Abstract
Results following fusion for chronic low back pain (CLBP) are unpredictable and generally not very satisfying. The major reason is the absence of a detailed description of the symptoms of patients with pain, if present, in a motion segment of the spine. Various radiological findings have been attributed to discogenic pain, but if these radiological signs were really true signs of such pain, fusion would have been very successful. If discogenic pain exists, it should be possible to select these patients from all others within the CLBP population. Even if this selection were 100% perfect, however, identification of the painful segment would remain, and at present there is no reliable test for doing so. Regardless of whether an anterior or posterior type of fusion is performed, or even if artificial discs are used, solving the puzzle of pain associated with the presumed segmental disorder must be the primary goal.
Keywords: Spinal fusion, low back pain, symptoms, discography, pseudarthrosis, artificial disc.
INTRODUCTION
In cases of pronounced chronic low back pain with or without nonspecific leg pain (CLBP) where the patient does not respond to conservative treatment, a discussion about spinal fusion may ultimately take place. The results following this surgery are varying, controversial and not very satisfying [1-3]. Since the specific indication for fusion in these cases is not yet determined, the question is whether spinal fusion should really be performed [2, 4-6].
A number of different conditions may present with symptoms of low back pain. Homogeneous groups of patients, each with a specific reason for their pain, are mixed with other groups of patients whose pain is also of homogeneous origin. Nevertheless, we do not know exactly what these different reasons are for the pain. This could be likened to a situation in which patients are collected under the diagnosis of abdominal pain, but specific disorders such as gall bladder disease, gastric ulcer and appendicitis are not distinguishable. Clearly, a spinal fusion would be of no help in cases of muscular or inflammatory pain or if the pain originates in the sacroiliac joints. Spinal fusion might, however, be of value in facet joint pain, discogenic pain and “instability”, provided these patients could be selected with certainty from the whole population of patients with CLBP.
SELECTION OF PATIENTS, SYMPTOMS
In most reports back pain is never specified, and is referred to simply as chronic low back pain. This is also true in recent randomised studies [7-10]. Analogous with the example of abdominal pain and with the intention of finding a specific pain pattern, back pain ought to be more thoroughly analysed according to localization (lateral or midline), character (diffuse or restricted, continuous or intermittent, dull, burning, stabbing, etc.), and situations that aggravate or alleviate the pain. Similarly, leg pain should be analysed as to whether it is diffuse, radicular, pseudoradicular, dull, burning, continuous or intermittent, and whether it is coupled with numbness or paresthesia. Specific questions about any bladder disturbance such as urinary retention, urgency, hesitancy, or terminal dribbling should also be posed. We have to complete the puzzle regarding pain associated with the condition to be treated using spinal fusion. The ideal condition for this treatment would be pain in one motion segment of the spine, i.e. pain in the motion between two vertebrae, or segmental pain. If such a condition exists, its clinical pain presentation would be the segmental pain syndrome.
RADIOLOGY HAS FAILED
In efforts to select patients suitable for treatment with fusion surgery, radiological findings have not provided a solution. Osteoarthritis of the facet joints is seen as early as at 20 years of age and in almost every person by the age of 50 [11, 12], and concentric as well as radiating tears appear even in young discs [13]. Over the years these and other radiological findings such as marked disc degeneration, spondylotic spurs, internal disc disruption and annular tears seen on MRI or discography, high intensity zones and Modic signs have been attributed to low back pain and also more specifically to discogenic pain [14-17]. However, this opinion has been contradicted by others [18-20]. If these radiological signs were actually true signs of discogenic pain spinal fusion would have been extremely successful in cases of CLBP because all these signs are easily recognized on plain X-rays, MRI and discography. Since this is not the case, the search for pathoanatomic changes that serve as landmarks of low back pain has so far been unsuccessful. If in some cases the origin of unspecific low back pain is discogenic, which is supported to some extent by the findings of low back pain provocation by direct palpation and pressure on the discs of awake individuals undergoing surgery under local anaesthesia [21, 22], the question may be posed as to whether there has to be any specific radiological disc abnormality at all.
