Abstract
Pre-eclamptic toxaemia (PET) may be associated with both endothelial dysfunction (ED) and sleep-disordered breathing (SDB). It was hypothesised that females with PET would demonstrate both SDB and ED, and that a correlation between these two would suggest a potential causative association.
A total of 17 females with PET and 25 matched females with uncomplicated pregnancy were studied. They underwent a nocturnal ambulatory sleep study (using Watch_PAT100) and noninvasive evaluation of endothelial function utilising the reactive hyperaemia test (using Endo_PAT 2000). A higher ratio of post- to pre-occlusion pulse-wave amplitude (endothelial function index (EFI)) indicated better endothelial function.
Females with PET had a significantly higher respiratory disturbance index (RDI) and lower EFI than controls (18.4±8.4 versus 8.3±1.3·h−1, and 1.5±0.1 versus 1.8±0.1, respectively). Blood pressure significantly correlated with RDI and with EFI. EFI tended to correlate with RDI.
In conclusion, these results suggest that both sleep-disordered breathing and endothelial dysfunction are more likely to occur in females with pre-eclamptic toxaemia than in females with uncomplicated pregnancies. The current authors speculate that respiratory disturbances contribute to the functional abnormality of the blood vessels seen in females with pre-eclamptic toxaemia, although causality cannot be determined based on this study.
Keywords: Endothelial dysfunction, hypertension, pre-eclampsia, sleep-disordered breathing
Pre-eclamptic toxaemia (PET) is a pregnancy-specific disorder characterised by hypertension, proteinuria and oedema. It is estimated to affect 7–10% of all pregnancies in the USA [1], and is considered a major cause of foetal and maternal morbidity and mortality. Recent evidence has suggested that vascular endothelial dysfunction (ED) may play an important role in the pathogenesis of PET [2, 3]. One of the vascular endothelial functions is the ability to release endothelium-derived relaxing factors (the most important of which is nitric oxide) to induce vasodilation and prevent organ ischaemia in specific circumstances. The loss of these athero-protective properties of the normal endothelium is termed ED, which results in abnormal regulation of blood vessel tone [4–6], peripheral vasoconstriction and, potentially, hypertension [7]. ED has recently been associated with adverse cardiovascular outcome, above and beyond conventional risk factors [8–10].
In addition to the cardiovascular changes during normal pregnancy and during pregnancy-induced hypertension, pregnancy may be associated with altered breathing during sleep. Several changes in the respiratory system occur during pregnancy, particularly during the third trimester, which can alter respiratory function during sleep and increase the incidence and severity of sleep-disordered breathing (SDB). These include reduced functional residual capacity due to weight gain and changes in the shape of the diaphragm and thorax, and increases in nasal congestion presumably secondary to increased oestrogen levels [11]. These physiological changes may be particularly pronounced in females with PET [12–14]. PET has been associated with marked sympatho-excitation and an increased risk of subsequent cardiovascular disease [15, 16]. Similarly, obstructive sleep apnoea (OSA) is a common breathing disorder, characterised by repetitive apnoeas and hypoxaemia caused by recurrent upper airways collapse during sleep [17]. One of the most important consequences of OSA is hypertension [18, 19], which may be mediated by hypoxaemia and increased sympathetic activation [20, 21].
Thus, two different mechanisms may theoretically lead to hypertension in PET females: ED and SDB. The objective of the current study was to examine these potential associations. It was hypothesised that females with PET would demonstrate both ED and SDB (compared with control females with uncomplicated pregnancies), and that a correlation between these two would suggest a potential causative association. An additional goal was to examine whether ED and/or SDB are associated with the birthweight of the newborn.
METHODS
Participants
A total of 17 pre-eclamptic females (PET group) and 25 females with uncomplicated normal pregnancy (control group) were enrolled in the study. Those with pre-existing hypertension or collagen-vascular diseases were excluded. PET was defined as having a blood pressure (BP) of >140/90 mmHg with proteinuria (>0.3 g·24 h−1). Females with PET were recruited through the Dept of Obstetrics and Gynaecology (Rambam Medical Center, Haifa, Israel) and control females were recruited by advertising in the hospital and university (Technion - Israel Institute of Technology), and among colleagues of the research team.
Age, gestational age and clinical parameters of the participants are demonstrated in table 1.
TABLE 1.
