of the cell wall for induction of the HR has also been reported recently in the interaction between harpin isolated from P. syringae pv syringae and tobacco suspension cells (Hoyos et al., 1996). Whereas tobacco suspension cells reacted to both P. syringae pv syringae and harpin by alkalinization of the extracellular medium, tobacco protoplasts alkalinized the medium at much reduced levels in response to P. syringae pv syringae and did not alkalinize the medium in response to harpin. Neither the current study nor that of Hoyos et al. (1996) observed evidence for an HR in challenged protoplasts, suggesting that close contact between the pathogen and the host cell wall may be required for the induction of the HR in suspension-cultured cells. Evidence that potato tuber protoplasts produce HO2·/O2− when elicited with hyphal wall components of P. infestans (Doke, 1983b) indicates that the cell wall may not be required for recognition of some nonspecific elicitors.
We have developed both a tissue culture system that is suitable for the study of gene-specific tobacco-Ppn interactions and a reliable and quantitative O2− assay using the novel tetrazolium dye XTT. XTT has a relatively low background reducibility, and the inhibition of XTT reduction by SOD or the scavenger Mn(III)desferal indicates the release of O2− during the incompatible interaction. By comparison, the Cyt c assay was a less sensitive indicator of HO2·/O2− generation. One likely reason is that Cyt c may be unable to diffuse to the plasmalemma surface. We also observed apparent binding of ferrocytochrome c to the outer surface of host cell walls, thus lowering the concentration of the indicator in harvested supernatants.
The use of XTT and Mn(III)desferal offers a means of quantifying O2− production in this system. Doke et al. (1983a, 1983b, 1985) estimated the rate of HO2·/O2− reduction of Cyt c in potato tissues to be on the order of 1 to 2 μmol min−1 mg−1 protein. Subsequently, Moreau and Osman (1989) provided convincing evidence that these rates were significantly overestimated because of calculation errors. In tobacco cells treated with XTT, the amount of HO2·/O2− produced during the second burst (8–10 h postinoculation) can be calculated from Figures 2 and 3 as 9.1 × 10−9 mol HO2·/O2− min−1 mg−1 protein or 9.9 × 10−15 mol HO2·/O2− cell−1 min−1.
Recently, several groups have attempted to quantify ROS production in cell cultures responding to nonspecific elicitors (Legendre et al., 1993; Nürnburger et al., 1994) by estimating H2O2 production. In soybean cells elicited with polygalacturonic acid and at the height of the defense response, 3 × 10−14 mol of H2O2 cell−1 min−1 were produced (Legendre et al., 1993). If it is assumed that virtually all HO2·/O2− dismutates to H2O2, then at the height of the defense response in tobacco cells (8–10 h postinoculation), 4.95 x 10 −15 mol of H2O2 cell−1 min−1 are produced. Given the difficulties of drawing comparisons between different host-pathogen and host-elicitor systems, these measurements are in broad agreement. Work currently under way in our laboratory is examining both the sources of ROS production in resistant tobacco cells and the respective yields of HO2·/O2− and H2O2 in a variety of physiological environments.
Abbreviations:
- HR
hypersensitive response
- MS
Murashige and Skoog
- NBT
nitroblue tetrazolium
- Ppn
Phytophthora parasitica var nicotianae
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- XTT
sodium,3′-[1-[phenylamino-carbonyl]-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene-sulfonic acid hydrate
Footnotes
This work was supported by the Australian Research Council (grant no. A19601127).
