Abstract
Background
We tested the hypothesis that central α2A-adrenergic receptor (α2AAR) signaling plays a key role in clonidine-ethanol evoked synergistic behavioral impairment.
Methods
Male Sprague-Dawley rats, with intracisternal and jugular vein cannulae implanted 6 days earlier, were tested for drug-induced behavioral impairment. The latter was assessed as the duration of loss of righting reflex (LORR) and rotorod performance every 15 minutes until the rat recovered to the baseline walk criterion (180 seconds). In a separate cohort, c-Fos expression in locus coeruleus (LC) and cerebellum was determined as a marker of neuronal activity following drug treatment.
Results
Rats that received clonidine (60 μg/kg, i.v.) followed by ethanol (1 g/kg, i.v.) exhibited synergistic impairment of rotorod performance and LORR. The mixed α2AAR and I1-imidazoline receptor agonist clonidine (30, 60, and 90 μg/kg) synergistically and dose-dependently enhanced behavioral impairment elicited by ethanol (1 g/kg). Possible involvement of I1-imidazoline receptors was ruled out because selective I1-agonist rilmenidine (300 μg/kg, i.v.) did not cause behavioral impairment alone or enhance ethanol-evoked behavioral impairment. Pharmacological blockade of central α2AAR (RX821002, 0.3 mg i.c.) abolished the synergy between clonidine and ethanol; the behavioral response caused by the drug combination was similar to that caused by ethanol alone. Conversely, involvement of central α2BAR in the interaction was ruled out because blockade of central α2BAR (ARC-239) independently evoked a strong sedative effect. Clonidine (60 μg/kg) or ethanol (1 g/kg) alone increased, but their combination decreased, c-Fos levels in LC, while inconsistent c-Fos responses were observed in cerebellum.
Conclusions
Central α2AAR, but not I1-imidazoline or α2BAR, signaling is implicated in the synergistic enhancement of ethanol-evoked behavioral impairment by clonidine. Although the mechanism of c-Fos response remains to be investigated, this neurochemical response highlights the LC as a neuroanatomical target for clonidine-ethanol behavioral interaction.
Keywords: Ethanol, Clonidine, Behavioral Impairment, Rotorod, Loss of Righting Reflex
Interactions between alcohol and prescription drug medications, such as the antihypertensive drug clonidine, represent a serious concern for public health. The antagonistic hemodynamic interaction between ethanol and clonidine, which renders the antihypertensive effect of clonidine ineffective, has been documented (El-Mas and Abdel-Rahman, 1999, 2001; Mao and Abdel-Rahman, 1998); however, an equally important behavioral interaction between these two drugs also produces a clinically serious adverse effect. The synergistic behavioral impairment elicited by clonidine-ethanol interaction has been reported (Mao and Abdel-Rahman, 1996) and supported by findings from interactions with related drug classes (Czarnecka et al., 1986; Durcan et al., 1991; Idanpaan-Heikkila et al., 1995; Kushikata et al., 2002). Although the sedative effect of clonidine is utilized in some clinical applications, such as analgesia, anesthesia, or treatment of withdrawal symptoms (Fauler and Verner, 1993; Khan et al., 1999), the combined use of ethanol and clonidine and the resultant synergistic behavioral impairment is a greater concern for patients taking clonidine unsupervised as an antihypertensive agent. Little is known, however, about the mechanisms underlying this synergistic interaction. Furthermore, clonidine is a mixed α2/I1-receptor agonist that induces its hypotensive effect primarily via the I1-imidazoline receptor (El-Mas and Abdel-Rahman, 2001; Head and Mayorov, 2006) and its sedative effects primarily via the α2A-adrenergic receptor (AR) (Hunter et al., 1997; Lahdesmaki et al., 2003; Lakhlani et al., 1997). There is, nonetheless, some cross-over in this system, such that both receptor types also contribute to the nonprimary function. For example, hypotension is partly mediated by α2AR activation (Reis, 1996), while the rostral ventrolateral medulla (RVLM) sends I1-responsive projections to the locus coeruleus (LC), where clonidine is thought to produce its behavioral effects via α2AR (Meana et al., 1995; Ruiz-Durantez et al., 2002; Ruiz-Ortega and Ugedo, 1997). Therefore, while α2AAR signaling is likely implicated in clonidine–ethanol behavioral interaction, direct evidence in support of this notion is lacking. Further, the possibility has not been investigated that other molecular targets of clonidine (α2BAR or I1-receptor) might be implicated in the interaction.
It is also important when undertaking mechanistic studies to investigate neurochemical responses that might identify the neuroanatomical substrate(s) implicated in the behavioral response. Induction of immediate early genes, such as c-fos and its protein c-Fos, can serve as a marker of neuronal activity (Dragunow and Faull, 1989). In many brain areas, induction or inhibition of c-Fos expression has been associated with ethanol-evoked behavioral responses (Ryabinin, 1998; Sharpe et al., 2005; Thiele et al., 2000). In the Edinger-Westphal nucleus (EW), ethanol-induced c-Fos expression was modulated by α2AR activation (Bachtell et al., 2002). In the few studies that described the effect of clonidine on c-Fos or its gene, clonidine was shown to decrease induced c-fos mRNA expression in the nucleus tractus solitarius (NTS) and RVLM (El-Mas and Abdel-Rahman, 2000). Importantly, the studies that have implicated the α2AR in the modulation of the neurochemical (c-Fos expression) effects of ethanol did not directly evaluate the effect of α2AR activation on c-Fos expression in the brainstem or investigate the associated behavioral effects of ethanol in the presence of α2AR blockade (Bachtell et al., 2002). Equally important, no studies have evaluated changes in c-Fos expression as a result of clonidine–ethanol combination, particularly in the LC, which is an obvious target for clonidine action due to its high density of α2AR binding sites (Aghajanian and VanderMaelen, 1982).
Indeed, when injected into the LC, clonidine or the α2AR agonist dexmedetomidine produces α2AR mediated sedative effects by inhibition of LC firing, which likely disinhibits GABAergic neuronal activity downstream to cause behavioral impairment (Aghajanian and VanderMaelen, 1982; Correa-Sales et al., 1992; De Sarro et al., 1987; Guo et al., 1996; Pudovkina et al., 2001). Likewise, ethanol has also been shown to induce behavioral impairment by inhibition of LC firing, and it has been suggested that the pre-existing level of LC activity (level of arousal) may affect responsiveness to ethanol (Palmer and Granholm, 1992; Verbanck et al., 1991), possibly accounting for some inter-individual differences in observed behavioral responses to ethanol. Overall, clonidine and ethanol produce sedation independently by inhibiting LC firing; however, it is yet unclear whether the neurochemical effects of their combination in the LC could explain the additive/synergistic behavioral interaction between both drugs.
Therefore, the major goal of the present studies was to test the hypothesis that central α2AAR signaling plays a key role in the synergistic enhancement of behavioral impairment caused by ethanol in the presence of clonidine and that the LC is implicated in the interaction. To test this hypothesis, we investigated the effect of clonidine pretreatment on the behavioral response (impaired rotorod performance and loss of righting reflex, LORR) elicited by moderate doses of ethanol. Pharmacological interventions were used to identify the α2AR subtype implicated in the interaction and whether the I1-receptor, which is also activated by clonidine, plays a role in the interaction. In these studies we utilized selective α2A or α2BAR antagonist and the I1-receptor agonist rilmenidine. Finally, we measured the changes in c-Fos in the LC elicited by a moderate dose of ethanol in the presence or absence of clonidine; whether similar changes in c-Fos occurred in other brain areas (e.g., cerebellum) was investigated.
METHODS
Animals
Male Sprague-Dawley rats were obtained from Taconic Farms (Germantown, New York) and housed individually in a controlled environment room with a constant temperature of 23 ± 1°C, humidity of 50 ± 10%, and a 12:12 hr light–dark cycle. Food (Prolab Rodent Chow, Prolab RMH 3000, Granville Milling, Creedmoor, NC) and water were available ad libitum. At least 2 days were allowed for acclimatization prior to surgical or experimental manipulation. Animals weighed approximately 325 to 375 g on the day of behavioral testing. All surgical, experimental, and animal care procedures were performed in accordance with National Institutes of Health and institutional animal care and use committee guidelines.