Demographic data of the participants
| Control | PET | |
|---|---|---|
| Age yrs | 30±6 | 30±7 |
| BMI prior to pregnancy kg·m−2 | 23±6 | 24±5 |
| BMI at the time of the study kg·m−2 | 27±7 | 30±6 |
| Gestational age weeks | 29±6 | 32±4 |
| Systolic BP range mmHg | 90–120 | 140–160 |
| Diastolic BP range mmHg | 50–80 | 80–105 |
| Urinary protein g·24 h−1 | <0.3 | 2.2±1* |
| Birth weight of the infant g | 3150±825 | 1786±561* |
Data are presented as mean±SD, unless otherwise stated. PET: pre-eclamptic toxaemia; BMI: body mass index; BP: blood pressure.
p<0.05.
The study was approved by the Rambam Medical Center Helsinki committee and all participants signed an informed consent prior to participation. Clinical data were collected from the patients, personal charts in the hospital and through questionnaires.
Participants underwent clinical examination (including BP and urinalysis), endothelial function assessment (daytime), and ambulatory nocturnal sleep study in that night.
Endothelial function testing
This part of the study was performed in the morning. Subjects were instructed to fast starting the night before testing and to refrain from smoking, ingesting alcohol or caffeine.
The ED evaluation was assessed utilising the Endo_PAT device (Itamar Medical Ltd, Caesarea, Israel). This is a noninvasive technology that captures a beat-to-beat plethysmographic recording of the finger arterial pulse-wave amplitude (PWA) with pneumatic probes. A finger probe is placed on the index finger of each hand, and the peripheral arterial tone (PAT) is recorded from both hands throughout the study. Endothelial function was assessed using the reactive hyperaemia technique, with the participant sitting in a special comfortable chair with both hands located at the level of the heart. Briefly, measurements were obtained for 5 min of baseline (at rest), followed by 5 min of occlusion of one arm, with cuff inflated on the upper arm to suprasystolic pressure (50 mmHg above systolic pressure) and then released to induce reactive (flow-mediated) hyperaemia, measured for 10 min. The other hand remained unoccluded as a reference to correct for potential systemic changes.
The endothelial function is calculated as the ratio between the magnitude of the average post-obstructive PWA (1.5–2.5 min after release of the arterial occlusion) and average 5 min of baseline PWA (pre-occlusion baseline period), corrected to systemic changes (seen at the nonobstructed arm). The Endo_PAT measurements were analysed with a computerised, automated algorithm (Itamar Medical Ltd). Therefore, there is no intra-observer or interobserver variability. A value of ≤1.67 is considered as ED (determined in a population at risk for ischaemic heart disease). This technology has been validated and shown to be accurate in comparison with other technologies [22, 23]. It should be considered, however, that it was not validated in pregnant females with or without PET. Figure 1 exemplifies normal and pathological studies.
FIGURE 1.
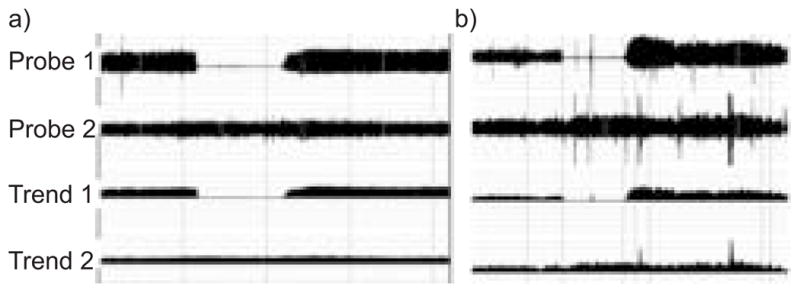
Endothelial dysfunction (ED) evaluation by the reactive hyperaemia test. Four channels are presented as follows: raw data of the occluded finger (probe 1); raw data of the unoccluded finger (probe 2); moving time average of the occluded finger (trend 1); and moving time average of the unoccluded finger (trend 2). a) shows an individual with ED (there is a lack of vasodilatation in post-obstruction period), whereas b) shows a normal endothelial response (there is a marked increase in pulse-wave amplitude indicating vasodilation shortly following the removal of the obstruction). In both cases, there is no systemic response as indicated by the stable signal in the nonoccluded arm.