REPORTED INDICATIONS FOR FUSION
In textbooks and reviews concerning indications for fusion in cases of CLBP the following can be found: translational instability, segmental instability, postlaminectomy syndrome, internal disc disruption, painful disc degeneration, isolated disc resorbtion, persistent low back pain after lumbar discectomy, discogenic back pain, annular tear, failed back syndrome, primary large central disc herniation and recurrent disc herniation [23, 24]. The results following spinal fusion surgery with regard to these indications have been reported by Turner et al. [1] who concluded, ”For several low back disorders no advantage has been demonstrated for fusion over surgery without fusion”. Further, Chou et al. [6] stated recently that “For nonradicular pain with common degenerative changes, fusion is no more effective than intensive rehabilitation.” Only one out of four rather recent randomised studies reported that the results following fusion surgery were better than in the control group [7-10].
We therefore face a situation in which the decision to perform a spinal fusion operation in cases of CLBP is based in most cases only on the presence of chronic low back pain and some very unspecific radiological signs. In every other kind of surgery there is an effort to rely on specific and relevant symptoms for each surgical intervention, e.g. symptoms of appendicitis for appendectomy, of gall bladder disease for cholecystectomy and so on. Abdominal surgery is not generally performed on the indication of diffuse abdominal pain. It is therefore an absolute necessity to search for the clinical symptoms and the pain description indicating a condition that is treatable by spinal fusion, and not to look for various radiological signs.
PINPOINTING THE PAINFUL SEGMENT, IF PRESENT
Logically, provocative discography would be the most suitable test for pinpointing a painful disc, and several reports defend this opinion [16, 25-27] while others do not [26, 28, 29]. A positive discographic test is usually based on concordant pain reaction of significant magnitude in one disc that is not found in an adjacent disc [27, 30]. The test has been questioned on the basis that pain may also be provoked during disc injection in normal subjects [31] and in subjects without low back pain but with ongoing chronic pain of non-lumbar origin [32]. However, by definition these persons should be unable to report about concordance. Similarly, it is difficult to understand how patients with iliac crest pain following bone harvesting can report concordant pain during discography [33]. Early reports on pain provocation at lumbar discography invariably noted that the pain was located in the lumbar spine, but also, in declining frequency, in the buttock, thigh or lower leg [34]. A recent report strongly supports the view that a positive provocation test in discography is of little or no value in detecting the pain generator, e.g. the presumed painful disc, if performed in a non-specific group of patients suffering from CLBP [35]. Whether the disc provocation test would be of value in selecting the pain-generating disc in a homogeneous population of patients actually suffering from discogenic pain is an open question, since no such patient selection has yet been possible on clinical or other grounds. Some other testing techniques have also been developed and used as possible methods for finding the painful segment, if present, including the bony vibration test [36], but they are apparently not specific enough [20]. The open mechanical provocation test that has been described [37] is likewise not validated. In summary, as recently stated by Hancock et al. [20], “The usefulness of all these tests in clinical practice, particularly for guiding treatment selection, remains unclear.”