Surgical Procedures
All surgical procedures were conducted under sterile conditions. Animals were anesthetized with sodium pentobarbital (60 mg/kg, i.p.). A stainless steel guide cannula (23 G) was inserted approximately 6 mm into the skull between the occipital bone and the cerebellum so that its tip protruded into the cisterna magna. The cannula was secured in place with small metal screws and dental acrylic cement (Durelon, Thompson Dental Supply, Raleigh, NC) as described previously (El-Mas and Abdel-Rahman, 2001). Stainless steel wire (0.012″ diameter) inside the guide cannula served as a block until the day of the experiment. Intracisternal cannulation was not performed in animals used for neurochemical studies. All animals were vascularly catheterized for intravenous (i.v.) administration of drugs. For behavioral studies, the jugular vein was isolated, and a polyethylene-50, gas sterilized catheter filled with heparinized saline (100 U/ml) was inserted approximately 2 cm into the vessel. For neurochemical studies, the femoral vein was isolated, and 2 smaller catheters (polyethylene-10 tubing connected to polyethylene-50 tubing, gas sterilized) filled with heparinized saline were inserted into the abdominal vena cava. All catheters were: (i) secured by thread to the blood vessel and muscle tissue, (ii) tunneled subcutaneously (s.c.) and exteriorized at the back of the neck between the shoulder blades, (iii) flushed with heparinized saline, and (iv) closed with sterilized metal pins. The neck or leg wound was swabbed with povidone-iodine solution and closed by wound clips. All rats received postoperative care that comprised buprenorphine hydrochloride (0.03 mg/kg, s.c.) and penicillin G benzathine/penicillin G procaine (100,000 units/kg, s.c.). Following recovery from anesthesia, the rats were provided with wet food (rat chow softened in water) to facilitate food intake and gain in body weight postsurgery. Rats were allowed to recover for approximately 6 days before the experiment following intracisternal cannulation or 2 days following femoral catheterization only as in our reported studies (El-Mas and Abdel-Rahman, 2001; Mao and Abdel-Rahman, 1996).
Behavioral Testing Protocol
Behavioral impairment was assessed by performance on a rotorod (Treadmill for Rats 7700, Ugo Basile Biological Research Apparatus, Comeriova, Italy) operated at constant speed (~11 RPM). Prior to surgery, rats were trained to walk on the rotorod for 180 seconds consecutively without falling off as in reported studies (Dar, 1998). All animals were successfully trained to this baseline criterion. On the day of the experiment, all animals were retested to ensure that they could still meet the baseline criterion post-surgery. Animals received an intracisternal (i.c.) injection (pretreatment) of pharmacological inhibitor or vehicle (artificial cerebrospinal fluid, aCSF) followed by 2 i.v. injections of drug (clonidine or rilmenidine, ethanol, or their combination) and/or vehicle (saline) depending on the experiment. The duration of pretreatment varied depending on the pharmacological inhibitor used. Briefly, after removing the block, a 30 G stainless steel injector was inserted into the guide cannula to deliver the pharmacological inhibitor or aCSF to the cisterna magna by a microsyringe connected to the injector with polyethylene tubing. A volume of 4 μl was delivered by hand over 30 to 60 seconds. After 5 minutes, the injector was removed and the block was replaced for the duration of testing. The 2 i.v. injections of drug and/or saline were followed by saline flush to ensure complete drug delivery. An injection interval of no more than 10 minutes between clonidine and ethanol is required to produce synergistic behavioral impairment (Bender and Abdel-Rahman, 2006, 2007) and was employed throughout these studies. LORR was assessed immediately after the 2 i.v. injections by placing the animal on its back and recording the duration (in minutes) before it righted itself (all 4 paws touching the floor). Rotorod performance was assessed every 15 minutes after the 2 i.v. injections until recovery to the baseline criterion was achieved or until the end of the experiment (3 hours max). Each assessment consisted of a maximum of 3 walk trials, with a 180 seconds cutoff time as reported (Dar, 1998). The highest walk time (180 seconds max.) was recorded for each time point. Steps were taken to ensure that animals were not injured by falling. No cardiovascular parameters (e.g., blood pressure) were measured in these studies. Animals were euthanized by pentobarbital overdose at the end of the experiment. Correct placement of the intracisternal cannula was determined by i.c. injection of fast green dye.
Western Blot Analysis
Brains were collected for neurochemical studies 15 minutes after the 2 i.v. drug and/or saline injections—the time corresponding to peak drug-induced behavioral impairment (Bender and Abdel-Rahman, 2006, 2007). Animals received a lethal dose of sodium pentobarbital (i.p.), and following decapitation, brains were removed, flash frozen in 2-methylbutane (cooled on dry ice for at least 30 minutes), and stored at −80°C until use. Brains were equilibrated to −20°C and sectioned with a cryostat (HM 505E; Microm International GmbH, Waldorf, Germany) rostrally to the locus coeruleus (LC) according to atlas coordinates (Paxinos and Watson, 1998). Tissue from the locus coeruleus (LC) was collected bilaterally using a 0.75-mm punch instrument as described in other studies (e.g., Mouledous et al., 2007) from approximately −10.04 mm to −9.30 mm from bregma (Paxinos and Watson, 1998). Bilateral cerebellum (CB) tissue punches were collected for Western blot analysis at the level of the LC in the same animals. Tissue was homogenized on ice by sonication in cell lysis buffer (20 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM activated sodium orthovanadate) containing protease inhibitor cock-tail tablet (Roche Diagnostics, Indianapolis, IN). After centrifugation at 4°C at 10,000 RPM for 20 minutes, protein in the supernatant was quantified using a standard Bio-Rad protein assay system (Bio-Rad Laboratories, Hercules, CA). Protein extracts (20 μg per lane) were denatured at 97°C for 5 to 10 minutes, separated on NuPAGE Novex Bis-Tris 4 to 12% SDS–PAGE gels (Invitrogen, Carlsbad, CA) using MOPS NuPAGE running buffer, and electroblotted to nitrocellulose membranes (cold transfer buffer: 230 mM glycine, 25 mM Tris, 0.7 mM SDS, 20% methanol). Positive controls for c-Fos (A431 + EGF cell lysate; BD Transduction/BD Biosciences, San Jose, CA) and actin (Sigma, St. Louis, MO) as well as a marker (SeeBlue® Plus2 Pre-Stained Standard, Invitrogen) were loaded on each gel as appropriate. Non-specific binding sites on the membranes were blocked at room temperature in wash buffer (10 mM Tris, 150 mM NaCl, 0.2% 0.5 M EDTA pH 8.0, 0.01% Triton X-100) containing 5% non-fat milk for 1 to 2 hour. The membranes were then incubated overnight at 4°C with rabbit polyclonal antibodies to c-Fos (1:200, Sigma) in block solution. The blots were washed 4X then incubated for 60 minutes at room temperature with anti-rabbit IgG horseradish peroxidase-linked secondary antibody (1:2,000, GE Healthcare, Piscataway, NJ). After 4 more washes, protein was detected on the blots by enhanced chemiluminescence and exposure to x-ray film. Equivalent sample loading was confirmed by stripping the membranes with Blot Fresh Stripping Reagent (SignaGen, Gaithersburg, MD) and reprobing with rabbit anti-actin antibody (1:2,000, Sigma); all data were expressed as values normalized to actin. Although multiple gels were run, samples from control and treated groups were loaded on each gel.
Protocols and Experimental Groups
To ensure comparison of behavioral responses under the same conditions, all rats used in behavioral studies were subjected to intra-cisternal (i.c.) cannulation. This permitted i.c. pretreatment with the selective α2AAR antagonist RX821002 (as described below) or its vehicle, aCSF. The dose of ethanol (1 g/kg) used in these studies produced blood ethanol concentration compatible with moderate ethanol consumption (El-Mas and Abdel-Rahman, 1999).
Experiment 1. Effect of Clonidine, Ethanol, or Their Combination on Rotorod Performance and Righting Reflex
In this experiment, we investigated whether the enhancement by clonidine of the behavioral impairment caused by a moderate dose (1 g/kg) of ethanol is dose dependent. Based on preliminary studies, 4 groups of rats were used to investigate the effect of clonidine or ethanol given alone or in combination on the behavioral tests (rotorod and LORR). In these studies, each rat received 2 i.v. injections: (i) saline + saline, (ii) clonidine (60 μg/kg) + saline, (iii) saline + ethanol (1 g/kg), or (iv) clonidine (60 μg/kg) + ethanol (1 g/kg). The rats in these 4 groups received i.c. aCSF pretreatment prior to i.v. drug or saline injection and served as controls for the data generated following pretreatment with the α2AAR antagonist described in Experiment 3. To investigate whether the dose of clonidine influences the magnitude of the behavioral impairment caused by ethanol, 2 additional groups of rats received i.v. injections of: (i) clonidine (30 μg/kg) + ethanol (1 g/kg) or (ii) clonidine (90 μg/kg) + ethanol (1 g/kg). The effect of the lower and higher dose (30 or 90 μg/kg) of clonidine on behavioral performance when administered alone was not investigated because the objective of these studies was to investigate the dose-related effects of clonidine on ethanol-evoked behavioral impairment. Further, the additional pharmacological and neurochemical studies detailed below were conducted in rats that received the middle dose (60 μg/kg) of clonidine and ethanol (1 g/kg) alone or in combination.