Sleep-disordered breathing evaluation
This part of the study took place in the hospital during the night for the pre-eclamptic females, and at home for the control females. In both cases, a careful attempt was made to provide the participants with a quiet and calm environment during the night. SDB was quantified using the ambulatory system Watch_PAT100 (Itamar Medical Ltd). This system consists of four channels monitoring the following: actigraphy; peripheral arterial tone; oxygen saturation; and pulse rate. It has been shown to accurately detect SDB in both normal patients and patients with sleep apnoea [24]. However, It should be considered that it was not validated in pregnant females with or without PET. The device was applied for one night to each participant, storing the data during the night and automatically scoring and analysing the data on the following morning. The automatic algorithm of the Watch_PAT100 to detect SDB events has been previously described [24]. Briefly, it is based on the PAT signal amplitude, heart rate and oxygen saturation. The sleep/wake detection is based on data recorded by the built-in actigraph. The automatic algorithm scores a respiratory disturbance event if either a substantial digital vasoconstriction occurs (>50%) or substantial arterial oxygen desaturation occurs (>4%), or a milder degree of vasoconstriction (>30%) is detected with concurrent pulse rate acceleration (>10%) or subthreshold arterial oxygen desaturation (>3%). Based on the actigraphical data, periods of sleep and wakefulness are identified, and the automatic algorithm respiratory disturbance index (RDI) is calculated per hour of detected sleep. Since most SDB events are terminated by an arousal response (with sympathetic surge), this method is relatively sensitive for both apnoeas/hypopnoeas and respiratory effort-related arousals.
Statistical analysis
Individual data of the ED or sleep study of the participants were blinded and automatic. Data are expressed as mean ± SEM, unless stated otherwise. Comparison between the PET group and the control group was performed using the unpaired t-test. Correlation analyses were performed to assess the association between the RDI and the ED, as well as between ED or RDI and birthweight of the infant, using Pearson’s correlation coefficient. For all data, a p-value of <0.05 was considered significant.
RESULTS
The average age, body mass index (BMI), gestational age of the females (at the time of the study), and birthweight of the newborns of the females with PET and controls are presented in table 1. By definition, BP ranges and urinary protein differed between the groups. In addition, the birthweight of the newborns in the PET group was significantly lower than the control group. This was partially secondary to premature labour, but not solely. Out of the 17 neonates of the PET group, 12 were small for the gestational age (71%). Age, BMI prior to pregnancy, BMI at the time of the study and gestational age did not differ significantly between the groups, although there was a trend towards a higher BMI (predominantly at the time of the study) and gestational age (at the time of the study) in the PET group.
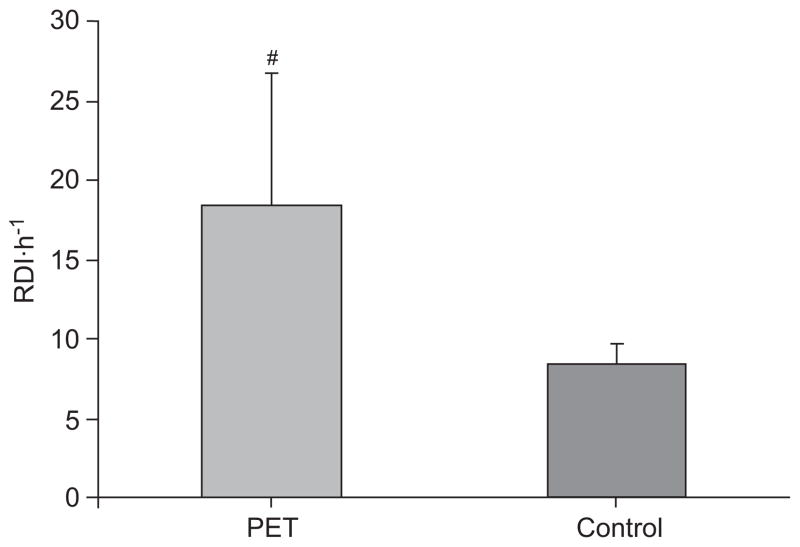
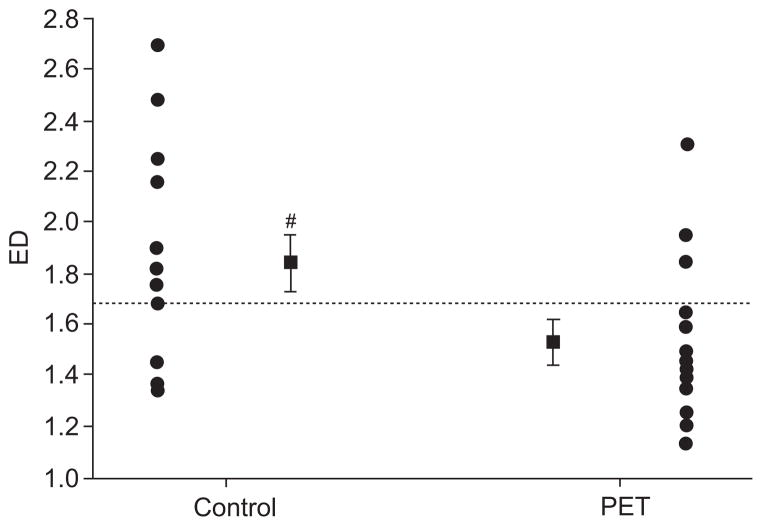
