Abstract
Introduction
Monoaminergic pathways, impinging an α2-adrenoceptors and 5-HT3 serotonin receptors, modulate nociceptive transmission, but their mechanisms and interactions after neuropathic injury are unknown. Here we examine these interactions in rodents after nerve injury.
Methods
Male Sprague-Dawley rats following L5-L6 spinal nerve ligation (SNL) were used for either behavioral testing, in vivo microdialysis for γ-amino butyric acid (GABA) and acetylcholine release, or synaptosome preparation for GABA release.
Results
Intrathecal administration of the α2-adrenoceptor agonist (clonidine) and 5-HT3 receptor agonist (chlorophenylbiguanide) reduced hypersensitivity in SNL rats via GABA receptor-mediated mechanisms. Clonidine increased GABA and acetylcholine release in vivo in the spinal cord of SNL rats but not in normal rats. Clonidine-induced spinal GABA release in SNL rats was blocked by α2-adrenergic and nicotinic cholinergic antagonists. The 5-HT3 receptor antagonist ondansetron decreased and chlorophenylbiguanide increased spinal GABA release in both normal and SNL rats. In synaptosomes from the spinal dorsal horn of SNL rats, pre-synaptic GABA release was increased by nicotinic agonists and decreased by muscarinic and α2-adrenergic agonists. Spinally administered ondansetron significantly reduced clonidine-induced anti-hypersensitivity and spinal GABA release in SNL rats.
Conclusion
These results suggest that spinal GABA contributes to anti-hypersensitivity from intrathecal α2-adrenergic and 5-HT3 receptor agonists in the neuropathic pain state, that cholinergic neuroplasticity after nerve injury is critical for α2-adrenoceptor-mediated GABA release, and that blockade of spinal 5-HT3 receptors reduces α2-adrenoceptor-mediated anti-hypersensitivity via reducing total GABA release.
Introduction
Nearly 50 years ago, Melzack and Wall proposed a theory of pain transmission wherein activity of touch-sensitive afferents could inhibit transmission by nociceptive afferents at the spinal level.1 They further speculated that this gate control was powerfully regulated by pathways descending from higher centers. In the 1970s, two monoamine pathways, one releasing noradrenaline and another releasing serotonin were identified as inhibitory of nociceptive neurotransmission.2 The purpose of this study was to address key gaps in our understanding about the role of these pathways after neuropathic injury and their mechanisms of action. We focused on their regulation of γ-amino butyric acid (GABA), a key inhibitory neurotransmitter of projecting neurons in the spinal cord.
Noradrenaline is released by bulbospinal noradrenergic axons originating from the locus coeruleus and adjacent nuclei in the brainstem,3,4 and suppresses pain transmission via inhibitory α2-adrenoceptors on sensory terminals and projecting neurons, and via excitatory α1-adrenoceptors on GABA interneurons.2 Although noradrenergic inhibition exists in the normal state, peripheral nerve injury induces drastic changes in anatomy and function of this pathway. Peripheral nerve injury results in an increase in noradrenergic axon density in the spinal cord and induces more noradrenaline release when this pathway is stimulated.5,6 In synaptosomes prepared from the lumbar dorsal horn, activation of α2-adrenoceptors, which inhibits acetylcholine release in the normal state, facilitates acetylcholine release after peripheral nerve injury via Gs-protein mediated mechanisms.7,8 This facilitation of spinal acetylcholine release after nerve injury is critical for analgesia from intrathecal injection of α2-adrenoceptor agonists such as clonidine.9,10 In the normal state, stimulation of nicotinic and muscarinic receptors on GABA neurons increases GABA release to inhibit pain transmission in the spinal cord.11-17 In the neuropathic pain state, however, the roles of α2-adrenergic and cholinergic receptors on spinal GABA release are not known. We hypothesized that α2-adrenoceptor –mediated analgesia after nerve injury depends on cholinergic mediated GABA release.
Spinal serotonin is released from descending serotonergic axons that originate from the rostral ventral medulla,18 and has both inhibitory or facilitatory effects on pain transmission in the spinal cord.2 Spinal administration of the 5-hydroxytryptophan-3 (5-HT3) receptor antagonist ondansetron inhibits pain-related behaviors and activity of spinal neurons in rats following peripheral formalin injection,19,20 suggesting a facilitatory role of endogenous serotonin in spinal pain transmission via 5-HT3 receptors. After nerve injury, however, the role of 5-HT3 receptors is less clear, since the antagonist ondansetron has inconsistent effects21,22 and since the 5-HT3 receptor selective agonist chlorophenylbiguanide (m-CPBG) reduced hypersensitivity.23 The behavioral effect of m-CPBG after nerve injury is blocked by GABA antagonists,23 and 5-HT3 agonists induce pre-synaptic GABA release in normal rats,24,25 suggesting a role for GABA in its action. We hypothesized that m-CPBG would increase and ondansetron would decrease spinal GABA release in both normal and spinal nerve ligation (SNL) rats. Finally, since intrathecal clonidine produces analgesia in patients with neuropathic pain and since some studies suggest that ondansetron should also be analgesic, we examine the behavioral effects of this combination. Surprisingly, we observed antagonism of clonidine’s effect by ondansetron, and tested whether this could be explained by opposing actions on GABA release.
Materials and Methods
Animals and surgeries
Male Sprague-Dawley rats (weighing 180-250 g) from Harlan Industries (Indianapolis, IN) and Japan SLC Inc. (Hamamatsu, Japan), housed under a 12-h light-dark cycle with food and water ad libitum, were used. All experiments were approved by Animal Care and Use Committee at Wake Forest University (Winston Salem, NC) and Gunma University (Maebashi, Japan). Microdialysis study for acetylcholine release was performed at Gunma university and other experiments were performed at Wake Forest university.
L5-L6 (SNL) was performed as previously described.26 Briefly, animals were anesthetized with 2 % isoflurane in oxygen and the right L5 and L6 spinal nerves were tightly ligated with 5-0 silk sutures. One week after SNL, intrathecal catheterization was performed as previously described.27 Briefly, animals were anesthetized with 2-3 % isoflurane. A small puncture was made in the atlanto–occipital membrane of the cisterna magnum and a polyethylene catheter (ReCathCO LLC, Allison Park, PA), 7.5 cm, was inserted. After surgery, animals were allowed to recover for a week.
Behavioral tests
In SNL animals, paw withdrawal threshold in response to light touch with calibrated von Frey filaments (Stoelting, Wood Dale, IL) was determined using an up–down statistical method.28 Filaments were applied to the bending point for 5 s, and a brisk paw withdrawal was considered a positive response.
In normal animals, withdrawal threshold to pressure applied to the hind paw, expressed in grams, was measured using an analgesimeter (Ugo Basile, Comerio, Italy) as previously described.29 The device applies increasing pressure to the hind paw. When the animal withdrew the paw or vocalized, the pressure was immediately released and the withdrawal threshold read on a scale. Training of animals for this test was performed for 3-5 days before the drug treatment. A cut-off of 250 g was used to avoid potential tissue injury. Withdrawal threshold was measured two times in the right and left hind paws and these values were averaged for each animal.
On the day of the experiment, animals received an intrathecal injection of test drug at a volume of 10 μL followed by 10 μL saline. We used these normal and SNL animals two or three times on different days. Experiments in the same animals were separated by at least 5 days. Drugs and their doses were randomly assigned. The person performing the behavioral test was blinded to drug and treatment.