LITERATURE CITED
- Adam A, Farkas T, Somlyai G, Hevesi M, Kiraly Z. Consequence of superoxide generation during a bacterially induced hypersensitive reaction in tobacco: deterioration of membrane lipids. Physiol Mol Plant Pathol. 1989;34:13–26. [Google Scholar]
- Anderson AA, Rogers K, Tepper CS, Blee K, Cardon J. Timing of molecular events following elicitor treatment of plant cells. Physiol Mol Plant Pathol. 1991;38:1–13. [Google Scholar]
- Atkinson MM, Huang J, Knopp JA. Hypersensitivity of suspension-cultured tobacco cells to pathogenic bacteria. Phytopathology. 1985;75:1270–1274. [Google Scholar]
- Babior BM, Kipnes RS, Curnette JT. Biological defense mechanisms: the production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CJ, Mock NM. An improved method for monitoring cell death in cell suspension and leaf disc assays using Evans Blue. Plant Cell Tissue Org Cult. 1994;39:7–12. [Google Scholar]
- Baker CJ, Orlandi EW. Active oxygen in plant pathogenesis. Annu Rev Phytopathol. 1995;33:299–321. doi: 10.1146/annurev.py.33.090195.001503. [DOI] [PubMed] [Google Scholar]
- Bielski BHJ, Cabelli DE, Arudi RL, Ross AB. Reactivity of HO2/O2− radicals in aqueous solution. J Phys Chem Reference Data. 1985;14:1041–1100. [Google Scholar]
- Bradford MM. Quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chaplin JF. Transfer of black-shank resistance from Nicotiana plumbaginifolia to flue-cured Nicotiana tabacum. Tob Sci. 1962;6:182–187. [Google Scholar]
- Collins GB, Legg PD, Litton CC, Stokes GW. Locus homology in two species of Nicotiana. J Hered. 1971;62:288–290. [Google Scholar]
- Davies E. Action potentials as multifunctional signals in plants: a unifying hypothesis to explain apparently disparate wound responses. Plant Cell Environ. 1987;10:623–651. [Google Scholar]
- Davis D, Merida J, Legendre L, Low PS, Heinstein P. Independent elicitation of the oxidative burst and phytoalexin formation in cultured plant cells. Phytochemistry. 1993;32:607–611. [Google Scholar]
- Degousee N, Triantaphylides C, Montillet JL. Involvement of oxidative processes in the signaling mechanisms leading to the activation of glyceollin synthesis in soybean. Plant Physiol. 1994;104:945–952. doi: 10.1104/pp.104.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin WS, Gustine DL. Involvement of the oxidative burst in phytoalexin accumulation in the hypersensitive reaction. Plant Physiol. 1992;100:1189–1195. doi: 10.1104/pp.100.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Lamb CJ. Molecular communication in interactions between plants and microbial pathogens. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:339–367. [Google Scholar]
- Doke N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol Plant Pathol. 1983a;23:345–357. [Google Scholar]
- Doke N. Generation of superoxide anion by potato tuber protoplasts during the hypersensitive response to hyphal wall components of Phytophthora infestans and specific inhibition of the reaction by suppressors of hypersensitivity. Physiol Plant Pathol. 1983b;23:359–367. [Google Scholar]
- Doke N. NADPH-dependent O2− generation in membrane fractions isolated from wounded potato tubers inoculated with Phytophthora infestans. Physiol Plant Pathol. 1985;27:311–322. [Google Scholar]
- Doke N, Chai HB. Activation of superoxide generation and enhancement of resistance against compatible races of Phytophthora infestans in potato plants treated with digitonin. Physiol Plant Pathol. 1985;27:323–334. [Google Scholar]
- Faulkner KM, Stevens RD, Fridovich I. Characterisation of Mn(III) complexes of linear and cyclic desferrioxamines as mimics of superoxide dismutase activity. Arch Biochem Biophys. 1994;310:341–346. doi: 10.1006/abbi.1994.1176. [DOI] [PubMed] [Google Scholar]
- Friend J. Host pathogen interactions: current questions. In: Lucas JA, Shattock RC, Shaw DS, Cooke LR, editors. Phytophthora. Cambridge, UK: Cambridge University Press; 1993. pp. 46–49. [Google Scholar]