The average PAT RDI (PRDI) was 18.4±8.4 in the PET group versus 8.3±1.3·h−1 in the controls (p<0.05; fig. 2). The oxygen desaturation index was 11.2±6.5·h−1 and 0.65±0.3·h−1 in the PET and control groups, respectively (p<0.05). The average endothelial function index (EFI) was 1.5±0.1 in the PET group versus 1.8±0.1 in the controls (p<0.05; fig. 3). Females with PET had significantly earlier delivery (34±6.6 versus 38±2.6 weeks; p<0.05), and the birthweight of their infants was significantly smaller (1,790±560 g versus 3,150±800 g; p<0.05). There was a weak negative trend towards a correlation between PRDI and the EFI, indicating a tendency towards an inverse relationship between the severity of SDB and endothelial function (r=−0.28; p<0.1). There was a weak trend towards a correlation between ED and oxygen desaturation index (r=−0.22; p<0.2). There was a significant correlation between RDI and mean BP (r=0.41; p<0.05), and between EFI and mean BP (r=−0.41; p<0.05). In addition, the birthweight of the newborns correlated positively with the endothelial function score (r=0.37; p<0.05), and tended to negatively correlate with RDI (r=−0.19; p<0.2). When correcting birth-weight to the week of delivery (expressing birthweight in percentiles), these correlations deteriorated (r=0.1; r=−0.1 respectively; p=NS).
FIGURE 2.
Respiratory disturbance index (RDI) of pre-eclamptic toxaemia (PET) and controls. The RDI of the PET group was significantly higher than in the control group. #: p=0.03.
FIGURE 3.
The endothelial dysfunction (ED) score of the two groups is presented, indicating the averages (■) and the individual data (●). The ED score of the pre-eclamptic toxaemia (PET) group was significantly lower than in the control group. Most females with PET had an ED score below the threshold of normality (······=1.67), whereas most controls were above it. #: p=0.03.
DISCUSSION
The current data show that both SDB and ED occur in females with PET more than in females with uncomplicated pregnancies. There was a trend towards a negative correlation between RDI and ED, suggesting a possible association between these two measures. It is speculated that respiratory disturbances may contribute to or perpetuate the functional abnormality of the blood vessels seen in females with PET, although causality cannot be determined by the present study. The significant positive correlation of RDI with mean BP, and negative correlation of ED with mean BP indicate both RDI and ED can contribute to the hypertension measured in these females. The correlation of the newborn’s birthweight with maternal ED and negative correlation with SDB suggest that these measures may affect clinical outcome (although not via one pathway, but probably partially by trend to advance delivery and by trend to inhibit intra-uterine growth).
PET is a disease of pregnancy, which presents with de novo hypertension and proteinuria developing after 20 weeks of gestational age. The disease is characterised by generalised vasoconstriction, an increase in peripheral resistance, platelet activation, reduced plasma volume and organ hypoperfusion [25, 26]. The aetiology is still unclear, although recent evidence suggests that increments in blood pressure may reflect ED, the inability of the blood vessel endothelial cells to release relaxing factors that cause vasodilatation [27, 28]. ED is now believed to lead to some of the widespread clinical features of the disease. The current results, therefore, are in concert with several previous studies that have found ED in females with PET [27, 29]. Furthermore, a significant correlation between ED and mean BP was found, supporting a potential mechanistic association between these two.