Microdialysis
GABA study
Animals were anesthetized with 2% isoflurane and then maintained with 1.25-1.5% isoflurane during the study. A heating blanket was used to maintain rectal temperature (36.5 ± 0.5°C) and the right jugular vein was cannulated for saline infusion (1.2 ml/kg/hr). The L3-L6 level of spinal cord was exposed by the T13-L1 laminectomy. A microdialysis probe (OD = 0.22 mm, ID = 0.20 mm, length= 1 mm, CX-I-8-01, EICOM, Kyoto, Japan) was inserted from just lateral to the right dorsal root 1 hr prior to the study and perfused with Ringer’s solution. Fractions were collected every 30 min for 2.5 hr starting 1 hr prior to drug treatment and samples were kept at −80 °C until assay for GABA. GABA content in the microdialysates was measured by a high pressure liquid chromatography system with electrochemical detection (HTEC-500, EICOM). GABA in samples were derivatized with 2-mercaptoethanol and o-phthaldialdehyde (4 mM) in 0.1 M carbonate buffer (pH=9.5). The o-phthaldialdehyde derivatives were then separated on the column (3.0 mm x 150 mm, SC-5ODS, EICOM) at 30°C, using a mobile phase consisting of 50 mM phosphate buffer (pH=2.8) and methanol (1:1 vol/vol) containing 5 mg/ml EDTA-2Na at a flow rate of 0.5 ml/min. The limit of detection of GABA assay in the current study was 1.5 pg per injection (15 μl) and the inter-assay coefficient of variation at 100 pg per injection was 7.2 %.
Acetylcholine study
Anesthesia was induced with urethane (1.2-1.5 g/kg, intraperitoneal) and then maintained with 0.5% isoflurane. The left femoral vein was cannulated for saline infusion, rectal temperature was maintained at 36.5 ± 0.5°C, and the L3-L6 level of spinal cord was exposed. A microdialysis probe (OD = 0.22 mm, ID = 0.20 mm, length= 2 mm, AI8-02, EICOM) was inserted from just lateral to the dorsal root and advanced at a 30° angle to a depth of 2 mm and perfused with Ringer’s solution (1.0 μl/min). After 120 min of constant perfusion, fractions were collected into a sample-injector (EAS-20, EICOM) connected with HTEC-500 analyzing system every 30 min for 2.5 hr starting 1 hr prior to drug treatment. The sample was then separated on the column (2.0 mm × 150 mm; EICOMPAC AC-GEL, EICOM) at 33°C, using a mobile phase consisted of 50 mM carbonate buffer containing 300 mg/l sodium decane-1-sulfonate (pH=8.5) and 50 mg/l EDTA-2Na at a flow rate of 0.15 ml/min. The limit of detection of acetylcholine assay in the current study was 10 fg per injection (30 μl) and the inter-assay coefficient of variation at 30 pg per injection was 8.2%.
GABA release from synaptosomes
Crude synaptosomes from the lumbar spinal dorsal horn ipsilateral to SNL surgery were prepared as previously described.7 Under deep anesthesia with 5% isoflurane, animals were killed by decapitation, the spinal lumbar enlargement was quickly removed and placed in ice-cold sucrose (0.32 M)-HEPES (10 mM) buffer, pH = 7.4. Each synaptosome preparation contained unilateral dorsal quadrants of spinal cord from 3 SNL rats. The initial homogenate was centrifuged at 1,000xG for 3 min and the resulting supernatant was centrifuged again at 10,000xG for 13 min. The supernatant was discarded and the pellet was resuspended in Krebs buffer (in mM: NaCl 124, KCl 3, MgSO4 2, CaCl2 2, NaH2PO4 1.25, NaHCO3 25, and glucose 10, saturated with 95% O2-5% CO2, pH = 7.4). [3H]-GABA release from synaptosomes was measured as previously described, 30 with minor modifications. After incubation with [3H]-GABA and unlabeled GABA (final concentration of 0.25 μCi/ml and 1 μM, respectively) for 20 min at 37°C, the synaptosome-containing solution was centrifuged at 10,000xG for 5 min and the pellet was resuspended in Krebs buffer. Each synaptosome preparation was divided into six equal aliquots and transferred to Whatman filters in individual temperature controlled perfusion chambers (SF-12, Brandel, Gaithersburg, MD). Synaptosomes were perfused with Krebs buffer (0.8 ml/min) for 25 min to remove free radioactivity, and then fractions were collected every 5 min for 20 min. After a 10 min baseline collection, synaptosomes were stimulated with 12 mM KCl-Krebs buffer (in mM: NaCl 115, KCl 12, MgSO4 2, CaCl2 2, NaH2PO4 1.25, NaHCO3 25, and glucose 10, pH = 7.4) containing test drug for 3 min. In a separate experiment, synaptosomes were perfused with ondansetron for 2 min and then stimulated with 12 mM KCl-Krebs buffer containing nicotine with ondansetron for 3 min. The inhibitor of GABA transporters 1,2,5,6-tetrahydro-1-[2-[[(diphenylmethylene)amino]oxy]ethyl]-3-pyridinecarboxylic acid hydrochloride (NNC-711, 1 μM, Tocris Bioscience, Ellisville, MO) was present during perfusion to inhibit reuptake of GABA. 30 Amount of radioactivity of each fraction was measured by a liquid scintillation spectrometry (LS6500, Beckman Coulter Inc., Fullerton, CA).
Test drugs
Clonidine hydrochloride, ondansetron hydrochloride, dolasetron mesylate, bicuculline methiodide, atropine sulfate, mecamylamine hydrochloride, idazoxan hydrochloride, (-)-nicotine, epibatidine dihydrochloride, and muscarine chloride were obtained from Sigma-Aldrich (St. Louis, MO). Dexmedetomidine hydrochloride, 3-Aminopropyl-diethoxymethyl phosphinic acid hydrate (CGP 35348), and 1-(3-Chlorophenyl)-biguanide hydrochloride (mCPBG) were obtained from Tocris bioscience. Each drug was dissolved in saline, Ringer’s solution, or Krebs buffer. A higher concentration of ondansetron was used in microdialysis than in synaptosome experiments, due to barriers of drug diffusion in the former.
Statistical analysis
Unless otherwise stated, data are presented as mean ± SE. Behavioral and microdialysis data were analyzed using one way or two way repeated measures analysis of variance (ANOVA) followed by Tukey post-hoc test. Synaptosome data were analyzed by one-way ANOVA followed by Dunnet post-hoc test. P<0.05 was considered significant.