Experiment 2. Effect of the I1-Selective Agonist Rilmenidine on Ethanol-Induced Behavioral Impairment
Two groups of rats received the following i.v. drug injections: (i) rilmenidine (300 μg/kg) + saline, or (ii) rilmenidine (300 μg/kg) + ethanol (1 g/kg). The dose of rilmenidine was chosen because it produces hypotension comparable to clonidine (30 μg/kg) while maintaining I1-receptor selectivity based on reported findings (Mao et al., 2003) and preliminary experiments.
Experiment 3. Effect of α2A or α2B Adrenergic Receptor Blockade on Clonidine-Ethanol Induced Behavioral Impairment
Four groups of rats were used in this experiment to elucidate the α2AR subtype implicated in clonidine-evoked enhancement of the behavioral impairment caused by ethanol. Following a 10 minute i.c. pretreatment with 0.3 mg RX821002 (selective α2AAR antagonist), the rats received the following i.v. injections: (i) saline + saline, (ii) clonidine (60 μg/kg) + saline, (iii) saline + ethanol (1 g/kg), or (iv) clonidine (60 μg/kg) + ethanol (1 g/kg). The dose and pretreatment time for RX821002 were selected based on reported studies (Bachtell et al., 2002) and scaled down for i.c. administration. The selective α2BAR antagonist, ARC-239, was reported to have sedative behavioral effects per se (Bachtell et al., 2002); therefore, we investigated in two animals the effect of a 10 minute i.v. pretreatment of 10 mg/kg ARC-239 on rotorod performance. ARC-239 was dissolved in saline and given systemically (i.v.) as in the reported study (Bachtell et al., 2002), because it was insoluble in aCSF.
Experiment 4. Effect of Clonidine, Ethanol, and Their Combination on c-Fos Expression in the Locus Coeruleus and Cerebellum
In this experiment, the effects of clonidine, ethanol, and their combination on c-Fos expression in the locus coeruleus (LC) were investigated. The rats were killed 15 minutes after treatment, the time that coincides with peak behavioral synergy between clonidine and ethanol as assessed by the rotorod test. Four groups of rats received i.v. drug/vehicle administration as follows: (i) saline + saline, (ii) clonidine (60 μg/kg) + saline, (iii) saline + ethanol (1 g/kg), or (iv) clonidine (60 μg/kg) + ethanol (1 g/kg). Brains were collected and analyzed for c-Fos protein levels in the LC by Western blot as described. Western blot analyses were also performed on cerebellum tissue collected from the same animals to determine whether the changes in c-Fos caused by clonidine, ethanol, and their combination in the LC and another brain area are similar.
Drug Preparation
All drugs and chemicals, except for rilmenidine, were obtained commercially. Ethanol (Pharmco, Brookfield, CT) was given as a 100% solution. Ethanol was not diluted, as in earlier studies from our lab (e.g., Mao and Abdel-Rahman, 1996), to circumvent any potential “volume” effect on the cardiovascular system. Clonidine hydrochloride (Sigma), ARC-239 dihydrochloride (Tocris Bioscience, Ellisville, MO), and rilmenidine dihydrogen phosphate (gift from Technologie Servier, Neuilly Sur Seine, France) were prepared in sterile saline (0.9% sodium chloride, Abbott Labs, North Chicago, IL). Saline injections were given at 0.1 ml/100 g body weight. Artificial CSF was prepared as: 123 mM NaCl, 0.86 mM CaCl2, 3 mM KCl, 0.89 mM MgCl2 • 6 H2O, 0.5 mM NaH2PO4, 0.25mM Na2HPO4, and 25 mM NaHCO3. RX821002 hydrochloride and fast green dye (Sigma) were prepared in aCSF.
Data Analysis and Statistical Procedures
Data were input into Excel and Prism for graphical analysis and calculation of averages ± SEM and area under the curve (AUC). Statistical analyses were performed using SPSS software (SPSS, Inc., Chicago, IL). Rotorod data were analyzed by repeated measures ANOVA to determine differences in time course trends. Differences at each time point were determined by multiple one-way ANOVA’s. Treatment averages were compared by one-way ANOVA or unpaired t-test (two-tailed) for LORR and rotorod AUC. For post hoc comparisons, Tukey’s test was used when data had equal variance, and the Games-Howell test was used when data had unequal variance. Behavioral synergy was evaluated with rotorod AUC data, where a linear combination of means was used within the ANOVA setting (to take advantage of the pooling of error terms) to compare the sum of the single drug treatment groups (clonidine or ethanol) to combination treatment groups. The linear combination of means was achieved through the use of a contrast coefficient, where the sum of the 2 single drug groups (each denoted “1”) could be compared by ANOVA with the combination group (denoted “−1”). Western blot bands were scanned and quantified by measuring the integrated density (mean density × area) using NIH Image software (version 1.37). Data were normalized in relation to actin, tested for normality (Shapiro–Wilk test) to identify outlier values, and analyzed by univariate ANOVA which included a blocking factor to remove variability associated with running samples on multiple gels. Treatment differences were determined by least significant difference (LSD) post hoc tests. For graphical presentation, group means are shown as % saline control. In all analyses, p < 0.05 was considered statistically significant.
RESULTS
Effect of Clonidine, Ethanol, or Their Combination on Rotorod Performance and Righting Reflex
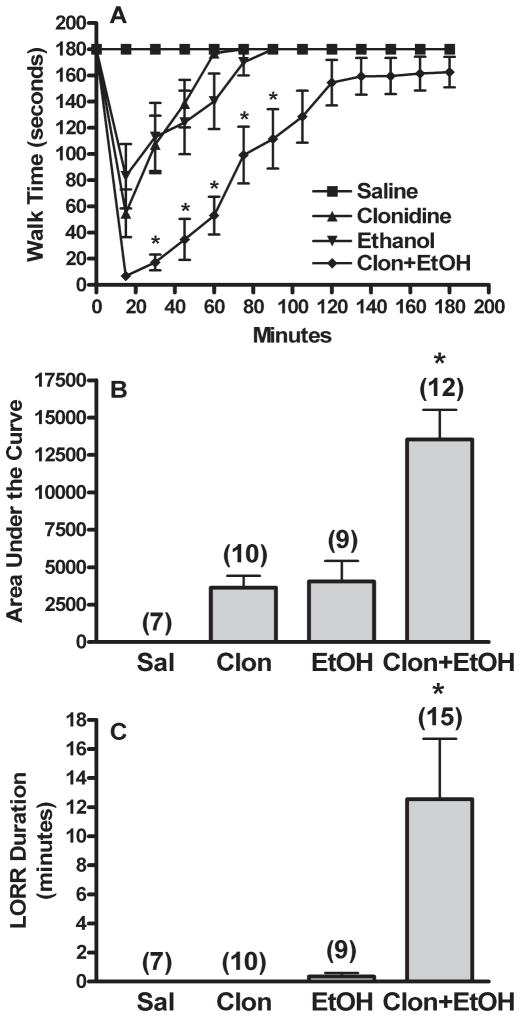
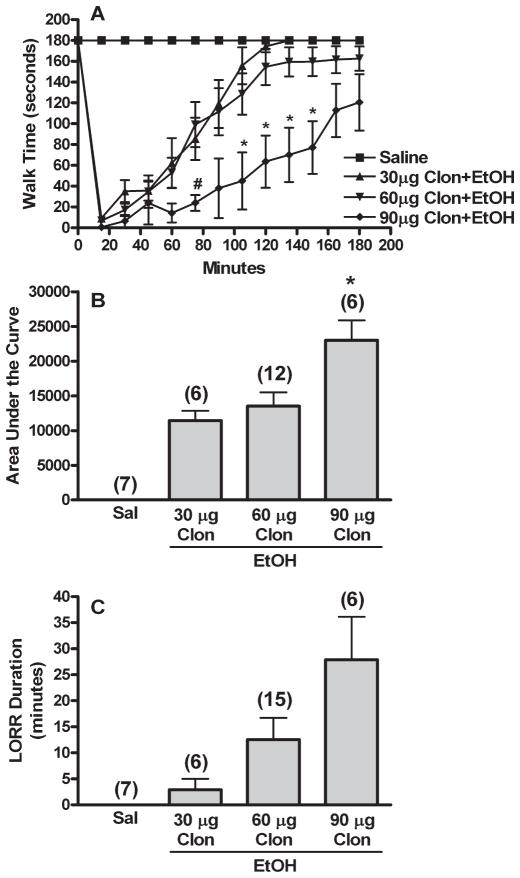
Vehicle (aCSF or saline) treated animals exhibited no behavioral deficits. Clonidine or ethanol treatment alone evoked similar degrees of impairment on the rotorod (Fig. 1A,B); however, while ethanol elicited brief LORR, clonidine, in the dose range used, produced no LORR (Fig. 1C). Combination treatment produced significantly greater rotorod impairment [AUC, F(3,34) = 16.218, p < 0.001] and LORR duration [F(3,37) = 4.973, p < 0.05] than either single drug or saline treatment. The time course for clonidine-ethanol treated animals also differed significantly from the other groups in trend (repeated measures ANOVA) and at several time points (multiple ANOVA’s, Fig. 1A). All ANOVA testing was followed by Games-Howell post hoc tests to determine which groups differed (p < 0.05). As shown in Fig. 1, clonidine synergistically enhanced ethanol-evoked impairment of rotorod performance and righting reflex [ANOVA with contrast, 1 = sum of clonidine and ethanol AUC, −1 = clonidine-ethanol AUC, F(2,28) = 13.597, p < 0.001]. Furthermore, clonidine enhancement of ethanol-evoked behavioral impairment was dose-dependent. As shown in Fig. 2, clonidine (30, 60, and 90 μg/kg) dose-dependently increased behavioral impairment elicited by a moderate dose of ethanol (1 g/kg) in rotorod and LORR testing. Behavioral impairment obtained in the 3 combination treatment groups was compared as described above for Fig. 1 [AUC, F(2,21) = 6.088, p < 0.05; LORR, F(2,24) = 3.970, Games-Howell post hoc test for data with unequal variance, p > 0.05; see Fig. 2A for significant time point differences in rotorod time courses].