In addition to the involvement of alterations in the maternal vascular endothelium, some recent studies have suggested that females with PET may suffer from inspiratory flow limitation, a phenomenon seen in OSA [12–14]. Averages of 8.3 respiratory events per hour in the control group and 18.4 in the PET group were found in the present study. The oxygen desaturation index was 11·h−1 in females with PET versus 0.7·h−1 in the controls. The increased SDB in PET is, therefore, in agreement with these previous studies [12–14], which all indicate increases in SDB in PET, but usually not to a severe extent. The 8.3 events per hour of sleep with an oxygen desaturation index of only 0.7·h−1 found in the control group is slightly higher than expected normally, and may be attributable to pregnancy itself [30, 31].
In the current study, the authors chose to measure ED by a noninvasive technique. Although the gold standard is considered to be direct assessments of blood vessels via arterial catheterisation [32], this is an invasive method, very cumbersome, and not realistic in the present study involving pregnant females. In the current study, the system used has the advantage of assessing the ED automatically by a computerised system, and, more importantly, the advantage of correcting for systemic changes. In some cases during the study in general, and while inflating the cuff in particular, there may be systemic responses that can alter the assessment of endothelial function in the tested arm. Therefore, detecting the systemic changes in the nonoccluded arm provides important information that can lead to a more accurate measure of the ED. One such example is presented in figure 4. Thus, monitoring the PWA in the control arm is a major advantage of this technique.
FIGURE 4.
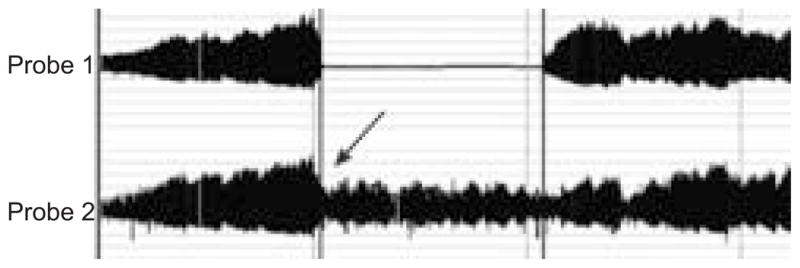
An example of normal endothelial function, with vasoconstrictive systemic response. In this example, the post-occlusion pulse-wave amplitude (PWA) in the occluded arm (occlusion duration 5.2 min, probe 1) is similar to the pre-occluded values, which could have been misinterpreted as abnormal endothelial function. However, looking at this individual systemic response (assessed in the control arm, probe 2), it can be seen that this individual had a vasoconstrictive (stress) response with the cuff inflation (arrow), and, therefore, the “unchanged” PWA seen in the tested arm actually results from local vasodilatation, indicating a normal endothelial function (endothelial dysfunction score 2.16). This example emphasises the advantage of monitoring systemic response during the reactive hyperaemia test.
The evaluation of breathing during sleep was carried out utilising the Watch_PAT100, which is a simple, reliable and accurate noninvasive device for ambulatory assessment of SDB [24].
Nevertheless, it measures autonomic channels and detects SDB by autonomic activity in the termination of SDB events, and not by directly assessing respiration during sleep. While this methodology could be considered a limitation of the study, this is also its advantage. Since the system is simple and easy to use, it was well tolerated by the participants. Those with PET who had to stay in the hospital wore the device in the obstetrics department and the control females took it to their home. Since the differences expected between the groups may be subtle and based mainly on flow limitation and not necessarily complete apnoeas, the current methodology represents a relative advantage as it is very sensitive to autonomic changes, such as those seen in the termination of flow limitation periods [33]. In fact, the current authors did attempt a study using full overnight polysomnography, but discontinued it due to poor enrolment. Thus, the autonomic assessment was useful from both a methological and practical standpoint.