SEGMENTAL PAIN AND PAIN DISTRIBUTION
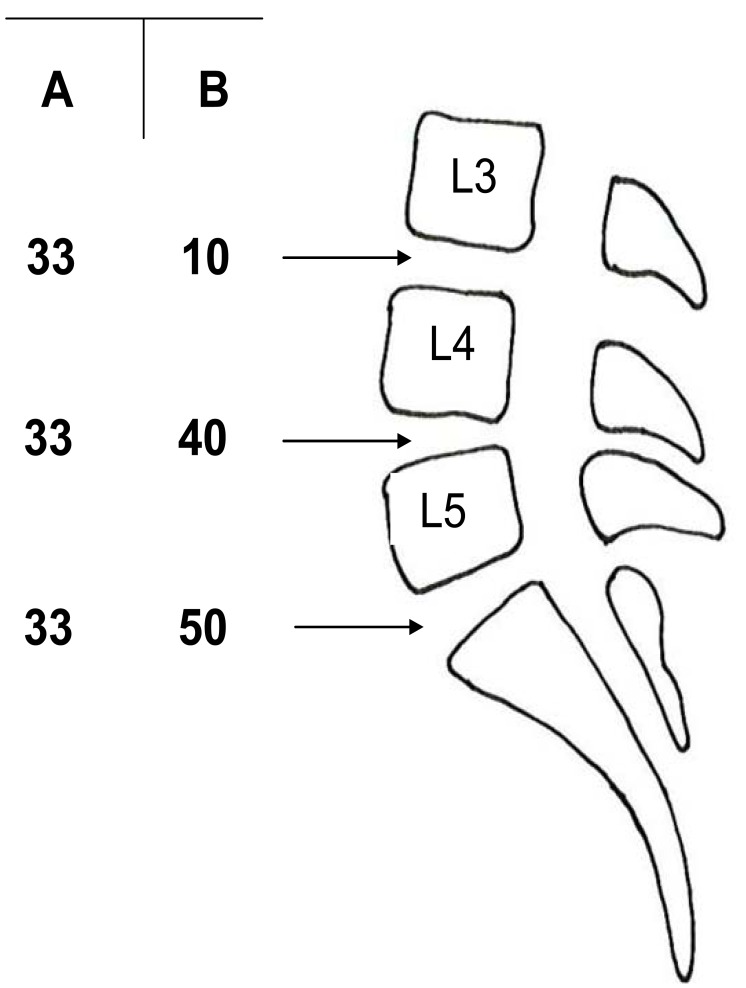
If we assume that segmental pain really exists and can express itself clinically in a specific segmental pain syndrome, it would be possible to select these patients from all others within the population suffering from CLBP. Even if this selection of patients were to be 100 % perfect, we would still have to identify the segment producing the pain. Pain might have its origin in one single segment, L3-L4, L4-L5 or L5-S1, but possibly also in two or even three of these segments. Without any reliable test to pinpoint a painful segment [20] the probability of fusing the appropriate painful segment or segments would depend on the distribution of segmental pain at different levels. Let us assume that patients in a given population have segmental discogenic pain localized to only one segment and with an equivalent distribution among the three lower lumbar segments, i.e. one third of the selected patients have their segmental pain at L3-L4, one third have it at L4-L5 and one third at L5-S1 (column A in Fig. 1). In this situation a segmental fusion at a single level would have a 33% probability of curing the patient from his/her low back pain. If we assume another distribution of segmental pain, e.g. 10% with their discogenic pain at L3-L4, 40% at L4-L5 and 50% at L5-S1 (column B in Fig. 1), which probably would be more relevant, a mono-segmental fusion at L5-S1 would have a 50% probability of success, the remaining half of the patient population would have their pain at higher levels. If we add to this discussion the possibility that segmental pain might exist at two levels simultaneously, e.g. in both the L4-L5 and L5-S1 segments, and assume this to be the case in say 20% of the patients, the probability of curing the patient with a mono-segmental fusion declines to 40% in the L5-S1 example just mentioned, since one fifth of these patients would still have pain from the L4-L5 segment. It must be remembered that the prerequisite for these figures is the assumption that it is possible to select all these patients with exclusively segmental pain. If only half of the patients selected truly suffer from discogenic pain, the figures just discussed would decrease by half to around a 20-25% probability of curing the L5-S1 patient discussed above with a mono-segmental fusion, a figure actually found in a well-designed study by Carragee et al. [35].
Fig. (1).
Examples of different percent distributions, A and B, of segmental pain in the lower lumbar levels in a conceived patient population.