Results
Spinal GABA contributes to α-2 adrenoceptor-mediated anti-hypersensitivity
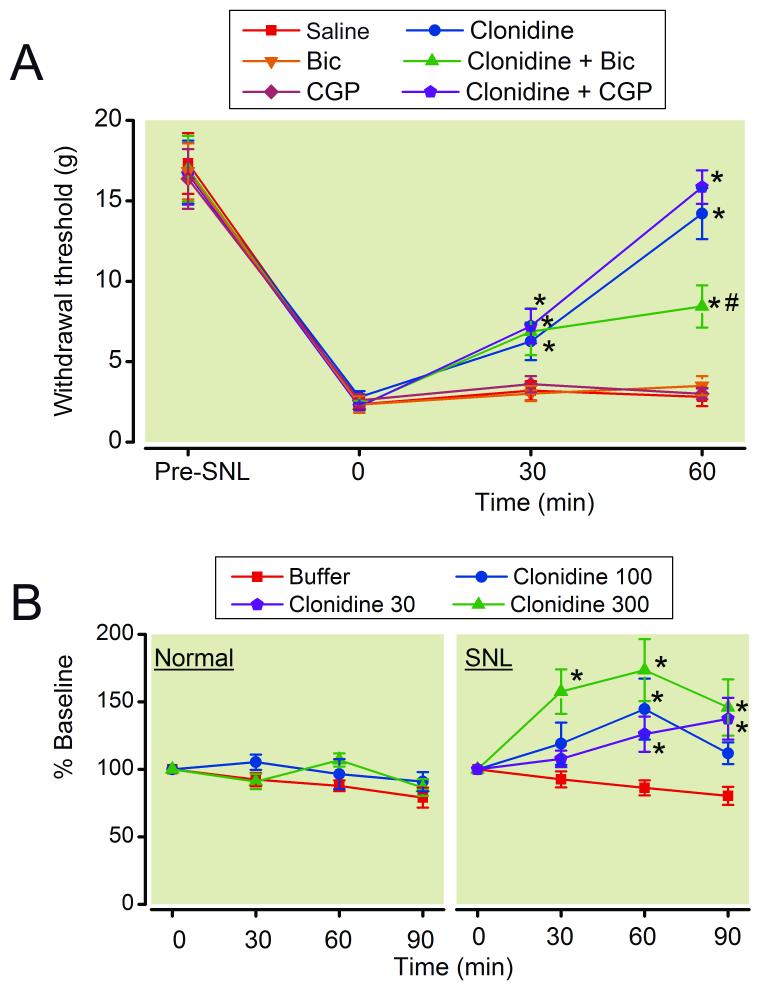
SNL strongly reduced the withdrawal threshold in the paw ipsilateral to surgery from 16.7 ± 4.3 g to 1.8 ± 1.0 g (mean ± SD, n=165, p<0.0001) at 2 weeks after surgery. Intrathecal injection of clonidine (15 μg/rat) increased withdrawal threshold in the paw ipsilateral to SNL compared to saline in a time-dependent manner (Fig. 1A, p<0.05). This clonidine effect was significantly reduced by the GABAA receptor antagonist bicuculline (0.25 μg/rat) but not by the GABAB antagonist CGP 35348 (15 μg/rat) at 60 min after injection. Neither GABA antagonist alone affected withdrawal threshold in SNL rats. The doses of bicuculline and CGP35348 were determined from a previous study. 23
Figure 1.
(A) Effects of γ-amino butyric acid (GABA) antagonists on clonidine-induced analgesia in spinal nerve ligated (SNL) rats. Animals received intrathecal injection of saline, clonidine (15 μg/rat), bicuculline (Bic, 0.25 μg/rat), CGP35348 (CGP, 15 μg/rat), or their combination. Withdrawal threshold in the paw ipsilateral to SNL is presented over the time. N = 6-8. * p < 0.05 versus saline. # p < 0.05 versus clonidine alone. (B) Microdialysis for spinal GABA release. Buffer or clonidine (30-300 μM) was perfused into the spinal dorsal horn through the microdialysis probe in normal and SNL rats. N = 7-9. Data are presented over time as percentage of baseline. * p < 0.05 versus buffer.
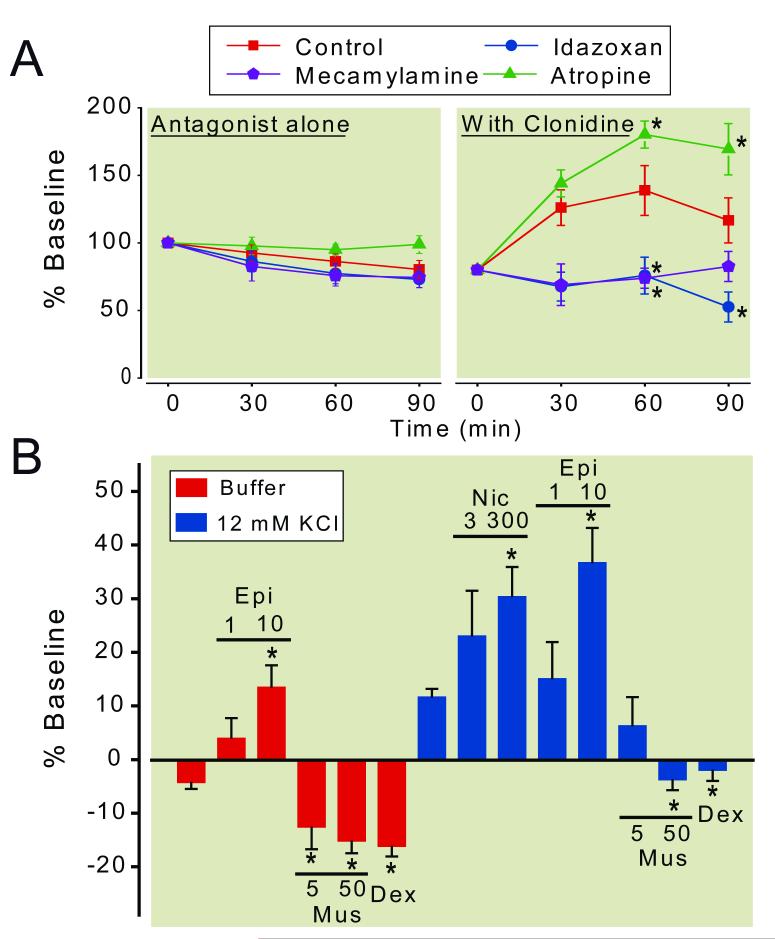
Consistent with previous observations in rats after peripheral nerve or spinal cord injury,31-33 baseline concentrations of GABA in microdialysates from the spinal dorsal horn were significantly less from SNL rats (36.8 ± 3.3 pg /30 μL, n=89) compared to normal rats (76.8 ± 9.7 pg/30 μL, n=38, p<0.001). Clonidine did not affect GABA concentrations in microdialysates from the spinal dorsal horn in normal rats but increased them in SNL rats (Fig. 1B). This clonidine-induced GABA release in SNL rats peaked at 100 μM clonidine in the perfusate and decreased at 300 μM clonidine. At 100 μM clonidine, the GABA concentrations in the dialysates from the SNL spinal cord increased within 30 min, peaked at 60 min, and decreased at 90 min. Based on this result, we selected 100 μM clonidine for the following experiments. Neither α2-adrenoceptor antagonist idazoxan (300 μM), muscarinic antagonist atropine (100 μM), nor nicotinic antagonist mecamylamine (100 μM) affected GABA concentrations in microdialysates from the spinal dorsal horn of SNL rats (Fig. 2A). Co-perfusion of mecamylamine or idazoxan with clonidine significantly reduced clonidine-induced GABA release at 60-90 min in the spinal dorsal horn of SNL rats (P<0.05). On the other hand, atropine significantly enhanced clonidine-induced GABA release at 60-90 min in the spinal dorsal horn of SNL rats (P<0.05).
Figure 2.
