Abstract
Background
Several minimally invasive technologies are available to treat common soft tissue lesions including symptomatic hemorrhoids. The use of energy to deliver heat and coagulate target lesions is commonly practiced. This study compares the histologic effects produced on intestinal tissues by two energy-based systems which employ different approaches of heat delivery.
Methods
Two heat delivery systems were evaluated in vivo in a single porcine subject: infrared coagulator and bipolar tissue ligator utilizing constant tissue compression and temperature guidance. Eighteen treatment sites divided into three groups of six were assessed. Treatment site temperature was measured and the effects of thermal treatment in the mucosa, submucosa, submucosal vessels, and muscularis layer were scored. Lateral thermal spread beyond the energy application site was also assessed.
Results
Treatment site temperatures were much lower in the bipolar ligator group than in the infrared coagulator group. The mucosal and submucosal tissue changes observed in tissues treated with infrared energy and bipolar energy at 55°C were similar. Both the mucosal and submucosal tissue changes with bipolar energy at 50°C were significantly less.
Conclusion
Both devices achieved similar histologic results. However, the unique design of the bipolar ligator, which allows consistent capture, constant compression, and temperature monitoring of target tissue, accomplished the desired histologic changes with less muscular damage at much lower temperatures than the infrared coagulator. The use of bipolar ligation could offer clinical advantages such as reduced patient pain and a minimized chance of heat-related collateral tissue damage.
Keywords: bipolar ligator, internal hemorrhoids, tissue manipulation, ligation
Introduction
Symptomatic Grade 1 and 2 hemorrhoids rarely require surgical intervention and are usually treated with less invasive technologies – most frequently with rubber band ligation or infrared coagulation. Rubber band ligation is associated with frequent post-procedural pain and discomfort,1–6 as well as other, sometimes very serious, complications.3,7–14 Infrared coagulation causes much fewer complications than rubber band ligation, but produces inconsistent clinical results and is frequently ineffective.2,6,15–19 The purpose of this study was to compare histologic findings between the Infrared Coagulator (IRC 2100, Redfield Corporation, Rochelle Park, NJ, USA) and the HET™ Bipolar Ligator (HET Systems, Northvale, NJ, USA) – a new bipolar device for tissue manipulation and coagulation.
Technology and methods
The HET™ Bipolar Ligator System is comprised of an innovative tissue ligator and an associated temperature monitor. The HET™ Bipolar Ligator has a unique, constant tissue compression mechanism, a temperature sensor adjacent to the bipolar electrodes, and integral LED-based illumination. The HET™ Bipolar Ligator’s unique tissue clamp and bipolar radio-frequency-based tissue coagulation allow compression and ligation of the target tissue with a constant force and predictable energy delivery. When this operator-independent force is applied parallel to the rectal wall, the associated superior hemorrhoid vasculature in the formed tissue fold is predictably occluded and ligated (Figure 1). Continuous tissue temperature monitoring during use of the HET™ Bipolar Ligator provides the operator with objective, real-time feedback about the treated tissue temperature.20
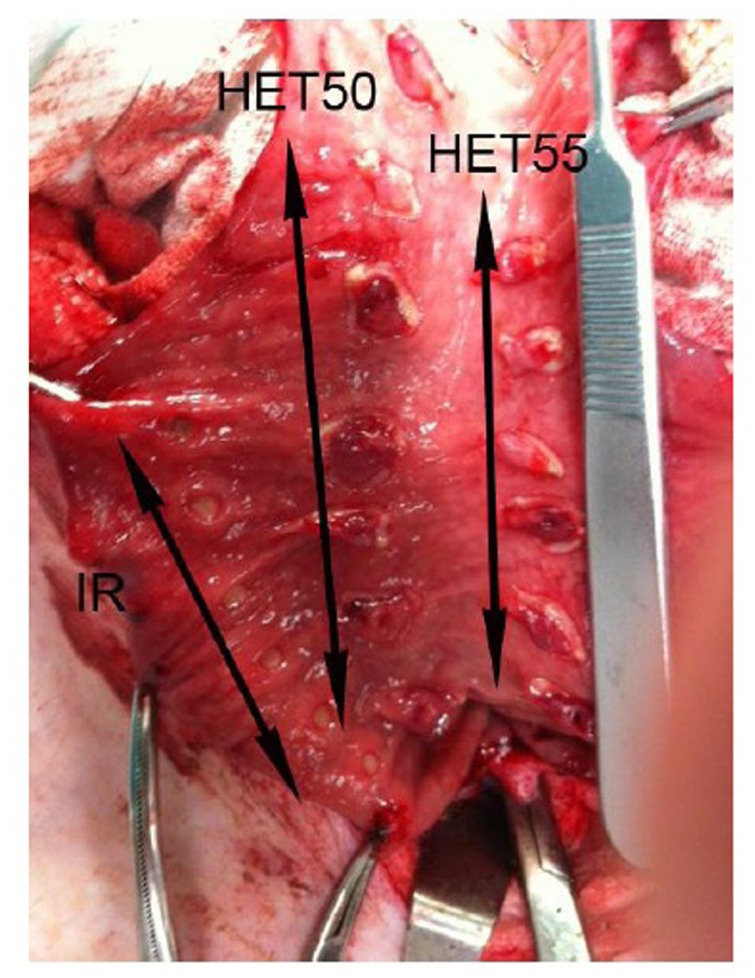
Figure 1.