Fig. 1.
Clonidine synergistically enhances ethanol-evoked behavioral impairment. Rotorod time courses (A), corresponding area under the curve (AUC, B), and LORR durations (C) are shown for groups pretreated i.c. with aCSF prior to 2 i.v. injections of: (i) saline + saline (Sal or Saline), (ii) 60 μg/kg clonidine + saline (Clon or Clonidine), (iii) saline + 1 g/kg ethanol (EtOH or Ethanol), or (iv) 60 μg/kg clonidine + 1 g/kg ethanol (Clon + EtOH). The number of animals per group is shown in parentheses above the bar graphs. (Note: A few additional animals were tested for LORR only in the Clon + EtOH group.) p < 0.05, *differs from Sal, Clon, and EtOH (rotorod trends also differed from Clon + EtOH). Clon + EtOH AUC was significantly greater than the sum of the AUC for Clon and EtOH (synergistic interaction).
Fig. 2.
Clonidine causes dose-related enhancement of ethanol induced behavioral impairment. Rotorod time courses (A), corresponding area under the curve (AUC, B), and LORR durations (C) are shown for groups pretreated i.c. with aCSF prior to 2 i.v. injections of 30, 60, or 90 μg/kg clonidine (30, 60, or 90 μg Clon) followed by 1 g/kg ethanol (EtOH). Sal (Saline) and 60 μg Clon (Clonidine) groups are replotted from Fig. 1. The number of animals per group is shown in parentheses above the bar graphs. p < 0.05, *differs from 30 μg Clon (rotorod trends also differed); #differs from 60 μg Clon. LORR data did not reach significance due to unequal variance.
Effect of the I1-Selective Agonist Rilmenidine on Ethanol-Induced Behavioral Impairment
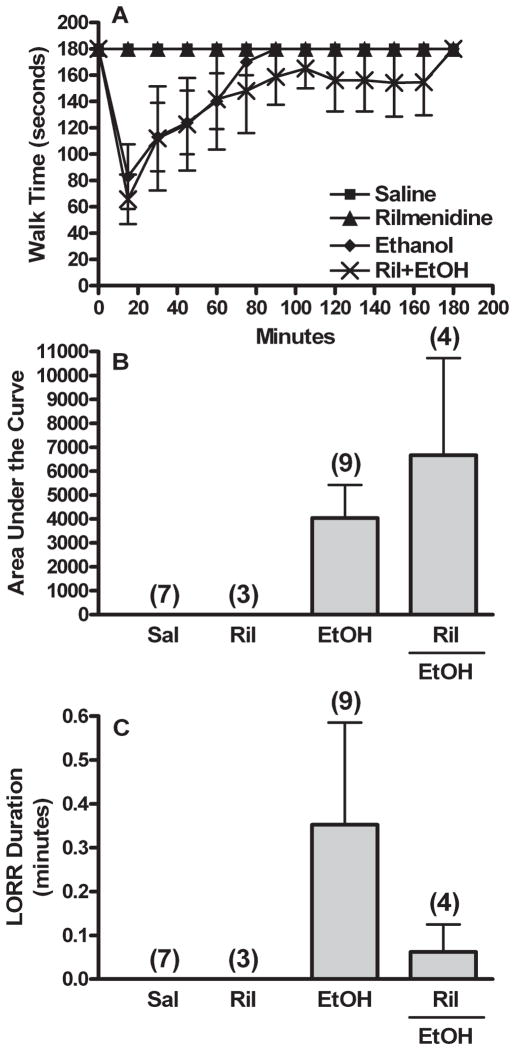
Rotorod performance and righting reflex were evaluated following treatment with I1-receptor agonist rilmenidine (300 μg/kg) alone or in combination with 1 g/kg ethanol. Rilmenidine alone did not elicit behavioral impairment assessed as rotorod performance or LORR (Fig. 3). Furthermore, by contrast to clonidine enhancement of ethanol-evoked behavioral impairment (Fig. 1), selective I1-receptor activation (rilmenidine) had no significant influence on ethanol-evoked impairment of rotorod performance [Fig. 3A,B; AUC (ethanol vs. rilmenidine + ethanol), t(11) = −0.792, p > 0.05, or LORR, Fig. 3C; t(11) = 0.804, p > 0.05].
Fig. 3.
Clonidine-ethanol synergistic behavioral interaction does not depend on activation of the I1-imidazoline receptor. Rotorod time courses (A), corresponding area under the curve (AUC, B), and LORR durations (C) are shown for groups pretreated i.c. with aCSF prior to 2 i.v. injections of: (i) 300 μg/kg rilmenidine (I1 agonist) + saline (Ril or Rilmenidine) or (ii) 300 μg/kg rilmenidine (I1 agonist) + 1 g/kg ethanol (Ril + EtOH). The Sal and EtOH groups are replotted from Fig. 1. The number of animals per group is shown in parentheses above the bar graphs. LORR data did not reach significance due to unequal variance.