(A) Effects of α2-adrenergic and cholinergic antagonists on clonidine-induced γ-amino butyric acid (GABA) release in the spinal dorsal horn ipsilateral to spinal nerve ligation (SNL). Buffer (control), idazoxan (300 μM), atropine (100 μM), or mecamylamine (100 μM) in the absence or presence of clonidine (100 μM) was perfused into the spinal dorsal horn through the microdialysis probe. Data are presented over time as percentage of baseline. N = 6-9. * p < 0.05 versus control. (B) Effects of α2-adrenergic and cholinergic agonists on GABA release in the synaptosomes from the spinal dorsal horn ipsilateral to SNL surgery. Synaptosomes were treated with epibatidine (Epi, 1-10 μM), nicotine (Nic, 30-300 μM), muscarine (Mus, 5-50 μM), or dexmedetomidine (Dex, 10 nM) in the presence or absence of 12 mM KCl. Data are presented as percentage of baseline. N = 6-10. * p < 0.05 versus buffer or KCl alone.
The roles of cholinergic and α2-adrenergic receptors on pre-synaptic GABA release were examined in synaptosomes prepared from the lumbar spinal dorsal horn of SNL rats. Basal or 12 mM KCl-evoked GABA release in the synaptosomes from SNL rats was increased by the nicotinic agonists, epibatidine and nicotine, but decreased by muscarine in a concentration-dependent manner (Fig. 2B). In contrast to the microdialysis results from spinal cord in vivo with intact circuits, stimulation of α2-adrenoceptors in isolated GABA terminals by the highly selective agonist dexmedetomidine (10 nM) significantly reduced basal and KCl-evoked GABA release in SNL rats.
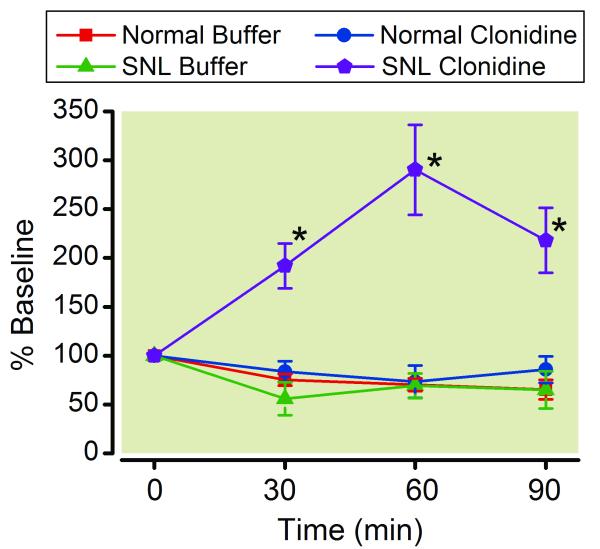
Baseline concentrations of acetylcholine in microdialysates from the spinal dorsal horn did not differ between normal (4.7±0.7 pg /30 μL, n=13) and SNL (4.5±0.71 pg /30 μL, n=13) rats. Similar to the GABA microdialysis results, clonidine increased spinal acetylcholine release in SNL rats but not in normal rats (Fig. 3). The acetylcholine concentrations in the dialysates from the SNL spinal cord increased within 30 min, peaked at 60 min, and decreased at 90 min during clonidine perfusion.
Figure 3.
Microdialysis for spinal acetylcholine release. Buffer or clonidine (100 μM) was perfused into the spinal dorsal horn through the microdialysis probe in normal and spinal nerve ligated (SNL) rats. Data are presented over time as percentage of baseline. N = 6-7. * p < 0.05 versus buffer.
Spinal GABA contributes to 5-HT3 receptor-mediated anti-hypersensitivity
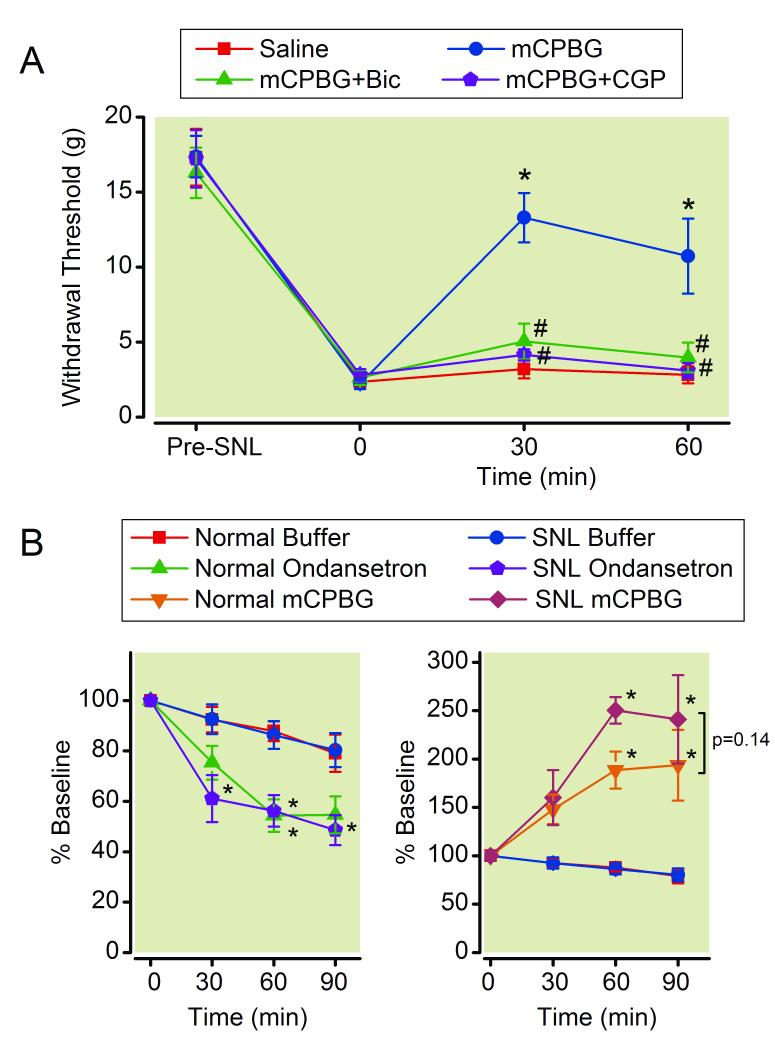
As previously reported,23 intrathecal injection of mCPBG (15 μg/rat) in SNL rats produced an anti-hypersensitivity effect which was blocked by bicuculline and CGP 35348 (Fig 4A).
Figure 4.
(A) Effects of γ-amino butyric acid (GABA) antagonists on chlorophenylbiguanide (mCPBG)-induced analgesia in spinal nerve ligated (SNL) rats. Animals received intrathecal injection of saline, mCPBG (15 μg/rat), mCPBG + bicuculline (Bic, 0.25 μg/rat), or mCPBG + CGP35348 (CGP, 15 μg/rat). Withdrawal threshold in the paw ipsilateral to SNL is presented over the time. N = 7-8. * p < 0.05 versus saline. # p < 0.05 versus mCPBG alone. (B) Microdialysis for spinal GABA release. Buffer, ondansetron (100 μM), or mCPBG (100 μM) was perfused into the spinal dorsal horn through the microdialysis probe in normal and SNL rats. N = 6-8. Data are presented over time as percentage of baseline. * p < 0.05 versus buffer.
We then examined effects of blockade or stimulation of 5-HT3 receptors on spinal GABA release in normal and SNL rats. Ondansetron (100 μM) reduced but mCPBG (100 μM) increased GABA concentrations in microdialysates from the spinal dorsal horn in both normal and SNL rats in time-dependent manners (Fig 4B).