One may argue that a two-level fusion in the example just given, where 40% of the patients had segmental pain at L4-L5 and 50% at L5-S1, would have a 90% chance of success. Two-level fusions, however, are often performed using a postero-lateral technique. Even after successful bony healing this type of fusion may permit some movement in the disc region [38, 39]. Therefore, if discogenic pain were of a mechanical nature, pain might still exist despite a healed postero-lateral fusion due to the lever arm anterior to the disc, making slight axial movement still possible and thereby the possibility of persisting pain. If discogenic pain really exists, the appropriate fusion would therefore be an interbody fusion [40-42].
PSEUDARTHROSIS
It has been argued that good results following spinal fusion can also be seen in cases of pseudarthrosis in both interbody and postero-lateral fusions [2, 43]. If discogenic pain exists and is of a mechanical nature, it is, however, reasonable to assume that the sensitivity for pain in the discs might differ considerably in different patients. There might be very sensitive discs that react to the slightest movement or compression in the disc region, whereas in other patients the disc might only react after considerably more disc movement or compression. This might be one explanation for why some patients present with very good clinical results despite pseudarthrosis. Another possible explanation is that the pain was not discogenic. The real but unknown reason for the pain might have subsided, leading to apparent success of the fusion without any connection whatsoever to the operation and bony healing.
THE PROBLEM
The problem we must deal with involves, first of all, the selection of those patients who really suffer from segmental pain; secondly we have to find the painful segment(s); and thirdly we have to perform a fusion that best alleviates the pain, with all probability an interbody fusion that does not permit any axial movement [39, 40].
Spine surgeons of all kinds have tried to contribute knowledge to this issue but most published studies do not specify the symptomatology of the patients included in the study; it is generally described simply as chronic low back pain with or without nonspecific leg pain. This tells us nothing about whether the pain originates in the sacro-iliac joints, if it is of muscular origin or if it represents segmental pain. The development and use of various internal fixation devices might add some knowledge about how to improve fusion rates [3, 44, 45], but this is only one factor among several. Prospective randomised multicenter studies also encounter the same problem regarding patient selection, perhaps even more so since in these studies a large number of surgeons are responsible for the selection of patients. Of four such studies published recently, three describe the symptoms of the patients included in the study simply as “chronic low back pain” [8-10] and in the fourth study they are described as “back pain more pronounced than leg pain and no signs of nerve root compression” [7]. In all four studies the selection of the segment or segments to be fused was based on clinical and radiographic grounds, which we know to be of doubtful value.
ARTIFICIAL DISCS
Some surgeons and clinics advocate the use of artificial discs with the intention of preserving motion of the segment in question [46]. When the artificial disc is placed in the disc space, the outer layers of the annulus are with all probability left in place. These layers contain the nerve endings of the disc innervation [47, 48]. Therefore, if segmental pain is related to movement in the disc space, pain will probably remain following a movement-preserving operation with an artificial disc. The same argument holds true for so-called semi-rigid fixation [49].