Gross comparative mucosal changes in three rows of applications: IRC (IR) and HET with final tissue temperatures of 50°C and 55°C.
Abbreviations: IRC, infrared coagulator; HET, HET Bipolar Ligator.
The Redfield IRC 2100 includes a halogen lamp-based heat generator and an elongated probe, which interfaces with the target tissue and delivers heat by regulating the amount of time the device is activated. The manufacturer provides predetermined recommended time intervals for treatment. This approach does not control the amount of heat applied to the tissue; however, it controls the length of time the tissue will be exposed to varying temperatures. The IRC system does not provide a mechanism for controlling or gauging tissue compression, or a method for measuring the temperature of the treated tissue. Additionally, the pressure applied to the treatment site by the IRC device is operator dependent.
An in vivo single animal, multiple treatment sites study was conducted to compare the histologic effects of the two systems when used on the recto-sigmoid swine colon.
The objective of this study was to assess histopathologic changes following treatment with heat delivered to normal colonic tissues by the two devices. Since both the Redfield IRC 2000 and the HET™ Bipolar Ligator are applied to the normal colonic mucosa of humans during the treatment of internal hemorrhoids, and since the hemorrhoidal tissue is not directly affected by thermal energy, only normal animal colonic tissues were studied. The effects of thermal treatment in the mucosa, submucosa, submucosal vessels, and muscularis layer were evaluated. Lateral thermal spread beyond the energy application site was also assessed.
Following receipt of University Institutional Animal Care and Use Committee approval, the study was carried out on a single female Sus scrofa pig weighing approximately 54.5 kg. Preanesthesia of intramuscular ketamine (14.7 mg/kg) and intravenous acepromazine (1.5 mg/kg) was given. Surgical plane was maintained by inhalation of a 3% isoflurane solution. The pig was connected to a heart rate monitor, oxygen monitor, and ventilator. A midline abdominal incision was performed, and the sigmoid colon was exposed. A longitudinal incision was made on the antimesenteric side of the recto-sigmoid colon and wide access to the colonic mucosa was obtained.
Three similar segments of the colon were used to study three treatment groups with six areas per group. The HET™ Bipolar Ligator was used to treat two groups at two different temperatures: 55°C (HET55 group) and 50°C (HET50 group). The IRC device was used to treat one group (IRC group).
Both the IRC 2100 (IRC) and HET™ Bipolar Ligator (HET) devices were used according to the manufacturer’s instructions for use. The HET™ Bipolar Ligator was connected to a Conmed Hyfrecator 2000 bipolar radiofrequency generator (Conmed, Utica, NY, USA) with an output coagulation setting at 10 watts.
The tissue in the HET™ Bipolar Ligator was compressed parallel to the bowel wall, and bipolar radiofrequency energy was then delivered until the temperature reached 50°C (across six areas) or 55°C (across six areas).