Blockade of 5-HT3 receptors reduces clonidine-induced GABA release and antihypersensitivity in SNL rats
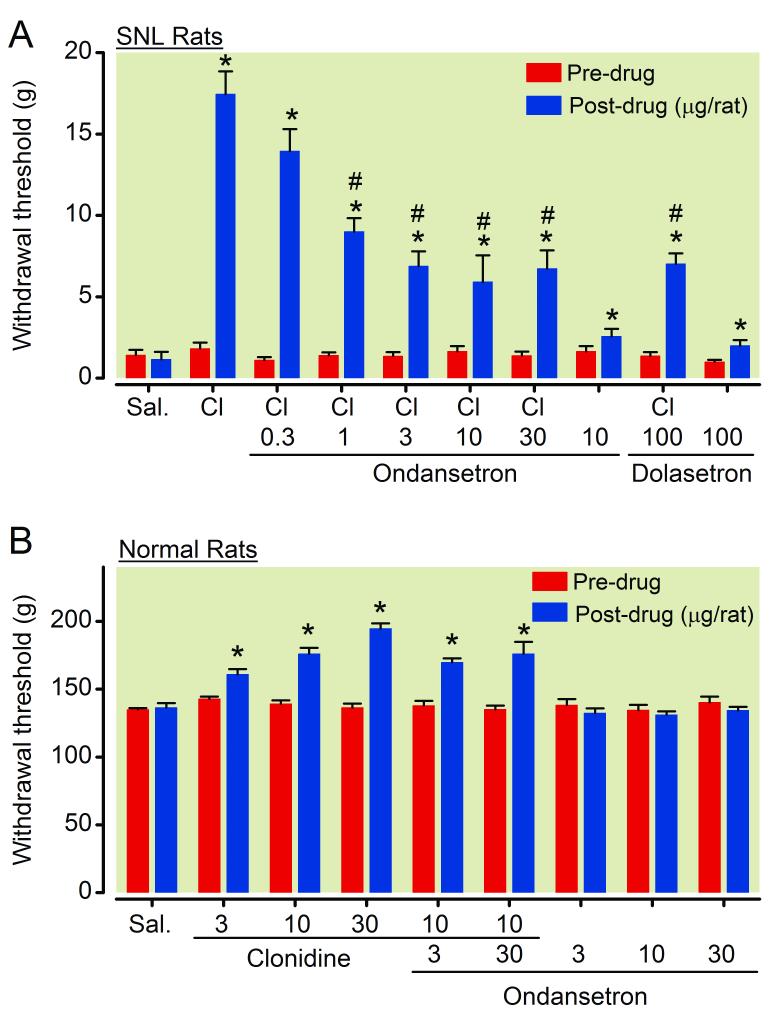
In SNL rats, an intrathecal injection of ondansetron (10 μg/rat) alone slightly but significantly increased withdrawal threshold in the paw ipsilateral to surgery compared to saline (Fig 5A, p<0.05). The antihypersensitivity effect of intrathecal clonidine (15 μg/rat) was partially reduced by ondansetron (0.3-30 μg/rat) in a dose-dependent manner. Another selective 5-HT3 receptor antagonist dolasetron (100 μg/rat) itself produced minor anti-hypersensitivity and also reduced clonidine’s effect in SNL rats. The dose of dolasetron was determined from our previous study.22
Figure 5.
(A) Effects of 5-HT3 antagonists on clonidine-induced analgesia in spinal nerve ligated (SNL) rats. Animals received intrathecal injection of saline (Sal.), clonidine (Cl, 15 μg/rat), ondansetron (0.3-30 μg/rat), dolasetron (100 μg/rat), or their combination 1 h prior to the von Frey test. Withdrawal threshold in the paw ipsilateral to SNL is presented. N = 6-8. * p < 0.05 versus saline. # p < 0.05 versus clonidine alone. (B) Effects of ondansetron on clonidine-induced antinociception in normal rats. Animals received intrathecal injection of saline (Sal.), clonidine (Cl, 3-30 μg/rat), ondansetron (3-30 μg/rat), or their combination 1 h prior to the Randall-Sellitto test. Average of withdrawal threshold in the both paws is presented. N = 8-14. * p < 0.05 versus saline.
In normal rats, intrathecal injection of clonidine (3-30 μg/rat) dose-dependently increased withdrawal threshold (Fig 5B). Ondansetron (3-30 μg/rat) failed to affect withdrawal threshold and clonidine-induced antinociception in normal rats, in contrast to its inhibition of clonidine’s effect after SNL.
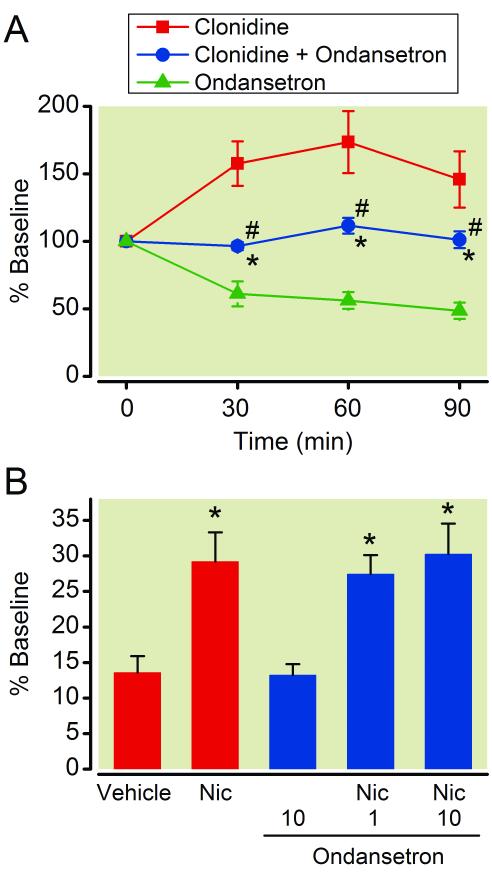
In the spinal dorsal horn of SNL rats, co-perfusion of ondansetron with clonidine significantly increased GABA concentrations in microdialysates compared to ondansetron alone and decreased them compared to clonidine alone (Fig. 6A, p<0.05).
Figure 6.
(A) Microdialysis for γ-amino butyric acid (GABA) release following perfusion of clonidine (100 μM), ondansetron (100 μM), or their combination in the spinal dorsal horn ipsilateral to spinal nerve ligation (SNL). Data are presented over time as percentage of baseline. N = 7. * p < 0.05 versus ondansetron alone. # p < 0.05 versus clonidine alone. (B) Effects of ondansetron on nicotine-induced GABA release in the synaptosomes from the spinal dorsal horn ipsilateral to SNL surgery. Synaptosomes were treated with vehicle, nicotine (Nic, 300 μM), ondansetron (1-10 μM), or their combination in the presence of 12 mM KCl. Data are presented as percentage of baseline. N = 9. * p < 0.05 versus vehicle alone.
Since nicotinic receptors are involved in clonidine-induced spinal GABA release in SNL rats, we lastly examined whether ondansetron affects nicotine-induced GABA pre-synaptic GABA release. In the spinal dorsal horn synaptosomes from SNL rats, ondansetron (1-10 μM) did not affect nicotine-induced GABA release (Fig. 6B).