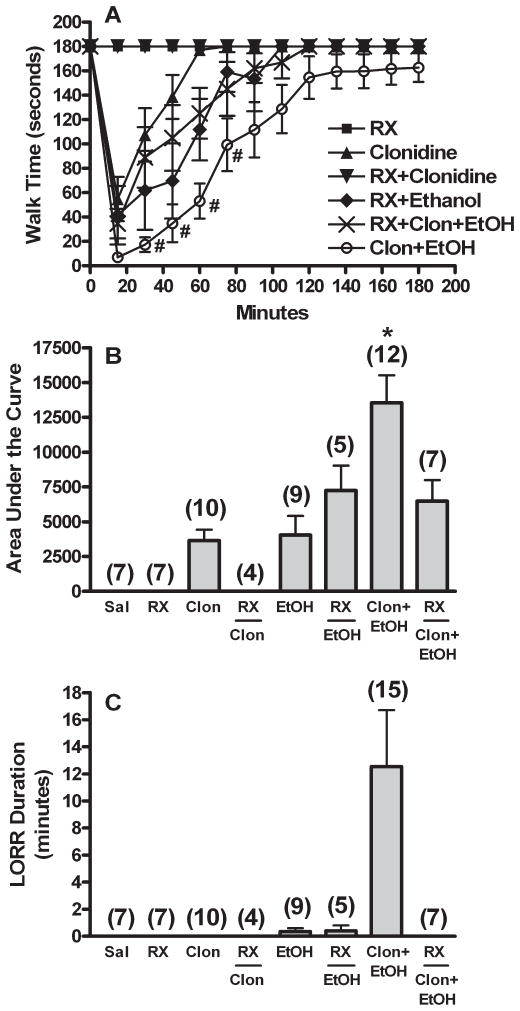
Central α2AAR Blockade Abolished Clonidine Enhancement of Ethanol Induced Behavioral Impairment
The effects of α2AAR blockade with RX821002 (0.3 mg, i.c.) on rotorod performance and LORR in rats that received saline, single drug (clonidine or ethanol), or clonidine-ethanol combination are shown in Fig. 4. Although a transient sedative effect, including in some cases 1 to 2 minutes LORR, was observed following intracisternal RX821002 injection, all animals recovered within the 10-minute pretreatment period, and RX821002 pretreatment alone had no effect on rotorod performance over the 3-hour observation period (Fig. 4). Clonidine (60 μg/kg), but not ethanol (1 g/kg), evoked behavioral impairment was significantly (p < 0.05) reduced by pretreatment with RX821002 [Fig. 4; ANOVA followed by Games-Howell post hoc tests on 8 groups; AUC, F(7,53) = 12.027, p < 0.001; LORR, F(7,56) = 3.902, unequal variance post hoc tests p > 0.05]. Further, RX821002 pretreatment abrogated the enhancement of behavioral impairment caused by clonidine-ethanol combination, since in this group behavioral impairment was similar to that produced by ethanol alone (Fig. 4). As reported (Bachtell et al., 2002), the selective α2BAR antagonist ARC-239 independently caused behavioral impairment on the rotorod (Fig. 5); however, no LORR was observed. This sedative effect of ARC-239 precluded the investigation in our model system of the potential involvement of α2BAR in clonidine enhancement of the behavioral impairment caused by ethanol.
Fig. 4.
Clonidine-ethanol synergistic behavioral interaction requires activation of the α2A-adrenergic receptor. Rotorod time courses (A), corresponding area under the curve (AUC, B), and LORR durations (C) are shown for groups pretreated with the selective α2A-receptor antagonist RX821002 (RX, 0.3 mg, i.c.) 10 min prior to 2 i.v. injections of: (i) saline + saline (RX), (ii) 60 μg/kg clonidine + saline (RX + Clonidine), (iii) saline + 1 g/kg ethanol (RX + Ethanol), or (iv) 60 μg/kg clonidine + 1 g/kg ethanol (RX + Clon + EtOH). All other aCSF i.c. pretreated groups (Sal, Clon, EtOH, and Clon + EtOH) are replotted for comparison from Fig. 1. The number of animals per group is shown in parentheses above the bar graphs. p < 0.05, * differs from Sal, RX, Clon, RX + Clon, and EtOH (rotorod trends also differed); #differs from Clon. LORR data did not reach significance due to unequal variance.
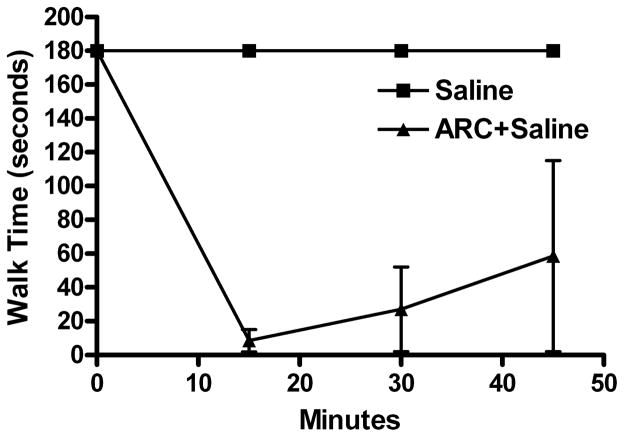
Fig. 5.
Clonidine-ethanol synergistic behavioral interaction does not appear to involve activation of the α2B-adrenergic receptor. Rotorod time courses are shown for saline (control, replotted from Fig. 1, n = 7) and animals given i.v. injections of ARC-239 (10 mg/kg) 10 min prior to 2 i.v. saline injections (ARC + Saline, n = 2). Although clearly sedated, neither animal exhibited LORR.
Effect of Clonidine, Ethanol, and Their Combination on c-Fos Expression in the Locus Coeruleus and Cerebellum
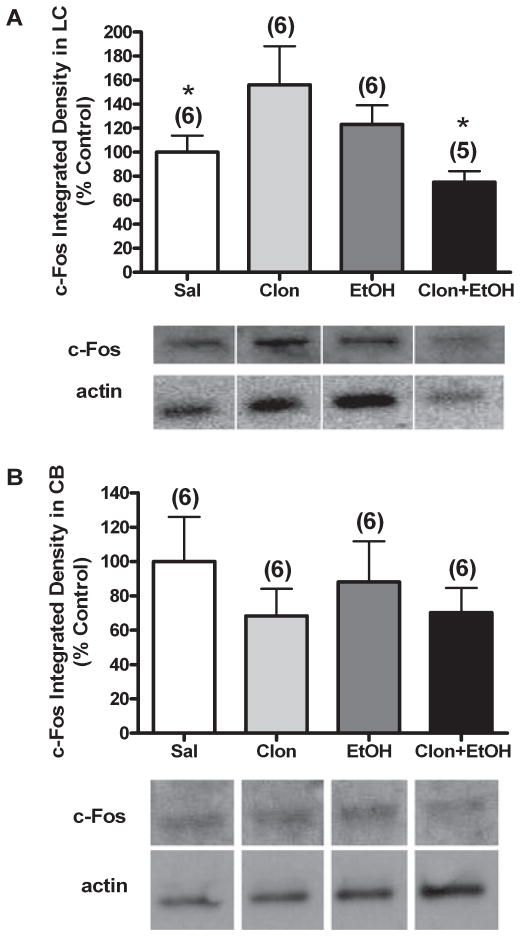
The neurochemical response reflected changes in c-Fos in LC or cerebellum 15 minute following clonidine, ethanol, or their combination; the time of sacrifice coincided with peak synergistic behavioral impairment elicited by the drug combination (Fig. 1). c-Fos protein expression in brain homogenates of locus coeruleus (LC) of animals treated with clonidine (60 μg/kg, i.v.) or ethanol (1 g/kg, i.v.) were increased compared to saline treated animals (Fig. 6A); the difference was only significant for clonidine treatment [univariate ANOVA, F(3,19) = 2.514, p < 0.05 with LSD post hoc as described in Methods]. Interestingly, in the LC of rats treated with clonidine- ethanol combination, the increase in c-Fos was abrogated and c-Fos level became lower than that found in control (saline-treated) animals [Fig. 6A (LSD post hoc, p < 0.05)]. Finally, Western blot analysis showed that in the cerebellum of the same animals (Fig. 6B) c-Fos level was not significantly different among groups and was not elevated by either clonidine or ethanol treatment [univariate ANOVA, F(3,20) = 0.954, p > 0.05].
Fig. 6.
Effect of clonidine, ethanol, or their combination on c-Fos level in the LC and cerebellum. Brain homogenates of LC (A) and cerebellum (B) were analyzed for levels of c-Fos by Western blot in animals treated with i.v. injections of: (i) saline + saline (Sal), (ii) 60 μg/kg clonidine + saline (Clon), (iii) saline + 1 g/kg ethanol (EtOH), or (iv) 60 μg/kg clonidine + 1 g/kg ethanol (Clon + EtOH). The number of animals per group is shown in parentheses above the bar graphs. One value for Clon + EtOH was excluded as an outlier in panel A (n = 5, test of normality failed). Samples were run on multiple gels due to the number of samples; however, control and treated samples were run on each gel. Data was therefore normalized to actin to account for variation in gel loading and graphed as % saline control for clearer comparison between groups. p < 0.05, *differs from Clon. Representative Western blot bands are presented below the graph for c-Fos and actin for each treatment in the LC and cerebellum. Note: Actin representative bands appear different because they are from different gels, as indicated by the white space between lanes; however, these bands correspond to the samples represented above them.