As mentioned previously, there was a trend towards an association between RDI and ED, which was somewhat weaker between the oxygen desaturation index and ED. These findings may suggest that there is an association between respiratory abnormalities in sleep and ED, although a causative relationship cannot be determined based on the present study. It is speculated that respiratory disturbances may contribute to or perpetuate the functional abnormality of the blood vessels seen in females with PET, and that it is not necessarily mediated by hypoxaemia. It has been well documented that SDB is associated with daytime hypertension [18, 34]. Therefore, the SDB that was observed in the PET group can potentially contribute to an increase in BP, as was evident by the significant correlation (r=0.41) between the two. Previous studies have demonstrated reduction of BP when hypertensive patients with OSA are treated using continuous positive airway pressure (CPAP). This has not been assessed in the current study, but the results suggest that CPAP may be clinically valuable for some females. Indeed, some recent studies have reported benefits of administering CPAP to females with PET [35, 36]. In theory, the rise in carbon dioxide tension that would occur during inspiratory flow limitation could lead to sympatho-excitation and marked hypertension due to progesterone-mediated upregulation of catecholamine receptors [37].
The correlation of the newborn’s birthweight to both ED (positive) and RDI (negative) suggests that these measures can influence clinical outcome. When the correlations of ED and RDI with week of delivery and with percentile at birth were examined, nonsignificant correlations were found. Thus, the correlation with birthweight probably represents a combined effect on intra-uterine growth retardation (trend) and premature delivery (trend in the same direction), and probably additional factors. The current authors did not find that ED or SDB can solely explain intra-uterine growth retardation or premature delivery, but, in combination, significant correlations between ED and SDB and the infant’s weight upon delivery were found. Several papers have shown that factors that may reduce placental blood flow, such as smoking or hypertension, can result in low birthweight [38, 39]. Thus, the present results (although not significant) are not surprising. The relatively weak correlations, however, clearly indicate that ED and/or RDI cannot be the sole reasons for this outcome. Whether CPAP treatment or another treatment to ameliorate endothelial function will result in increased birthweight has to be determined in future studies.
The present study has several limitations. First, as discussed previously, the gold-standard technology was not used to assess ED and SDB. However, the techniques used were more practical than the gold standards, and also offered some advantages over other techniques. Secondly, for the SDB, the PET females were studied in the hospital, whereas the females with uncomplicated pregnancies were studied in their homes. This should not impose a systematic difference between the groups as studies have not found a systematic change in SDB when laboratory studies were compared with home studies [40, 41]. Nevertheless, this is a difference that was not controlled. Finally, the current sample size was relatively small, which can emphasise the role of confounding factors. Although not statistically significant, the PET females were heavier and were studied somewhat later in their pregnancies (table 1). The present authors attempted to control these factors, but, nevertheless, mild differences in these variables were observed, mainly at the time of the study and not between BMIs prior to pregnancy. The increased BMI of the females with PET (especially during pregnancy itself) may result from increased fat, which could potentially affect both SDB and ED. However, the BMI differences between the PET and control group were not significant. BMI may also increase with increased extracellular fluids (oedema), which the present authors believe has less effect on endothelial function. However, since BMI is a risk factor for both PET and OSA, one possibility is that obesity may be a factor important in mediating the observed ED. Another possibility is that the increase in BMI (oedema) occurs as a result of the pre-eclampsia, rather than the converse. Obviously, larger-scale studies are required to investigate the role of potential confounders in the SDB and ED seen in females with PET.
In conclusion, despite the previously mentioned limitations, the current authors believe that this study demonstrates endothelial dysfunction and sleep-disordered breathing in females with pre-eclamptic toxaemia compared with controls, and suggests that, to some extent, sleep-disordered breathing may contribute to the hypertension in these females. The results also suggest that endothelial dysfunction and sleep-disordered breathing are associated with each other, although causality remains to be proven. Therefore, it is proposed that endothelial dysfunction and sleep-disordered breathing should be measured during pregnancy, and speculated that the current results encourage treatment with continuous positive airway pressure early in the pregnancy of females with endothelial dysfunction or sleep-disordered breathing.
Footnotes
SUPPORT STATEMENT: D. Yinon was supported with a scholarship from the Technion -Israel Institute of Technology, (Haifa, Israel) to run this study as an MSc project. Itamar Medical Ltd (Caesarea, Israel) supported the study with Watch_PAT100 and Endo_PAT devices.