Discussion
Descending serotonergic and noradrenergic pathways act on multiple targets to reduce and, in some circumstances, enhance excitation of primary sensory afferents and second order neurons in the spinal cord.2 The current study demonstrated, in rats after peripheral nerve injury, that some inhibitory actions of both pathways rely in part on spinal GABA for their behavioral antihypersensitivity effects and that α2-adrenoceptors require cholinergic neuronal plasticity following nerve injury to induce GABA release in the spinal dorsal horn. They also predict an adverse, antagonistic effect from ondansetron in patients receiving intrathecal clonidine for analgesia for neuropathic pain.
In both normal and neuropathic pain states, activation of α2-adrenoceptors reduces pre-synaptic release of pronociceptive neurotransmitters including substance P and glutamate from primary afferents, hyperpolarizes spinal neurons via activation of potassium channels subsequent to stimulation of Gi/o-proteins, as key mechanisms of antinociception.2,14,34 In addition to these mechanisms, the current study suggests that α2-adrenoceptors also reduce hypersensitivity after nerve injury by increasing GABA release. This is not a direct effect, but requires spinal cord circuits, since it was present in vivo in the microdialysis experiments but not in vitro in studies in isolated synaptosomes. Clonidine-induced GABA release in the spinal cord in vivo relies on acetylcholine release, since it was blocked by mecamylamine. These results are consistent with novel Gs-mediated facilitation of acetylcholine release from α2-adrenoceptor stimulation in the spinal dorsal horn after peripheral nerve injury.7 This novel excitatory action, previously demonstrated in vitro, is extended in the current study in vivo, using microdialysis. Together, these results suggest that stimulation of α2-adrenoceptors on cholinergic terminals results in acetylcholine release which in turn induces GABA release to reduce hypersensitivity after nerve injury. These data further extend our understanding of the α2-adrenoceptors and nicotinic and GABA receptors in analgesia. Taken together, they suggest that potency and efficacy of intrathecal clonidine is enhanced in neuropathic states due to novel cholinergic excitation (Figure 7, top panels). A limitation of the study design, which compared nerve injured animals and tissues to normal, was that we could not distinguish the effect of surgery and nerve injury compared to normal from the effect of a surgical procedure without nerve injury.
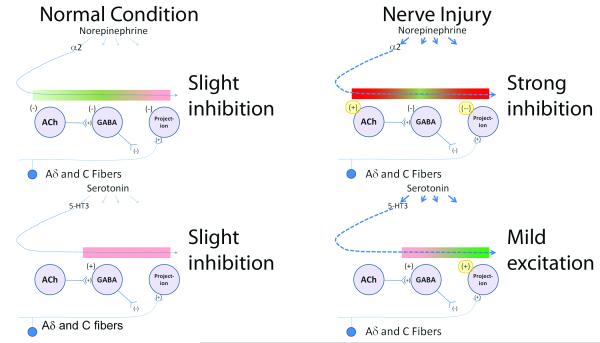
Figure 7.
Net effect of α2-adrenoceptor and 5-hydroxytryptamine-3 (5-HT3) receptors on nociceptive sensory transmission in the spinal cord of normal (left) and nerve injured (right) animals. The colored bar indicates the effect of each step in the proposed circuit of these neurotransmitters, with green depicting increased nociceptive neurotransmission and red depicting reduced nociceptive neurotransmission. Both norepinephrine and serotonin are released from pathways which descend to the spinal cord, and act on many receptor subtypes.
As regards α2-adrenoceptors, norepinephrine release is minimal in the normal condition, and it’s net effect is slight inhibition, due to and actions on primary afferent endings and projection neurons (upper left panel). This effect is only mildly counterbalanced by inhibition of acetylcholine (ACh) release which would drive γ-amino benzoic acid (GABA) release and by direct inhibition of GABA release. After nerve injury, norepinephrine release increases and the effect of α2-adrenoceptors on ACh shifts from inhibition to excitation and the expression of inhibitory α2-adrenoceptors on afferents and projection neurons increases (highlighted in yellow), resulting in strong inhibition (upper right panel).
As regards 5-HT3 receptors, serotonin release is minimal in the normal condition, and it’s net effect is slight inhibition, due to direct stimulation of GABA release (lower left panel). After nerve injury, serotonin release increases and the effect of 5-HT3 receptors results in enhanced nociception neurotransmission, due to increased excitatory signaling on afferents and projection neurons (highlighted in yellow, lower right panel).
Stimulation of nicotinic receptors in the spinal cord causes pre-synaptic GABA release and GABAA receptor-mediated analgesia in both normal and nerve-injured rats,13,15,35,36 consistent with the current behavioral and neurochemical observations. These results suggest that GABAergic mechanisms contribute to nicotinic receptor-mediated anti-hypersensitivity from intrathecal clonidine in SNL rats. In contrast, the GABAB antagonist CGP35348, at a dose which abolished anti-hypersensitivity from mCPBG, failed to affect anti-hypersensitivity from clonidine in SNL rats. Since both α2-adrenoceptors and GABAB receptors are expressed on primary sensory afferents and their stimulation reduces pronociceptive neurotransmitter release,2,14 activation of α2-adrenoceptors by clonidine might mask the effect of CGP35348.
We have previously demonstrated that peripheral nerve injury up-regulates inhibitory M2 muscarinic receptors in primary sensory afferents.37 Stimulation of M2 muscarinic receptors reduces pain-related behavior following formalin injection in rats, and inhibits heat- or capsaicin-evoked release of calcitonin gene-related peptide from primary sensory neurons.38,39 Stimulation of M2, M3, and M4 subtype of muscarinic receptors also results in pre-synaptic GABA release in the spinal cord and GABAB receptor-mediated analgesia in normal rats or those with diabetic neuropathy.11,12,16,17 However, the current study in SNL rats demonstrated that atropine enhanced clonidine-induced GABA release in the spinal dorsal horn and muscarine reduced pre-synaptic GABA release from the synaptosome. Although we did not examine which subtype(s) of muscarinic receptors contribute to the inhibition of GABA release and whether peripheral nerve injury alters the function or expression of muscarinic receptors on GABA neurons in the spinal dorsal horn, these results suggest that muscarinic receptors have an inhibitory role on GABA neurons in the spinal cord after peripheral nerve injury and that GABAergic mechanisms are less likely to contribute to muscarinic receptor-mediated anti-hypersensitivity from clonidine after nerve injury.
Stimulation of 5-HT3 receptors in the spinal cord can result in facilitation of pain transmission via increasing substance P release from the primary sensory afferents40 or inhibition by increasing GABA release.24,25 A facilitation of pain from the tonic activity of spinal 5-HT3 receptors has been described in several persistent pain states. As such, intrathecal injection of ondansetron in rats20,41 or genetic deletion of 5-HT3 receptors in mice42 reduced pain-related behavior following the formalin injection into the paw. Single or repeated intrathecal injection of ondansetron reduced mechanical hypersensitivity in rats after spinal cord or peripheral nerve injury.21,43,44 Paradoxically, 5-HT3 receptor agonists also reduce hypersensitivity in the same pain states where endogenous serotonin induces 5-HT3 receptor-mediated facilitation. As such, intrathecal administration of the 5-HT3 agonists resulted in reduction of formalin-evoked behavior in normal rats45 and GABA receptor-dependent antihypersensitivity in SNL rats.23 The current study confirmed both facilitatory and inhibitory roles of 5-HT3 receptors in the spinal dorsal horn in SNL rats. For the former, intrathecal injection of 5-HT3 receptor antagonists produced a small but significant anti-hypersensitivity. For the latter, spinally administered mCPBG increased GABA release in the spinal dorsal horn and produced GABA receptor-dependent anti-hypersensitivity. The current study also demonstrated that the spinally administered ondansetron reduced spinal GABA release in normal and SNL rats, consistent with a tonic 5-HT3 receptor-dependent enhancement of GABA release. Taken together, these results suggest that stimulation of 5-HT3 receptors results in activation of both facilitatory and inhibitory pathways in the spinal cord and that behavioral consequence of spinal 5-HT3 receptor activation in animals after nerve injury may depend on the balance between these pathways (Figure 7, lower panels).