- Glazener JA, Orlandi EW, Baker CJ. The active oxygen response of cell suspensions to incompatible bacteria is not sufficient to cause hypersensitive cell death. Plant Physiol. 1996;110:759–763. doi: 10.1104/pp.110.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazener JA, Orlandi EW, Harmon GL, Baker CJ. An improved method for monitoring active oxygen in bacteria-treated suspension cells using luminol-dependent chemiluminescence. Physiol Mol Plant Pathol. 1991;39:123–133. [Google Scholar]
- Gooding GV, Lucas GB. Factors influencing sporangial formation and zoospore activity in Phytophthora parasitica var. nicotianae. Phytopathology. 1959;49:277–281. [Google Scholar]
- Goodman RN, Novacky AJ (1994) The Hypersensitive Reaction in Plants to Pathogens. A Resistance Phenomenon. APS Press, St. Paul, MN
- Gross P, Julius C, Schmelzer E, Hahlbrock K. Translocation of cytoplasm and nucleus to fungal penetration sites is associated with depolymerisation of microtubules and defence gene activation in infected, cultured parsley cells. EMBO J. 1993;12:1735–1744. doi: 10.1002/j.1460-2075.1993.tb05821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest DI, Upton JCR, Rowan KS. Fosetyl-Al alters the respiratory response in Phytophthora nicotianae var. nicotianae-infected tobacco. Physiol Mol Plant Pathol. 1989;34:257–265. [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine, Ed 2. Oxford, UK: Clarendon Press; 1989. [Google Scholar]
- Hardham AR, Gubler F, Duniec J, Elliot J. A review of methods for the production and use of monoclonal antibodies to study zoosporic plant pathogens. J Microbiol. 1991;162:305–318. [Google Scholar]
- Helgeson JP, Haberlach GT. Disease resistance studies with tissue cultures. In: Ingram DS, Helgeson JP, editors. Tissue Culture Methods for Plant Pathologists. Oxford, UK: Blackwell Scientific Publications; 1980. pp. 179–184. [Google Scholar]
- Hoyos ME, Stanley CM, He SY, Pike S, Pu XA, Novacky A. The interaction of harpinpss with plant cell walls. Mol Plant-Microbe Interact. 1996;9:608–616. [Google Scholar]
- Jahnen W, Hahlbrock K. Cellular localisation of nonhost resistance reactions of parsley (Petroselinum crispum) to fungal infection. Planta. 1988;173:197–204. doi: 10.1007/BF00403011. [DOI] [PubMed] [Google Scholar]
- Keppler LD, Baker CJ. Superoxide-initiated lipid peroxidation in a bacteria-induced hypersensitive reaction in tobacco cell suspensions. Phytopathology. 1989;79:555–562. [Google Scholar]
- Keppler LD, Baker CJ, Atkinson MM. Active oxygen production during a bacteria induced hypersensitive reaction in tobacco suspension cells. Phytopathology. 1989;79:974–978. [Google Scholar]
- Keppler L, Novacky A. The initiation of membrane lipid peroxidation during bacteria-induced hypersensitive reaction. Physiol Mol Plant Pathol. 1987;30:233–245. [Google Scholar]
- Kitazawa K, Tomiyama K. Role of underlying healthy tissue in the hypersensitive death of a potato plant cell infected by an incompatible race of Phytophthora infestans. Ann Phytopathol Soc Jpn. 1973;39:85–89. [Google Scholar]
- Legendre L, Reuter S, Heinstein PF, Low PS. Characterization of the oligogalacturonide-induced oxidative burst in cultured soybean (Glycine max) cells. Plant Physiol. 1993;102:233–240. doi: 10.1104/pp.102.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthorst HJM. Pathogenesis-related proteins of plants. CRC Plant Sci. 1991;10:123–150. [Google Scholar]
- Low PS, Merida JR. The oxidative burst in plant defense. Function and signal transduction. Physiol Plant. 1996;96:533–542. [Google Scholar]
- Massey V. The microestimation of succinate and the extinction coefficient of Cyt c. Biophys Biochim Acta. 1959;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase. J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- Mehdy MC, Sharma YK, Sathasivan K, Bays NW. The role of activated oxygen species in plant disease resistance. Physiol Plant. 1996;98:365–374. [Google Scholar]
- Moreau RA, Osman SF. The properties of reducing agents released by treatment of Solanum tuberosum with elicitors from Phytophthora infestans. Physiol Mol Plant Pathol. 1989;35:1–10. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–479. [Google Scholar]
- Nemestothy GS, Guest DI. Phytoalexin accumulation, Phe ammonia lyase activity and ethylene biosynthesis in fosetyl-Al-treated resistant and susceptible tobacco cultivars infected with Phytophthora nicotianae var. nicotianae. Physiol Mol Plant Pathol. 1990;37:207–219. [Google Scholar]