CONCLUSION
In summary, therefore, it is absolutely necessary to find and describe the symptomatology of those patients who might suffer from segmental pain in order to be able to select just those patients for the surgical procedure. Following this selection a number of problems remain: selecting the proper segment for fusion without being able to recognize it visually, choosing a fusion procedure that best alleviates the pain (probably not the postero-lateral procedure), obtaining solid bony healing, and clearly understanding that after having made the right choice in each of these steps we still have a patient in great need of support and rehabilitation after, in most cases, having suffered from severe pain for many years.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
REFERENCES
- 1. Turner JA, Ersek M, Herron L, et al. Patient outcomes after lumbar spinal fusions. JAMA. 1992;268:907–11. [PubMed] [Google Scholar]
- 2. Deyo RA, Nachemson A, Mirza SK. Spinal-Fusion surgery – the case for restraint. New Eng J Med. 2004;350:722–6. doi: 10.1056/NEJMsb031771. [DOI] [PubMed] [Google Scholar]
- 3. Gibson JNA, Waddell G. Surgery for degenerative lumbar spondylosis: Updated Cochrane review. Spine. 2005;30:2312–20. doi: 10.1097/01.brs.0000182315.88558.9c. [DOI] [PubMed] [Google Scholar]
- 4.Hanley EN, Jr, David SM. Lumbar arthrodesis for the treatment of back pain. J Bone Joint Surg Am. 1999;81-A:716–30. doi: 10.2106/00004623-199905000-00015. [DOI] [PubMed] [Google Scholar]
- 5. Mirza SK, Deyo RA. Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine. 2007;32:816–23. doi: 10.1097/01.brs.0000259225.37454.38. [DOI] [PubMed] [Google Scholar]
- 6. Chou R, Baisden J, Carragee EJ, Resnick DK, Shaffer WO, Loeser JD. Surgery for low back pain. A review of the evidence for an American Pain Society clinical practice guideline. Spine. 2009;34:1094–109. doi: 10.1097/BRS.0b013e3181a105fc. [DOI] [PubMed] [Google Scholar]
- 7. Fritzell P, Hägg O, Wessberg P, Nordwall A, et al. Volvo award winner in clinical studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain. Spine. 2001;26:2521–34. doi: 10.1097/00007632-200112010-00002. [DOI] [PubMed] [Google Scholar]
- 8. Brox JI, Sörensen R, Friis A. Randomized clinical trial of lumbar instrumented fusion and cognitive intervention and exercises in patients with chronic low back pain and disc degeneration. Spine. 2003;28:1913–21. doi: 10.1097/01.BRS.0000083234.62751.7A. [DOI] [PubMed] [Google Scholar]
- 9. Fairbank J, Frost H, Wilson-MacDonald J, Yu L M, Barker K, Collins R. Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: the MRC spine stabilisation trial. Br Med J . 2005;330:1233–9. doi: 10.1136/bmj.38441.620417.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brox JI, Reikerås O, Nygaard Ö, et al. Lumbar instrumented fusion compared with cognitive intervention and exercises in patients with chronic back pain after previous surgery for disc herniation: A prospective randomized controlled study. Pain. 2006;122:145–55. doi: 10.1016/j.pain.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 11. Eubanks JD, Lee MJ, Cassinelli E, Ahn NU. Prevalence of lumbar facet arthrosis and its relationship to age sex and race. Spine. 2007;32:2058–62. doi: 10.1097/BRS.0b013e318145a3a9. [DOI] [PubMed] [Google Scholar]
- 12. Kalichman L, Li L, Kim DH, et al. Facet joint osteoarthritis and low back pain in the community-based population. Spine. 2008;33:2560–5. doi: 10.1097/BRS.0b013e318184ef95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vernon-Roberts B, Moore RJ, Fraser RD. The natural history of agerelated disc degeneration The pathology and sequelae of tears. Spine . 2007;32:2797–804. doi: 10.1097/BRS.0b013e31815b64d2. [DOI] [PubMed] [Google Scholar]
- 14. Macnab I. The traction spur An indicator of segmental instability. J Bone Joint Surg Am. 1971;53:663–70. [PubMed] [Google Scholar]