Based on the previously described analgesic effects of clonidine and ondansetron in various pain states, we originally hypothesized that combination of α2-adrenoceptor stimulation with blockade of 5-HT3 receptors in the spinal cord should produce additive or synergistic anti-hypersensitivity in SNL rats. Contrary to this hypothesis, ondansetron partially antagonized rather than enhanced anti-hypersensitivity from intrathecal clonidine in SNL rats, consistent with a reduction of clonidine-induced GABA release by ondansetron in the spinal cord. This is unlikely due to a direct interaction on α2-adrenoceptors by ondansetron since, to our knowledge, there is no evidence that this drug binds to these receptors. Since ondansetron did not affect nicotine-induced pre-synaptic GABA release from synaptosomes in SNL rats, this inhibitory effect of ondansetron on GABA release is not likely due to the direct inhibition of nicotinic receptors which mediate GABA release by clonidine. On the other hand, ondansetron did not affect antinociception from intrathecal clonidine in normal rats in which clonidine does not induce spinal GABA release. These results suggest that facilitatory influence of tonic 5-HT3 receptor activity on basal GABA tone in the spinal cord after nerve injury is important for α2-adrenoceptor-mediated antihypersensitivity which relies on spinal GABA release.
In summary, stimulation of α2-adrenergic and 5-HT3 receptors results in spinal GABA release and GABA receptor-mediated anti-hypersensitivity in rats after peripheral nerve injury, and cholinergic neuroplasticity after nerve injury is critical for the GABA release from α2-adrenoceptor activation in the spinal dorsal horn. These results suggest that spinal α2-adrenergic and 5-HT3 pathways share a common mechanism to reduce neuropathic pain after nerve injury. The current study also demonstrates that blockade of spinal 5-HT3 receptors reduces α2-adrenoceptor-mediated anti-hypersensitivity in SNL rats via reduction of basal GABA tone in the spinal cord. Since 5-HT3 receptor antagonists are commonly used for the treatment of nausea and vomiting 46 and since orally administered ondansetron crosses the blood-brain barrier,47 clinical trials in chronic pain patients should be performed to test whether 5-HT3 receptor antagonists affect efficacy of analgesics which rely on spinal α2-adrenoceptor action, including intrathecal clonidine and oral gabapentin.48
MS #201110096 Final Box Summary.
What we already know about this subject:
* Descending noradrenergic and serotonergic pathways inhibit nociceptive transmission in the spinal cord.
* The action is mediated through adrenoceptors on γ-amino butyric acid (GABA) interneurons.
What we know from this paper that is new:
* Following sciatic nerve ligation spinal GABA contributes to the antihypersensitivity effects mediated by α2 and serotonin receptors.
* In this model the effects of α2 adrenoceptors on GABA release is mediated, in part, by enhanced acetylcholine release.
* Blockade of spinal serotonin receptors reduces basal GABA tone and decreases α2 receptor mediated antihypersensitivity.
Summary Statement.
Spinal γ-amino butyric acid contributes to anti-hypersensitivity from stimulation of spinal α2-adrenergic and 5-HT3 receptors in rats after nerve injury. Blockade of spinal 5-HT3 receptors reduced α2-adrenoceptor-mediated anti-hypersensitivity via reducing spinal γ-amino butyric acid release in nerve-injured rats.
Acknowledgments
Funding: This work was supported by grants NS57594 to JE and DA27690 to KH from the National Institute of Health, Bethesda, Maryland.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Melzack R, Wall PD. Pain mechanisms: A new theory. Science. 1965;150:971–9. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 2.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 3.Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B, et al. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- 4.Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Hayashida K, Obata H, Nakajima K, Eisenach JC. Gabapentin acts within the locus coeruleus to alleviate neuropathic pain. Anesthesiology. 2008;109:1077–84. doi: 10.1097/ALN.0b013e31818dac9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashida KI, Clayton BA, Johnson JE, Eisenach JC. Brain derived nerve growth factor induces spinal noradrenergic fiber sprouting and enhances clonidine analgesia following nerve injury in rats. Pain. 2008;136:348–55. doi: 10.1016/j.pain.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashida K, Eisenach JC. Spinal alpha 2-adrenoceptor-mediated analgesia in neuropathic pain reflects brain-derived nerve growth factor and changes in spinal cholinergic neuronal function. Anesthesiology. 2010;113:406–12. doi: 10.1097/ALN.0b013e3181de6d2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashida KI, Eisenach JC. A Tropomyosine Receptor Kinase Inhibitor Blocks Spinal Neuroplasticity Essential for the Anti-Hypersensitivity Effects of Gabapentin and Clonidine in Rats With Peripheral Nerve Injury. J Pain. 2010;12:94–100. doi: 10.1016/j.jpain.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan HL, Chen SR, Eisenach JC. Intrathecal clonidine alleviates allodynia in neuropathic rats: interaction with spinal muscarinic and nicotinic receptors. Anesthesiology. 1999;90:509–14. doi: 10.1097/00000542-199902000-00027. [DOI] [PubMed] [Google Scholar]
- 10.Paqueron X, Conklin D, Eisenach JC. Plasticity in action of intrathecal clonidine to mechanical but not thermal nociception after peripheral nerve injury. Anesthesiology. 2003;99:199–204. doi: 10.1097/00000542-200307000-00030. [DOI] [PubMed] [Google Scholar]
- 11.Baba H, Kohno T, Okamoto M, Goldstein PA, Shimoji K, Yoshimura M. Muscarinic facilitation of GABA release in substantia gelatinosa of the rat spinal dorsal horn. J Physiol. 1998;508(Pt 1):83–93. doi: 10.1111/j.1469-7793.1998.083br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen SR, Pan HL. Spinal GABAB receptors mediate antinociceptive actions of cholinergic agents in normal and diabetic rats. Brain Res. 2003;965:67–74. doi: 10.1016/s0006-8993(02)04123-9. [DOI] [PubMed] [Google Scholar]
- 13.Genzen JR, McGehee DS. Nicotinic modulation of GABAergic synaptic transmission in the spinal cord dorsal horn. Brain Res. 2005;1031:229–37. doi: 10.1016/j.brainres.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 14.Pan HL, Wu ZZ, Zhou HY, Chen SR, Zhang HM, Li DP. Modulation of pain transmission by G-protein-coupled receptors. Pharmacol Ther. 2008;117:141–61. doi: 10.1016/j.pharmthera.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rashid MH, Ueda H. Neuropathy-specific analgesic action of intrathecal nicotinic agonists and its spinal GABA-mediated mechanism. Brain Res. 2002;953:53–62. doi: 10.1016/s0006-8993(02)03270-5. [DOI] [PubMed] [Google Scholar]