- Nürnberger T, Nennstiel D, Jabs T, Slacks WR, Hahlbrock K, Scheel D. High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell. 1994;78:449–460. doi: 10.1016/0092-8674(94)90423-5. [DOI] [PubMed] [Google Scholar]
- O'Connell RJ, Bailey JA, Richmond DV. Cytology and physiology of Phaseolus vulgaris by Colletotrichum lindemuthianum. Physiol Plant Pathol. 1985;27:75–98. [Google Scholar]
- Odermatt M, Röthlisberger A, Werner C, Hohl HR. Interactions between agarose-embedded plant protoplasts and germ tubes of Phytophthora. Physiol Mol Plant Pathol. 1988;33:209–220. [Google Scholar]
- Olson PD, Varner JE. Hydrogen peroxide and lignification. Plant J. 1993;4:887–892. [Google Scholar]
- Paull KD, Shoemaker RH, Boyd MR, Parsons JL, Risbood PA, Barbera W, Sharma MN, Baker DC, Hand E, Scuderio DA and others. The synthesis of XTT: a new tetrazolium reagent that is bioreducible to a water-soluble formazan. J Heterocycl Chem. 1988;25:911–914. [Google Scholar]
- Pavlovkin J, Novacky A, Ullrich-Eberuis CI. Membrane potential changes during bacteria induced hypersensitive reaction. Physiol Mol Plant Pathol. 1986;28:125–135. [Google Scholar]
- Pelissier B, Thibaud JB, Crignon C, Esquerre-Tugaye MT. Cell surfaces in plant microorganism interactions. VII. Elicitor preparations from two fungal pathogens depolarise plant membranes. Plant Sci. 1986;46:103–109. [Google Scholar]
- Pou S, Hassett DJ, Britigan BE, Cohen MS, Rosen GM. Problems associated with spin trapping oxygen-centred free radicals in biological systems. Anal Biochem. 1989;177:1–6. doi: 10.1016/0003-2697(89)90002-x. [DOI] [PubMed] [Google Scholar]
- Qian YC, Nguyen T, Murphy TM. Effect of washing on the plasma membrane and on stress reactions of cultured rose cells. Plant Cell Tissue Org Cult. 1993;35:245–252. [Google Scholar]
- Rabinowitch HD, Privalle CT, Fridovich I. Effects of paraquat on the green alga Dunaliella salina: protection by the mimic of superoxide dismutase, desferal-Mn(IV) Free Radical Biol Med. 1987;3:125–131. doi: 10.1016/s0891-5849(87)80007-2. [DOI] [PubMed] [Google Scholar]
- Razdan MK (1993) An Introduction to Plant Tissue Culture. Intercept, Andover, Hampshire, UK
- Robin C, Guest D. Characterization of pathogenicity of Phytophthora parasitica isolates by stem and detached-leaf inoculations in four tobacco cultivars. NZ J Crop Hortic Sci. 1994;22:159–166. [Google Scholar]
- Scuderio DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- Sutherland MW. The generation of oxygen radicals during host plant responses to infection. Physiol Mol Plant Pathol. 1991;39:79–93. [Google Scholar]
- Sutherland MW, Learmonth BA. The tetrazolium dyes MTS and XTT provide new quantitative assays for superoxide and superoxide dismutase. Free Radical Res. 1997;27:283–289. doi: 10.3109/10715769709065766. [DOI] [PubMed] [Google Scholar]
- Vera-Estrella R, Barkla BJ, Higgins VJ, Blumwald E. Plant defense response to fungal pathogens. Activation of host-plasma membrane H+-ATPase by elicitor-induced enzyme dephosphorylation. Plant Physiol. 1994;104:209–215. doi: 10.1104/pp.104.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Estrella R, Blumwald E, Higgins VJ. Effect of specific elicitors of Cladosporium fulvum on tomato suspension cells. Plant Physiol. 1992;99:1208–1215. doi: 10.1104/pp.99.3.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Estrella R, Blumwald E, Higgins VJ. Nonspecific glycopeptide elicitors of Cladosporium fulvum: evidence for involvement of active oxygen species in elicitor-induced effects on tomato cell suspensions. Physiol Mol Plant Pathol. 1993;42:9–22. doi: 10.1104/pp.99.3.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Goenner M, Schlosser E, Neubacher H. Evidence from electron-spin resonance for the formation of free radicals during infection of Avena sativa by Drechslera spp. Physiol Mol Plant Pathol. 1993;42:405–412. [Google Scholar]
- Wojtaszek P. Oxidative burst: an early response to pathogen infection. Biochem J. 1997;322:681–692. doi: 10.1042/bj3220681. [DOI] [PMC free article] [PubMed] [Google Scholar]