The IRC device delivers pulses of infrared light through a small contact tip applicator that is applied to the tissue. The light causes thermal coagulation which results in tissue necrosis.21 The amount of energy delivered is regulated by the amount of time the device is activated. As recommended by the manufacturer, the IRC timer was set in between 1.0–1.5 seconds for the treatment of hemorrhoids. The IRC heat pulse was delivered perpendicular to the mucosal surface to each of the six treatment mucosal areas. The temperature of the IRC probe was continuously measured during the heat delivery using a digital thermometer (Tektronix DTM920, Tektronix, Inc, Beaverton, Oregon, USA). The maximum temperature, observed in all cases at 1.5 seconds, was documented.
When all treatment groups were completed, the pig was euthanized, and the recto-sigmoid colon segment removed. The excised segment was placed in 10% buffered formalin for 72 hours.
All samples were sectioned perpendicular to the mucosa into two pieces and embedded in paraffin blocks. The blocks were sectioned in 4 μ cuts, and an additional step was performed approximately 150 μ from the first cut. All slides were stained with hematoxylin and eosin.
The thermal damage in the mucosa, submucosa, and muscularis layers was measured as follows: layer thinning was measured by an ocular micrometer comparing the treated area to an adjacent normal area, and was graded on a scale of 0 to +4 with 0 representing 0% thinning, +1 representing 25% thinning, +2 representing 50% thinning, +3 representing 75% thinning, and +4 representing total loss of the layer. Multiple areas were analyzed for each of the coagulation areas. Absolute dimensions were not used as the layer thickness can vary from place to place, and such measurements would not reflect the importance of the percent of layer damage. The percentage measurements used are a standard technique in assessing histological damage, including thermal damage.
The mucosa was graded based on the layer thinning, loss of glands and loss of basophilic staining. The submucosa was graded based on the layer thinning and the degree of the vascular injury. The muscularis layer was graded based on the increased eosinophilia, vacuolization and nuclear changes. Averages and standard error of the mean scores were calculated. The values were compared with the standard Students t-test.22 Collagen damaged was accessed by polarized light microscopy.
In the HET50 and HET55 groups, the treated tissues were also examined at different distances from the bipolar electrodes. The lateral thermal spread outside of the energy application site was evaluated as well.
Results
The treatment time and treatment site temperature readings are summarized by study group in Table 1. The treated tissue reached 50°C in 4–7 sec (HET50 group) and 55°C in 6–9 sec (HET55 group). The compressed tissue in both HET groups was 1–2 mm.
Table 1.
Tissue temperature following treatment in each study group
| Group | # treatment areas | Treatment site temperature (°C) | Treatment time (sec) |
|---|---|---|---|
| HET50 | 6 | 50 | 4–7 (4.8 ± 0.8 sec) |
| HET55 | 6 | 55 | 6–9 (6.2 ± 1.8 sec) |
| IRC | 6 | 127.8–155.7 (149.9 ± 11.1) | 1.5 |
Abbreviations: HET50, HET Bipolar Ligator at 50°C; HET55, HET Bipolar Ligator at 55°C; IRC, infrared coagulation.
In the IRC group, after 1.5 seconds of treatment, the temperature at the tip of the probe varied from 127.8°C to 155.7°C (149.9°C ± 11.1°C); with the final tissue temperature being highly dependent on the pressure applied by the device to the tissue. The gross, comparative changes between the IRC, HET55, and HET50 groups are shown in Figure 1.
Table 2 summarizes the comparative mucosal and submucosal changes between the IRC, HET55, and HET50 groups at sites immediately adjacent to or in contact with the treatment elements. The mucosal and submucosal tissue changes seen in the IRC and the HET55 groups were similar. Both the mucosal and submucosal tissue changes in the HET50 group were significantly milder than IRC and HET55 groups (P < 0.05). None of the samples displayed loss of tissue architecture or thermal damage of the full thickness of the bowel wall. The lateral thermal spread from the application site into the normal tissue in all cases was minimal.
Table 2.