- 16.Zhang HM, Chen SR, Cai YQ, Richardson TE, Driver LC, Lopez-Berestein G, Pan HL. Signaling mechanisms mediating muscarinic enhancement of GABAergic synaptic transmission in the spinal cord. Neuroscience. 2009;158:1577–88. doi: 10.1016/j.neuroscience.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang HM, Li DP, Chen SR, Pan HL. M2, M3, and M4 receptor subtypes contribute to muscarinic potentiation of GABAergic inputs to spinal dorsal horn neurons. J Pharmacol Exp Ther. 2005;313:697–704. doi: 10.1124/jpet.104.079939. [DOI] [PubMed] [Google Scholar]
- 18.Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 19.Green GM, Scarth J, Dickenson A. An excitatory role for 5-HT in spinal inflammatory nociceptive transmission; state-dependent actions via dorsal horn 5-HT(3) receptors in the anaesthetized rat. Pain. 2000;89:81–8. doi: 10.1016/S0304-3959(00)00346-8. [DOI] [PubMed] [Google Scholar]
- 20.Svensson CI, Tran TK, Fitzsimmons B, Yaksh TL, Hua XY. Descending serotonergic facilitation of spinal ERK activation and pain behavior. FEBS Lett. 2006;580:6629–34. doi: 10.1016/j.febslet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dogrul A, Ossipov MH, Porreca F. Differential mediation of descending pain facilitation and inhibition by spinal 5HT-3 and 5HT-7 receptors. Brain Res. 2009;1280:52–9. doi: 10.1016/j.brainres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Peters CM, Hayashida K, Ewan EE, Nakajima K, Obata H, Xu Q, Yaksh TL, Eisenach JC. Lack of analgesic efficacy of spinal ondansetron on thermal and mechanical hypersensitivity following spinal nerve ligation in the rat. Brain Res. 2010;1352:83–93. doi: 10.1016/j.brainres.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okazaki R, Namba H, Yoshida H, Okai H, Miura T, Kawamura M. The antiallodynic effect of Neurotropin is mediated via activation of descending pain inhibitory systems in rats with spinal nerve ligation. Anesth Analg. 2008;107:1064–9. doi: 10.1213/ane.0b013e31817e7a59. [DOI] [PubMed] [Google Scholar]
- 24.Fukushima T, Ohtsubo T, Tsuda M, Yanagawa Y, Hori Y. Facilitatory actions of serotonin type 3 receptors on GABAergic inhibitory synaptic transmission in the spinal superficial dorsal horn. J Neurophysiol. 2009;102:1459–71. doi: 10.1152/jn.91160.2008. [DOI] [PubMed] [Google Scholar]
- 25.Kawamata T, Omote K, Toriyabe M, Yamamoto H, Namiki A. The activation of 5-HT(3) receptors evokes GABA release in the spinal cord. Brain Res. 2003;978:250–5. doi: 10.1016/s0006-8993(03)02952-4. [DOI] [PubMed] [Google Scholar]
- 26.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–63. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 27.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–6. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 28.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 29.Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111:409–19. [PubMed] [Google Scholar]
- 30.Brawek B, Loffler M, Weyerbrock A, Feuerstein TJ. Effects of gabapentin and pregabalin on K+-evoked 3H-GABA and 3H-glutamate release from human neocortical synaptosomes. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:361–9. doi: 10.1007/s00210-008-0370-z. [DOI] [PubMed] [Google Scholar]
- 31.Eaton MJ, Plunkett JA, Karmally S, Martinez MA, Montanez K. Changes in GAD- and GABA- immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J Chem Neuroanat. 1998;16:57–72. doi: 10.1016/s0891-0618(98)00062-3. [DOI] [PubMed] [Google Scholar]
- 32.Gwak YS, Hulsebosch CE. GABA and central neuropathic pain following spinal cord injury. Neuropharmacology. 2011;60:799–808. doi: 10.1016/j.neuropharm.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Wolfe D, Hao S, Huang S, Glorioso JC, Mata M, Fink DJ. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain. Mol Ther. 2004;10:57–66. doi: 10.1016/j.ymthe.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Sonohata M, Furue H, Katafuchi T, Yasaka T, Doi A, Kumamoto E, Yoshimura M. Actions of noradrenaline on substantia gelatinosa neurones in the rat spinal cord revealed by in vivo patch recording. J Physiol. 2004;555:515–26. doi: 10.1113/jphysiol.2003.054932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeda D, Nakatsuka T, Papke R, Gu JG. Modulation of inhibitory synaptic activity by a non-alpha4beta2, non-alpha7 subtype of nicotinic receptors in the substantia gelatinosa of adult rat spinal cord. Pain. 2003;101:13–23. doi: 10.1016/s0304-3959(02)00074-x. [DOI] [PubMed] [Google Scholar]
- 36.Young T, Wittenauer S, Parker R, Vincler M. Peripheral nerve injury alters spinal nicotinic acetylcholine receptor pharmacology. Eur J Pharmacol. 2008;590:163–9. doi: 10.1016/j.ejphar.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayashida KI, Bynum T, Vincler M, Eisenach JC. Inhibitory M2 muscarinic receptors are upregulated in both axotomized and intact small diameter dorsal root ganglion cells after peripheral nerve injury. Neuroscience. 2006;140:259–68. doi: 10.1016/j.neuroscience.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Bernardini N, Reeh PW, Sauer SK. Muscarinic M2 receptors inhibit heat-induced CGRP release from isolated rat skin. Neuroreport. 2001;12:2457–60. doi: 10.1097/00001756-200108080-00034. [DOI] [PubMed] [Google Scholar]
- 39.Dussor GO, Helesic G, Hargreaves KM, Flores CM. Cholinergic modulation of nociceptive responses in vivo and neuropeptide release in vitro at the level of the primary sensory neuron. Pain. 2004;107:22–32. doi: 10.1016/j.pain.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25:613–7. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5:1319–26. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- 42.Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22:1010–9. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Oatway MA, Weaver LC. Blockade of the 5-HT3 receptor for days causes sustained relief from mechanical allodynia following spinal cord injury. J Neurosci Res. 2009;87:418–24. doi: 10.1002/jnr.21860. [DOI] [PubMed] [Google Scholar]
- 44.Oatway MA, Chen Y, Weaver LC. The 5-HT3 receptor facilitates at-level mechanical allodynia following spinal cord injury. Pain. 2004;110:259–68. doi: 10.1016/j.pain.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki M, Ishizaki K, Obata H, Goto F. Effects of 5-HT2 and 5-HT3 receptors on the modulation of nociceptive transmission in rat spinal cord according to the formalin test. Eur J Pharmacol. 2001;424:45–52. doi: 10.1016/s0014-2999(01)01117-7. [DOI] [PubMed] [Google Scholar]
- 46.Machu TK. Therapeutics of 5-HT3 receptor antagonists: Current uses and future directions. Pharmacol Ther. 2011;130:338–47. doi: 10.1016/j.pharmthera.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simpson KH, Murphy P, Colthup PV, Whelan P. Concentration of ondansetron in cerebrospinal fluid following oral dosing in volunteers. Psychopharmacology (Berl) 1992;109:497–8. doi: 10.1007/BF02247730. [DOI] [PubMed] [Google Scholar]
- 48.Hayashida K, DeGoes S, Curry R, Eisenach JC. Gabapentin activates spinal noradrenergic activity in rats and humans and reduces hypersensitivity after surgery. Anesthesiology. 2007;106:557–62. doi: 10.1097/00000542-200703000-00021. [DOI] [PubMed] [Google Scholar]