DISCUSSION
We investigated the role of central α2AR signaling as a potential mechanism underlying clonidine enhancement of ethanol-induced behavioral impairment. Given that clonidine is not selective for α2AR, we investigated whether other molecular targets of clonidine, such as the I1-imidazoline receptor (I1R), contributed to the interaction. Further, we tested the hypothesis that the LC constitutes a neuroanatomical substrate for the 2 drugs. The most important findings of these studies are: (i) clonidine dose-dependently increased ethanol-induced behavioral impairment, (ii) clonidine enhancement of ethanol behavioral effects required activation of α2AAR, but not I1-imidazoline or α2BAR, and (iii) c-Fos protein level in locus coeruleus (LC) was increased by clonidine or ethanol alone but decreased by clonidine-ethanol combination at a time that coincides with peak synergy of behavioral impairment; these neurochemical responses were not evident in the cerebellum. The pharmacological and neurochemical findings suggest a pivotal role for central α2AAR signaling in clonidine-evoked enhancement of behavioral impairment caused by ethanol and implicate the LC, at least partly, in the synergistic behavioral interaction between clonidine and ethanol.
We showed that clonidine synergistically and dose-dependently enhanced ethanol-induced rotorod impairment. These results complement similar findings on LORR, which agree with and extend our reported findings (Mao and Abdel-Rahman, 1996). Importantly, use of rotorod performance test (data expressed as time-course or area under the curve) permitted accurate evaluation of time of peak synergy and duration of behavioral impairment caused by clonidine, ethanol, and their combination. Together, LORR and rotorod data showed a similar outcome, synergistic behavioral impairment when clonidine and ethanol were combined. Further, the dose–response findings showed that the magnitude of ethanol-evoked behavioral impairment is positively related to the dose of clonidine. This relationship is clinically relevant, since patients taking higher doses of clonidine will be more affected by even moderate amounts of alcohol than patients on lower doses of clonidine; thus, patient history of alcohol consumption needs to be taken into consideration when prescribing clonidine or other drugs with similar mechanisms of action. Finally, the synergistic behavioral response is not caused by a pharmacokinetic interaction, since the presence of clonidine or similar drugs does not alter blood alcohol level or its rate of disappearance (Abdel-Rahman, 1994; Czarnecka et al., 1986). These findings suggest a key role for the molecular targets of clonidine in the enhancement of ethanol behavioral effects.
To identify which molecular target (receptor type/subtype) is activated by clonidine to cause enhancement of ethanol behavioral effects, selective α2AR agonists and antagonists were administered. This was important because clonidine activates different subtypes of adrenergic (α2A, α2B, and α2CAR) as well as nonadrenergic (I1) receptors (Khan et al., 1999). To determine whether the I1-receptor (I1R) is implicated in the interaction, the selective I1R agonist rilmenidine was used in a dose that elicits hypotension comparable to that caused by the lower dose of clonidine used in the present studies (Mao et al., 2003) while maintaining I1R selectivity. Rilmenidine, at the dose level tested, did not elicit behavioral impairment alone. More importantly, I1R activation did not influence behavioral impairment caused by ethanol, which suggests that clonidine enhancement of ethanol-evoked behavioral impairment is not dependent on central I1R signaling. This finding supports the notion that many of the behavioral effects of clonidine are produced through α2AAR activation (Hein, 2006; Hunter et al., 1997; Lahdesmaki et al., 2002, 2003; Lakhlani et al., 1997; Mizobe et al., 1996). Nevertheless, only one dose of rilmenidine was used in these studies, and while selective at the chosen dose, rilmenidine may still activate the α2AAR (Chan et al., 2007; Head and Burke, 2000; Mao et al., 2003). Indeed, this may explain the trend observed (Fig. 3B) for rilmenidine to slightly enhance the rotorod impairment elicited by ethanol. Therefore, we acknowledge that higher doses of rilmenidine may produce an interaction with ethanol similar to clonidine; however, under these circumstances, the activation of α2AAR by rilmenidine would be a major confounding factor. This issue could be unequivocally resolved when a highly selective I1R agonist becomes available. Importantly, while neither low dose (30 μg/kg) clonidine nor the comparable hypotensive dose (300 μg/kg) of rilmenidine caused behavioral impairment alone, only clonidine, but not rilmenidine, significantly enhanced ethanol-evoked behavioral impairment. These findings support the conclusion that α2AR signaling plays the major role in clonidine enhancement of ethanol-evoked behavioral impairment with little or no contribution by I1R signaling.
In order to identify the α2AR subtype implicated in clonidine enhancement of ethanol induced behavioral impairment, selective α2AAR (RX821002) and α2BAR (ARC-239) antagonists (Bylund et al., 1988) were used in the present study. Unlike in reported studies (Bachtell et al., 2002), we administered RX821002 intracisternally, scaling down from the reported systemic dose. The ability of the adopted dose regimen to adequately block central α2AAR was confirmed by the abolition of clonidine-evoked behavioral impairment in RX821002 pretreated rats. Equally important, blockade of central α2AAR abrogated clonidine enhancement of ethanol-evoked behavioral impairment; in RX821002 pretreated rats the impairment of rotorod performance caused by clonidine-ethanol combination was comparable to that produced by ethanol alone and LORR caused by the drug combination was abolished. These findings suggest that clonidine-ethanol behavioral synergy requires activation of central α2AAR. Further, the abolition of LORR suggests the full dependence of the synergistic enhancement of LORR caused by the drug combination on central α2AAR or other α2AR subtype signaling. It is also imperative to note that additional or separate mechanisms for inducing rotorod impairment may be employed by ethanol. The finding that rotorod impairment caused by ethanol was slightly increased in RX821002-pretreated rats (Fig. 4) may suggest modulation of ethanol effect, at least partly, by unopposed α2B/CAR signaling in the presence of α2AAR blockade.
While considered a selective α2AAR antagonist, RX821002 exhibits other pharmacological effects that might have contributed to the observed behavioral responses. First, it is possible that RX821002, acting as an inverse agonist (Murrin et al., 2000), further suppressed the clonidine component of the LORR response to clonidine-ethanol combination. Second, the ability of RX821002 to block α2CAR, which was demonstrated in vitro (Pauwels and Colpaert, 2000), makes it difficult to unequivocally rule out a role for α2CAR in the clonidine- ethanol behavioral interaction. α2CAR, found at a lower density in the brain (Bucheler et al., 2002), makes a small contribution to behavioral effects such as inhibition of locomotor activity and hypothermia (Hein, 2006; Lahdesmaki et al., 2003). Nonetheless, a selective α2CAR antagonist is not currently available, and α2CAR signaling may actually antagonize sedation mediated by α2AAR activation (Puolivali et al., 2002), which might explain, at least partly, the enhancement of rotorod impairment caused by ethanol in RX821002-pretreated rats (Fig. 4). The investigation of a possible role for α2BAR in the behavioral interaction was hampered by the ability of the antagonist (ARC-239) to cause impairment of rotorod performance indistinguishable from that produced by clonidine-ethanol combination (Fig. 5). This finding is in agreement with the reported sedative effect of ARC-239 (Bachtell et al., 2002). Thus, further studies with this compound were precluded. However, the confounding inherent behavioral effects of the α2BAR antagonist seem to rule out a role for α2BAR in clonidine enhancement of ethanol-induced rotorod behavioral impairment. Together, although further investigation of the involvement of both α2B and α2CAR seems warranted, the results of experiments with RX821002 provide clear evidence for the primary requirement of α2AAR activation in the synergistic behavioral interaction between ethanol and clonidine.
The expression of c-Fos was studied in an effort to identify a neurochemical mechanism that might underlie the synergistic behavioral interaction between ethanol and clonidine, since previous studies have associated c-Fos induction with behavioral responses (Paylor et al., 1994). In the present studies, ethanol slightly increased c-Fos expression above control (saline-treated) level in the LC but not the cerebellum. This finding agrees with reported findings (Thiele et al., 2000) and supports the hypothesis that ethanol induces behavioral impairment via an LC-dependent mechanism. Notably, in the reported studies, much higher doses of ethanol were used and c-Fos was measured by immunohistochemistry. The dose of ethanol used in the present study produced plasma ethanol levels comparable with that attained following social drinking (El-Mas and Abdel-Rahman, 1999). The induction of c-Fos in the LC by ethanol was dose-dependent (Thiele et al., 2000), which may explain why the increase in c-Fos produced by ethanol (1 g/kg) in the present study was not large enough to reach significance compared with the 1.5 and 3.5 g/kg doses of ethanol used in the reported study. Further, in a limited study analyzing c-Fos expression under the same conditions by immunohistochemistry, we observed a similar induction of c-Fos by ethanol (data not shown). Interestingly, we demonstrate, for the first time, that clonidine significantly increased LC c-Fos expression (Fig. 6A), which does not agree with reported studies where clonidine had no effect on c-Fos expression in the EW nucleus (Bachtell et al., 2002) or attenuated hypotension-induced c-fos mRNA expression in the RVLM (El-Mas and Abdel-Rahman, 2000). Furthermore, clonidine did not augment c-Fos expression in the cerebellum in the present studies (Fig. 6B). Although doses of clonidine used in the present and reported studies were similar, the disparity of findings might relate, at least partly, to measurement of c-Fos/c-fos in different brain regions. The present findings suggest that at the dose level used, LC c-Fos is increased by clonidine or ethanol when c-Fos is not induced by other mechanisms.