References
- 1.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of pre-eclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9:147–160. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 2.Davis KR, Ponnampalam J, Hayman R, Baker PN, Arulkumaran S, Donnelly R. Microvascular vasodilator response to acetylcholine is increased in women with pre-eclampsia. BJOG. 2001;108:610–614. doi: 10.1111/j.1471-0528.2001.00144.x. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida A, Nakao S, Kobayashi M, Kobayashi H. Flow-mediated vasodilation and plasma fibronectin levels in pre-eclampsia. Hypertension. 2000;36:400–404. doi: 10.1161/01.hyp.36.3.400. [DOI] [PubMed] [Google Scholar]
- 4.Celermajer D. Endothelial function: does it matter? Is it reversible? J Am Coll Cardiol. 1997;30:325–333. doi: 10.1016/s0735-1097(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 5.Vane J, Anggard E, Botting R. Regulatory function of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 6.Quyyumi AA, Dakak N, Andrews NP, et al. Nitric oxide activity in the human coronary circulation. Impact of risk factors for coronary atherosclerosis. J Clin Invest. 1995;95:1747–1755. doi: 10.1172/JCI117852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hedner T, Sun X. Measures of endothelial function as an endpoint in hypertension? Blood Press Suppl. 1997;2:58–66. [PubMed] [Google Scholar]
- 8.Chan SY, Mancini GB, Kuramoto L, Schulzer M, Frohlich J, Ignaszewski A. The prognostic importance of endothelial dysfunction and carotid atheroma burden in patients with coronary artery disease. J Am Coll Cardiol. 2003;42:1037–1043. doi: 10.1016/s0735-1097(03)00927-6. [DOI] [PubMed] [Google Scholar]
- 9.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 10.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 11.Edwards N, Middleton PG, Blyton DM, Sullivan CE. Sleep disordered breathing and pregnancy. Thorax. 2002;57:555–558. doi: 10.1136/thorax.57.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izci B, Riha RL, Martin SE, et al. The upper airway in pregnancy and pre-eclampsia. Am J Respir Crit Care Med. 2003;167:137–140. doi: 10.1164/rccm.200206-590OC. [DOI] [PubMed] [Google Scholar]
- 13.Connolly G, Razak AR, Hayanga A, Russell A, McKenna P, McNicholas WT. Inspiratory flow limitation during sleep in pre-eclampsia: comparison with normal pregnant and nonpregnant women. Eur Respir J. 2001;18:672–676. doi: 10.1183/09031936.01.00053501. [DOI] [PubMed] [Google Scholar]
- 14.Edwards N, Blyton DM, Kirjavainen TT, Sullivan CE. Hemodynamic responses to obstructive respiratory events during sleep are augmented in women with preeclampsia. Am J Hypertens. 2001;14:1090–1095. doi: 10.1016/s0895-7061(01)02190-2. [DOI] [PubMed] [Google Scholar]
- 15.Schobel HP, Fischer T, Heuszer K, Geiger H, Schmieder RE. Preeclampsia – a state of sympathetic overactivity. N Engl J Med. 1996;335:1480–1485. doi: 10.1056/NEJM199611143352002. [DOI] [PubMed] [Google Scholar]
- 16.Rodie VA, Freeman DJ, Sattar N, Greer IA. Pre-eclampsia and cardiovascular disease: metabolic syndrome of pregnancy? Atherosclerosis. 2004;175:189–202. doi: 10.1016/j.atherosclerosis.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 17.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 18.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnea syndrome as a risk factor for hypertension. BMJ. 2000;320:479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–1752. [PubMed] [Google Scholar]
- 20.Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 21.Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation. 1998;97:943–945. doi: 10.1161/01.cir.97.10.943. [DOI] [PubMed] [Google Scholar]