- 15. Vanharanta H, Sachs BL, Spivey MA, et al. The relationship of pain provocation to lumbar disc deterioration as seen by CT/Discography. Spine. 1987;12:295–8. doi: 10.1097/00007632-198704000-00019. [DOI] [PubMed] [Google Scholar]
- 16. Aprill C, Bogduk N. High-intensity zone a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65:361–9. doi: 10.1259/0007-1285-65-773-361. [DOI] [PubMed] [Google Scholar]
- 17. Braithwaite I, White J, Saifuddin A, Renton P, Taylor BA. Vertebral end-plate (Modic) changes on lumbar spine MRI: correlation with pain reproduction at lumbar discography. Eur Spine J. 1998;7:363–8. doi: 10.1007/s005860050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carragee EJ, Paragioudakis SJ, Khurana S. Volvo award winner in clinical studies. Lumbar high-intensity zone and discography in subjects without low back problems. Spine. 2000;25:2987–92. doi: 10.1097/00007632-200012010-00005. [DOI] [PubMed] [Google Scholar]
- 19. Deyo RA, Andersson GBJ, Jarvik JG. Diagnostic test performance: sensitivity, specificity, and predictive value. In: Frymoyer JW, Wiesel SW, editors. The adult and pediatric spine. Philadelphia. Lippincott: Williams & Wilkins; 2004. pp. 15–25. Chapter 2. [Google Scholar]
- 20. Hancock MJ, Maher CG, Latimer J, et al. Systematic review of tests to identify the disc SIJ or facet joint as the source of low back pain. Eur Spine J. 2007;16:1539–50. doi: 10.1007/s00586-007-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spurling RG, Grantham EG. Neurologic picture of herniations of the nucleus pulposus in the lower part of the lumbar region. Arch Surg . 1940;40:375–88. [Google Scholar]
- 22. Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica A report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am. 1991;22:181–7. [PubMed] [Google Scholar]
- 23. Gill K. Clinical indications for lumbar interbody fusion. In: Lin PM, Gill K, editors. Lumbar interbody fusion. Principles and techniques in spine surgery. Rockville, Maryland: Aspen publishers; 1989. pp. 35–53. Chapter 4. [Google Scholar]
- 24. Burkus JK. Interbody fusion devices: Biomechanics and clinical outcomes. In: Vaccaro AR, Betz RR, Zeidman SM, editors. Priciples and practice of spine surgery. USA: Mosby Inc; 2003. pp. 385–97. Chapter 30. [Google Scholar]
- 25. Simmons EH, Segil CM. An evaluation of discography in the localization of symptomatic levels in discogenic disease of the spine. Clin Orthop Relat Res. 1975;108:57–69. doi: 10.1097/00003086-197505000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Bogduk N, Modic MT. Controversy. Lumbar discography. Spine. 1996;21:402–4. doi: 10.1097/00007632-199602010-00031. [DOI] [PubMed] [Google Scholar]
- 27. Zdeblick TA. Discogenic back pain. In: Rothman RH, Simeone FA, editors. The Spine. Philadelphia: WB Saunders; 1999. pp. 749–65. Chapter 28. [Google Scholar]
- 28. Carragee EJ. Is lumbar discography a determinate of discogenic low back pain provocative discography reconsidered. Curr Rev Pain. 2000;4:301–8. doi: 10.1007/s11916-000-0107-2. [DOI] [PubMed] [Google Scholar]
- 29. Madan S, Gundanna M, Harley JM, Boeree NR, Sampson M. Does provocative discography screening of discogenic back pain improve surgical outcome? J Spinal Disord Tech. 2002;15:245–51. doi: 10.1097/00024720-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 30. Merskey H, Bogduk N, editors. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. 2nd ed. USA: IASP Press; 1994. pp. 175–206. [Google Scholar]
- 31. Holt EP. The question of lumbar discography. J Bone Joint Surg. 1968;50A:720–6. doi: 10.2106/00004623-196850040-00007. [DOI] [PubMed] [Google Scholar]
- 32. Carragee EJ, Alamin TF, Carragee JM. Low-pressure positive discography in subjects asymptomatic of significant low back pain illness. Spine. 2006;31:505–9. doi: 10.1097/01.brs.0000201242.85984.76. [DOI] [PubMed] [Google Scholar]