We show that, in combination, clonidine and ethanol decreased c-Fos level in the LC (Fig. 6A) but not the cerebellum (Fig. 6B). While no studies have investigated the effect of clonidine and ethanol in combination on c-Fos level, both drugs influence LC neuronal activity (Aghajanian and VanderMaelen, 1982; Verbanck et al., 1991). Activation of post-synaptic α2AAR present in the LC (Koss, 1986) by clonidine might underlie the significant increase in c-Fos in LC of clonidine-treated rats. Given that these α2AAR in the LC send inhibitory input to other brain areas such as the EW(Bachtell et al., 2002), clonidine would be expected to inhibit c-Fos in the EW. The lack of such effect in reported studies might be explained by the scant basal level of c-Fos in the EW(Bachtell et al., 2002). On the other hand, ethanol inhibits noradrenergic activity in the LC (Pohorecky and Brick, 1977). Therefore, it is plausible to assume that, in the LC, in the presence of enhanced noradrenergic activity by clonidine, the inhibitory effect of ethanol on noradrenergic activity might be exaggerated. This notion is supported by the significant reduction in c-Fos elicited by ethanol in the LC of clonidine pretreated rats. Nevertheless, further investigation is required to fully understand the role of c-Fos expression or other measures of LC neuronal activity in behavioral impairment caused by clonidine or ethanol, and the synergistic interaction caused by their combination. Although changes in c-Fos expression were not evident in the cerebellum in our studies, it is still possible that the interaction between clonidine and ethanol may involve other brain areas as part of the mechanism of their synergistic interaction. However, the present study has clearly demonstrated an important role for the LC as a neuroanatomical target for the behavioral interaction between clonidine and ethanol.
In summary, clonidine, a mixed α2AAR and I1-imidazoline receptor agonist, dose-dependently enhances ethanol-induced behavioral impairment through activation of central α2AAR. The findings with one dose of rilmenidine seem to rule out contribution of the I1-imidazoline receptor to this neurobiological interaction. The activation of the α2AAR by rilmenidine and other currently available I1R selective agonists circumvented the use of higher doses of these agents to more precisely evaluate the involvement of the I1R in the interaction. Further investigation into the role of α2B and α2CAR is also necessary, although their contribution to the synergistic interaction is probably minimal based on the present findings with the selective α2BAR antagonist and reported studies (Hein, 2006; Puolivali et al., 2002). Finally, in the LC, but not in the cerebellum, c-Fos level is decreased by clonidine-ethanol treatment, whereas either clonidine or ethanol treatment increases c-Fos. While it is clear that clonidine induces its behavioral effects and likely increases c-Fos in the LC via an α2AAR-dependent mechanism, the specific mechanisms that underlie the behavioral and neurochemical interaction utilized by ethanol in combination with clonidine may involve multiple pathways and/or other brain regions in addition to the LC. Nonetheless, the present findings yield insight into central mechanisms implicated in the synergistic and dose-dependent enhancement by clonidine of ethanol-evoked behavioral impairment.
Acknowledgments
The authors would like to thank Dr. Kevin Obrien for his valuable assistance with the statistical analyses used in these experiments. This study was supported by NIH grants 5F31AA015472 (TSB) and 2 R01 AA07839 (ARA).
References
- Abdel-Rahman AA. Alcohol abolishes the hypotensive effect of clonidine in spontaneously hypertensive rats. Hypertension. 1994;24:802–807. doi: 10.1161/01.hyp.24.6.802. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, VanderMaelen CP. Alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982;215:1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Alcohol-induced c-Fos expression in the Edinger-Westphal nucleus: pharmacological and signal transduction mechanisms. J Pharmacol Exp Ther. 2002;302:516–524. doi: 10.1124/jpet.102.036046. [DOI] [PubMed] [Google Scholar]
- Bender TS, Abdel-Rahman AA. Differential contribution of eNOS- and nNOS-derived NO to clonidine-ethanol induced behavioral impairment. Soc Neurosci Annu Meet. 2006;749:13. [Google Scholar]
- Bender TS, Abdel-Rahman AA. Clonidine-ethanol synergistic behavioral impairment requires activation of central {alpha}2A adrenergic receptors. FASEB J. 2007;21:A784. [Google Scholar]
- Bucheler MM, Hadamek K, Hein L. Two alpha(2)-adrenergic receptor subtypes, alpha(2A) and alpha(2C), inhibit transmitter release in the brain of gene-targeted mice. Neuroscience. 2002;109:819–826. doi: 10.1016/s0306-4522(01)00531-0. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Ray-Prenger C, Murphy TJ. Alpha-2A and alpha-2B adrenergic receptor subtypes: antagonist binding in tissues and cell lines containing only one subtype. J Pharmacol Exp Ther. 1988;245:600–607. [PubMed] [Google Scholar]
- Chan CK, Burke SL, Head GA. Contribution of imidazoline receptors and alpha2-adrenoceptors in the rostral ventrolateral medulla to sympathetic baroreflex inhibition by systemic rilmenidine. J Hypertens. 2007;25:147–155. doi: 10.1097/HJH.0b013e3280105ef0. [DOI] [PubMed] [Google Scholar]
- Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76:948–952. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- Czarnecka E, Pietkiewicz B, Skretkowicz J. Investigations of central interaction of ethanol and clonidine. Pol J Pharmacol Pharm. 1986;38:157–166. [PubMed] [Google Scholar]
- Dar MS. Involvement of kappa-opioids in the mouse cerebellar adenosinergic modulation of ethanol-induced motor incoordination. Alcohol Clin Exp Res. 1998;22:444–454. [PubMed] [Google Scholar]
- De Sarro GB, Ascioti C, Froio F, Libri V, Nistico G. Evidence that locus coeruleus is the site where clonidine and drugs acting at alpha 1- and alpha 2-adrenoceptors affect sleep and arousal mechanisms. Br J Pharmacol. 1987;90:675–685. doi: 10.1111/j.1476-5381.1987.tb11220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Durcan MJ, Lister RG, Linnoila M. Evidence for central alpha-2 adrenoceptors, not imidazoline binding sites, mediating the ethanol-attenuating properties of alpha-2 adrenoceptor antagonists. J Pharmacol Exp Ther. 1991;258:576–582. [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Ethanol counteraction of I1-imidazoline but not alpha-2 adrenergic receptor-mediated reduction in vascular resistance in conscious spontaneously hypertensive rats. J Pharmacol Exp Ther. 1999;288:455–462. [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Clonidine diminishes c-jun gene expression in the cardiovascular sensitive areas of the rat brainstem. Brain Res. 2000;856:245–249. doi: 10.1016/s0006-8993(99)02370-7. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Evidence for the involvement of central I1 imidazoline receptor in ethanol counteraction of clonidine hypotension in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2001;38:417–426. doi: 10.1097/00005344-200109000-00010. [DOI] [PubMed] [Google Scholar]
- Fauler J, Verner L. The pharmacokinetics of clonidine in high dosage. Eur J Clin Pharmacol. 1993;45:165–167. doi: 10.1007/BF00315500. [DOI] [PubMed] [Google Scholar]
- Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology. 1996;84:873–881. doi: 10.1097/00000542-199604000-00015. [DOI] [PubMed] [Google Scholar]
- Head GA, Burke SL. Comparison of renal sympathetic baroreflex effects of rilmenidine and alpha-methylnoradrenaline in the ventrolateral medulla of the rabbit. J Hypertens. 2000;18:1263–1276. doi: 10.1097/00004872-200018090-00013. [DOI] [PubMed] [Google Scholar]