- 22.Kuvin J, Patel A, Sliney K, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 23.Bonetti P, Barsness G, Keelan P, et al. Enhanced external counterpulsation improves endothelial function in patients with sympthomatic coronary artery disease. J Am Coll Cardiol. 2003;41:1761–1768. doi: 10.1016/s0735-1097(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 24.Bar A, Pillar G, Dvir I, Schnall R, Sheffy J, Lavie P. Evaluation of a portable device based on peripheral arterial tone (PAT) for unattended home sleep studies. Chest. 2003;123:695–703. doi: 10.1378/chest.123.3.695. [DOI] [PubMed] [Google Scholar]
- 25.Kenny LC, Baker PN, Kendall DA, Randall MD, Dunn WR. Differential mechanisms of endothelium-dependent vaso-dilator responses in human myometrial small arteries in normal pregnancy and pre-eclampsia. Clin Sci (Lond) 2002;103:67–73. doi: 10.1042/cs1030067. [DOI] [PubMed] [Google Scholar]
- 26.Vural P. Nitric oxide/endothelin-1 in pre-eclampsia. Clin Chim Acta. 2002;317:65–70. doi: 10.1016/s0009-8981(01)00751-3. [DOI] [PubMed] [Google Scholar]
- 27.Roberts JM, Taylor RN, Goldfien A. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. Am J Hypertens. 1991;4:700–708. doi: 10.1093/ajh/4.8.700. [DOI] [PubMed] [Google Scholar]
- 28.Haller H, Ziegler EM, Homuth V, et al. Endothelial adhesion molecules and leukocyte integrins in preeclamptic patients. Hypertension. 1997;29:291–296. doi: 10.1161/01.hyp.29.1.291. [DOI] [PubMed] [Google Scholar]
- 29.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 30.Guilleminault C, Querra-Salva M, Chowdhuri S, Poyares D. Normal pregnancy, daytime sleeping, snoring and blood pressure. Sleep Med. 2000;1:289–297. doi: 10.1016/s1389-9457(00)00046-0. [DOI] [PubMed] [Google Scholar]
- 31.Bourne T, Ogilvy AJ, Vickers R, Williamson K. Nocturnal hypoxaemia in late pregnancy. Br J Anaesth. 1995;75:678–682. doi: 10.1093/bja/75.6.678. [DOI] [PubMed] [Google Scholar]
- 32.Kuvin JT, Patel AR, Karas RH. Need for standardization of noninvasive assessment of vascular endothelial function. Am Heart J. 2001;141:327–328. doi: 10.1067/mhj.2001.113221. [DOI] [PubMed] [Google Scholar]
- 33.Pillar G, Bar A, Betito M, et al. An automatic ambulatory device for detection of AASM defined arousals from sleep: the WP100. Sleep Med. 2003;4:207–212. doi: 10.1016/s1389-9457(02)00254-x. [DOI] [PubMed] [Google Scholar]
- 34.Lavie P, Hoffstein V. Sleep apnea syndrome: a possible contributing factor to resistant hypertension. Sleep. 2001;24:721–725. doi: 10.1093/sleep/24.6.721. [DOI] [PubMed] [Google Scholar]
- 35.Blyton DM, Sullivan CE, Edwards N. Reduced nocturnal cardiac output associated with preeclampsia is minimized with the use of nocturnal nasal CPAP. Sleep. 2004;27:79–84. doi: 10.1093/sleep/27.1.79. [DOI] [PubMed] [Google Scholar]
- 36.Edwards N, Blyton DM, Kirjavainen T, Kesby GJ, Sullivan CE. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. Am J Respir Crit Care Med. 2000;162:252–257. doi: 10.1164/ajrccm.162.1.9905006. [DOI] [PubMed] [Google Scholar]
- 37.Edwards N, Wilcox I, Polo OJ, Sullivan CE. Hypercapnic blood pressure response is greater during the luteal phase of the menstrual cycle. J Appl Physiol. 1996;81:2142–2146. doi: 10.1152/jappl.1996.81.5.2142. [DOI] [PubMed] [Google Scholar]
- 38.Visscher WA, Feder M, Burns AM, Brady TM, Bray RM. The impact of smoking and other substance use by urban women on the birthweight of their infants. Subst Use Misuse. 2003;38:1063–1093. doi: 10.1081/ja-120017651. [DOI] [PubMed] [Google Scholar]
- 39.Xiong X, Fraser WD. Impact of pregnancy-induced hypertension on birthweight by gestational age. Paediatr Perinat Epidemiol. 2004;18:186–191. doi: 10.1111/j.1365-3016.2004.00553.x. [DOI] [PubMed] [Google Scholar]
- 40.White DP, Gibb TJ, Wall JM, Westbrook PR. Assessment of accuracy and analysis time of a novel device to monitor sleep and breathing in the home. Sleep. 1995;18:115–126. doi: 10.1093/sleep/18.2.115. [DOI] [PubMed] [Google Scholar]
- 41.Fry JM, DiPhillipo MA, Curran K, Goldberg R, Baran AS. Full polysomnography in the home. Sleep. 1998;21:635–642. doi: 10.1093/sleep/21.6.635. [DOI] [PubMed] [Google Scholar]