- 33. Carragee EJ, Tanner CM, Yang B, Brito JL, Truong T. False-positive findings on lumbar discography. Reliability of subjective concordance assessment during provocative disc injection. Spine. 1999;24:2542–7. doi: 10.1097/00007632-199912010-00017. [DOI] [PubMed] [Google Scholar]
- 34. Fernström U. A discographical study of ruptured lumbar intervertebral discs. Acta Chiru Scand Suppl. 1960. pp. 1–60. [PubMed]
- 35. Carragee EJ, Lincoln T, Parmar VS, Alamin T. A gold standard evaluation of the ”discogenic pain” diagnosis as determined by provocative discography. Spine. 2006;31:2115–23. doi: 10.1097/01.brs.0000231436.30262.dd. [DOI] [PubMed] [Google Scholar]
- 36. Yrjämä M, Vanharanta H. Bony vibration stimulation a new noninvasive method for examining intradiscal pain. Eur Spine J. 1994;3:233–5. doi: 10.1007/BF02221600. [DOI] [PubMed] [Google Scholar]
- 37.Nyström B. Open mechanical provocation under local anesthesia: a definitive method for locating the focus in painful mechanical disorder of the motion segment. Fifth international conference on lumbar fusion and stabilization; 1991; Osaka Japan. [Google Scholar]
- 38. Johnsson R, Selvik G, Strömqvist B, Sundén G. Mobility of the lower lumbar spine after posterolateral fusion determined by roentgen stereophotogrammetric analysis. Spine. 1990;15:347–50. doi: 10.1097/00007632-199005000-00001. [DOI] [PubMed] [Google Scholar]
- 39. Rolander SD. Motion of the lumbar spine with special reference to the stabilizing effect of posterior fusion. An experimental study on autopsy specimens. Acta Orthop Scand. 1966. pp. 1–144. [DOI] [PubMed]
- 40. Weatherley CR, Prickett CF, O´Brian JP. Discogenic pain persisting despite solid posterior fusion. J Bone Joint Surg Br. 1986;68:142–3. doi: 10.1302/0301-620X.68B1.2934399. [DOI] [PubMed] [Google Scholar]
- 41. Barrick WT, Schofferman JA, Reynolds JB, et al. Anterior lumbar fusion improves discogenic pain at levels of prior posterolateral fusion. Spine. 2000;25:853–7. doi: 10.1097/00007632-200004010-00014. [DOI] [PubMed] [Google Scholar]
- 42. Videbaek TS, Christensen FB, Soegaard R, et al. Circumferential fusion improves outcome in comparison with instrumented posterolateral fusion: Long-term results of a randomized clinical trial. Spine. 2006;31:2875–80. doi: 10.1097/01.brs.0000247793.99827.b7. [DOI] [PubMed] [Google Scholar]
- 43. Frymoyer JW, Hanley EN, Howe J, Kuhlmann D, Matteri RE. A comparison of radiographic findings in fusion and nonfusion patients ten or more years following lumbar disc surgery. Spine. 1979;4:435–40. doi: 10.1097/00007632-197909000-00008. [DOI] [PubMed] [Google Scholar]
- 44. Zdeblick TA. A prospective, randomized study of lumbar fusion. Spine. 1993;18:983–91. doi: 10.1097/00007632-199306150-00006. [DOI] [PubMed] [Google Scholar]
- 45. Sonntag VKH, Marciano FF. Is fusion indicated for lumbar spinal disorders? Spine. 1995;20:138S–42S. [PubMed] [Google Scholar]
- 46. Panjabi M, Malcolmson G, Teng E, Tominaga Y, Henderson G, Serhan H. Hybrid testing of lumbar Charité discs versus fusions. Spine. 2007;32:959–66. doi: 10.1097/01.brs.0000260792.13893.88. [DOI] [PubMed] [Google Scholar]
- 47. Roofe PG. Innervation of annulus fibrosus and posterior longitudinal ligament. Arch Neurol Psychiatr. 1940;44:100–3. [Google Scholar]
- 48. Palmgren T, Grönblad M, Virri J, Kääpä E, Karaharju E. An immunohistochemical study of nerve structures in the anulus fibrosus of human normal lumbar intervertebral discs. Spine. 1999;24:2075–9. doi: 10.1097/00007632-199910150-00002. [DOI] [PubMed] [Google Scholar]
- 49. Grob D, Benini A, Junge A, Mannion AF. Clinical experience with the Dynesys semirigid fixation system for the lumbar spine. Spine. 2005;30:324–31. doi: 10.1097/01.brs.0000152584.46266.25. [DOI] [PubMed] [Google Scholar]