- Head GA, Mayorov DN. Imidazoline receptors, novel agents and therapeutic potential. Cardiovasc Hematol Agents Med Chem. 2006;4:17–32. doi: 10.2174/187152506775268758. [DOI] [PubMed] [Google Scholar]
- Hein L. Adrenoceptors and signal transduction in neurons. Cell Tissue Res. 2006;326:541–551. doi: 10.1007/s00441-006-0285-2. [DOI] [PubMed] [Google Scholar]
- Hunter JC, Fontana DJ, Hedley LR, Jasper JR, Lewis R, Link RE, Secchi R, Sutton J, Eglen RM. Assessment of the role of alpha2-adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br J Pharmacol. 1997;122:1339–1344. doi: 10.1038/sj.bjp.0701520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idanpaan-Heikkila JJ, Bjorn M, Seppala T. The effects of ethanol in combination with the alpha 2-adrenoceptor agonist dexmedetomidine and the alpha 2-adrenoceptor antagonist atipamezole on brain monoamine metabolites and motor performance of mice. Eur J Pharmacol. 1995;292:191–199. doi: 10.1016/0926-6917(95)90012-8. [DOI] [PubMed] [Google Scholar]
- Khan ZP, Ferguson CN, Jones RM. Alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146–165. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- Koss MC. Pupillary dilation as an index of central nervous system alpha 2-adrenoceptor activation. J Pharmacol Methods. 1986;15:1–19. doi: 10.1016/0160-5402(86)90002-1. [DOI] [PubMed] [Google Scholar]
- Kushikata T, Hirota K, Yoshida H, Kubota T, Ishihara H, Matsuki A. Alpha-2 adrenoceptor activity affects propofol-induced sleep time. Anesth Analg. 2002;94:1201–1206. doi: 10.1097/00000539-200205000-00028. table of contents. [DOI] [PubMed] [Google Scholar]
- Lahdesmaki J, Sallinen J, MacDonald E, Kobilka BK, Fagerholm V, Scheinin M. Behavioral and neurochemical characterization of alpha(2A)-adrenergic receptor knockout mice. Neuroscience. 2002;113:289–299. doi: 10.1016/s0306-4522(02)00185-9. [DOI] [PubMed] [Google Scholar]
- Lahdesmaki J, Sallinen J, MacDonald E, Sirvio J, Scheinin M. Alpha2-adrenergic drug effects on brain monoamines, locomotion, and body temperature are largely abolished in mice lacking the alpha2A-adrenoceptor subtype. Neuropharmacology. 2003;44:882–892. doi: 10.1016/s0028-3908(03)00080-7. [DOI] [PubMed] [Google Scholar]
- Lakhlani PP, MacMillan LB, Guo TZ, McCool BA, Lovinger DM, Maze M, Limbird LE. Substitution of a mutant alpha2a-adrenergic receptor via “hit and run” gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proc Natl Acad Sci U S A. 1997;94:9950–9955. doi: 10.1073/pnas.94.18.9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Abdel-Rahman AA. Synergistic behavioral interaction between ethanol and clonidine in rats: role of alpha-2 adrenoceptors. J Pharmacol Exp Ther. 1996;279:443–449. [PubMed] [Google Scholar]
- Mao L, Abdel-Rahman AA. Ethanol counteraction of clonidine-evoked inhibition of norepinephrine release in rostral ventrolateral medulla of rats. Alcohol Clin Exp Res. 1998;22:1285–1291. [PubMed] [Google Scholar]
- Mao L, Li G, Abdel-Rahman AA. Effect of ethanol on reductions in norepinephrine electrochemical signal in the rostral ventrolateral medulla and hypotension elicited by I1-receptor activation in spontaneously hypertensive rats. Alcohol Clin Exp Res. 2003;27:1471–1480. doi: 10.1097/01.ALC.0000086062.95225.0C. [DOI] [PubMed] [Google Scholar]
- Meana JJ, Herrera-Marschitz M, Goiny M. In vivo modulation of norepinephrine and glutamate release through imidazoline receptors in the rat central nervous system. Ann N Y Acad Sci. 1995;763:490–493. doi: 10.1111/j.1749-6632.1995.tb32438.x. [DOI] [PubMed] [Google Scholar]
- Mizobe T, Maghsoudi K, Sitwala K, Tianzhi G, Ou J, Maze M. Antisense technology reveals the alpha2A adrenoceptor to be the subtype mediating the hypnotic response to the highly selective agonist, dexmedetomidine, in the locus coeruleus of the rat. J Clin Invest. 1996;98:1076–1080. doi: 10.1172/JCI118887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouledous L, Diaz MF, Gutstein HB. Extracellular signal-regulated kinase (ERK) inhibition does not prevent the development or expression of tolerance to and dependence on morphine in the mouse. Pharmacol Biochem Behav. 2007;88:39–46. doi: 10.1016/j.pbb.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrin LC, Gerety ME, Happe HK, Bylund DB. Inverse agonism at alpha(2)-adrenoceptors in native tissue. Eur J Pharmacol. 2000;398:185–191. doi: 10.1016/s0014-2999(00)00317-4. [DOI] [PubMed] [Google Scholar]
- Palmer MR, Granholm AC. Electrophysiological effects of ethanol on hippocampal and cerebellar neurons cografted with locus coeruleus in oculo: role of the noradrenergic circuitry. J Pharmacol Exp Ther. 1992;260:887–895. [PubMed] [Google Scholar]
- Pauwels PJ, Colpaert FC. Partial to complete antagonism by putative antagonists at the wild-type alpha(2C)-adrenoceptor based on kinetic analyses of agonist: antagonist interactions. Br J Pharmacol. 2000;131:1385–1390. doi: 10.1038/sj.bjp.0703726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson W. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Paylor R, Johnson RS, Papaioannou V, Spiegelman BM, Wehner JM. Behavioral assessment of c-fos mutant mice. Brain Res. 1994;651:275–282. doi: 10.1016/0006-8993(94)90707-2. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Brick J. Activity of neurons in the locus coeruleus of the rat: inhibition by ethanol. Brain Res. 1977;131:174–179. doi: 10.1016/0006-8993(77)90039-7. [DOI] [PubMed] [Google Scholar]
- Pudovkina OL, Kawahara Y, de Vries J, Westerink BH. The release of noradrenaline in the locus coeruleus and prefrontal cortex studied with dual-probe microdialysis. Brain Res. 2001;906:38–45. doi: 10.1016/s0006-8993(01)02553-7. [DOI] [PubMed] [Google Scholar]
- Puolivali J, Bjorklund M, Holmberg M, Ihalainen JA, Scheinin M, Tanila H. Alpha 2C-adrenoceptor mediated regulation of cortical EEG arousal. Neuropharmacology. 2002;43:1305–1312. doi: 10.1016/s0028-3908(02)00305-2. [DOI] [PubMed] [Google Scholar]
- Reis DJ. Neurons and receptors in the rostroventrolateral medulla mediating the antihypertensive actions of drugs acting at imidazoline receptors. J Cardiovasc Pharmacol. 1996;27(Suppl 3):S11–S18. doi: 10.1097/00005344-199627003-00003. [DOI] [PubMed] [Google Scholar]
- Ruiz-Durantez E, Ruiz-Ortega JA, Pineda J, Ugedo L. Effect of agmatine on locus coeruleus neuron activity: possible involvement of nitric oxide. Br J Pharmacol. 2002;135:1152–1158. doi: 10.1038/sj.bjp.0704556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ortega JA, Ugedo L. The stimulatory effect of clonidine on locus coeruleus neurons of rats with inactivated alpha 2-adrenoceptors: involvement of imidazoline receptors located in the nucleus paragigantocellularis. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:288–294. doi: 10.1007/pl00004945. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE. Role of hippocampus in alcohol-induced memory impairment: implications from behavioral and immediate early gene studies. Psychopharmacology (Berl) 1998;139:34–43. doi: 10.1007/s002130050687. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Tsivkovskaia NO, Ryabinin AE. Ataxia and c-Fos expression in mice drinking ethanol in a limited access session. Alcohol Clin Exp Res. 2005;29:1419–1426. doi: 10.1097/01.alc.0000174746.64499.83. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Cubero I, van Dijk G, Mediavilla C, Bernstein IL. Ethanol-induced c-fos expression in catecholamine- and neuropeptide Y-producing neurons in rat brainstem. Alcohol Clin Exp Res. 2000;24:802–809. [PubMed] [Google Scholar]
- Verbanck P, Seutin V, Massotte L, Dresse A. Yohimbine can induce ethanol tolerance in an in vitro preparation of rat locus coeruleus. Alcohol Clin Exp Res. 1991;15:1036–1039. doi: 10.1111/j.1530-0277.1991.tb05207.x. [DOI] [PubMed] [Google Scholar]