Comparison between mucosal and submucosal changes at treatment sites
| N (# samples) | IRC | HET55 | HET50 |
|---|---|---|---|
|
|
|
|
|
| 20 | 16 | 16 | |
| Mucosa | |||
| Layer thinning | 1.70 ± 0.14 | 2.00 ± 0.18 | 0.56 ± 0.18 |
| Loss of glands | 2.20 ± 0.09 | 1.50 ± 0.13 | 1.88 ± 0.09 |
| Loss of basophilic staining | 1.60 ± 0.11 | 1.62 ± 0.11 | 1.12 ± 0.09 |
| Submucosa | |||
| Layer thinning | 1.50 ± 0.10 | 2.00 ± 0.26 | 0.25 ± 0.17 |
| Degree of vascular injury | 2.40 ± 0.18 | 2.00 ± 0.26 | 1.25 ± 0.11 |
Note: Tissue damage scores graded 0 to 4 (avg ± SD).
Abbreviations: IRC, infrared coagulation; HET55, HET Bipolar Ligator at 55°C; HET50, HET Bipolar Ligator at 50°C; SD, standard deviation.
Mucosa layer
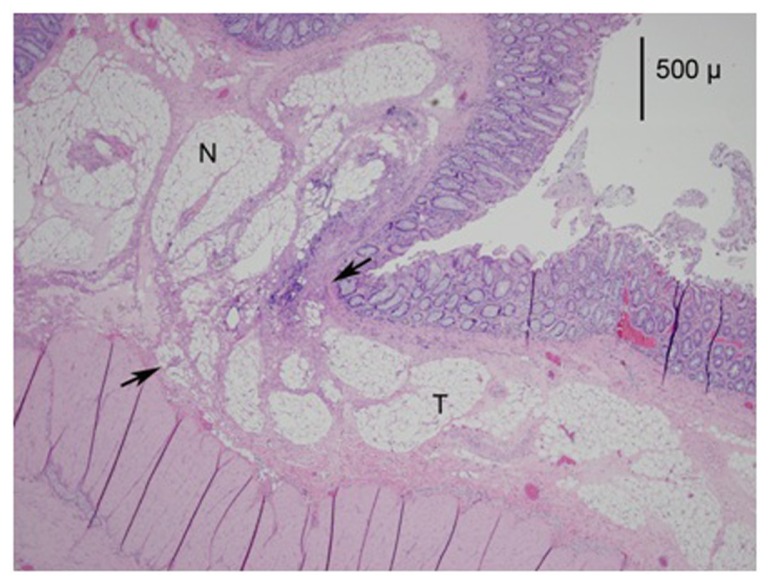
The treated mucosa displayed thinning caused by thermal desiccation and the loss of crypt glands (Figures 2 and 3). Some thinning in the HET specimens was also a result of compression from the tissue clamp of the device. The basophilic staining of the layer was also decreased (Figure 4). These effects, with the exception of glandular loss, were significantly less pronounced in the HET50 group. Some vascular congestion and small hemorrhaging were seen but were not prevalent.
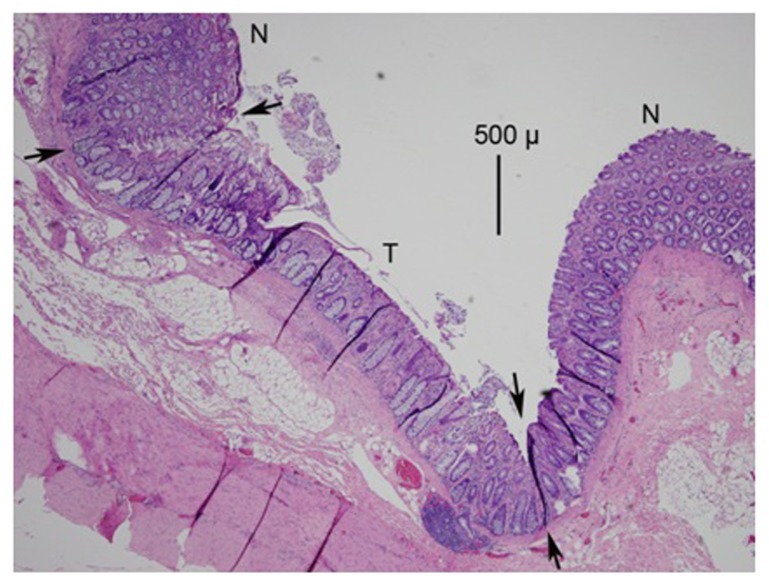
Figure 2.
A photomicrograph (mag 40×) from the HET55 group.
Notes: The treated area is between the arrows and marked as T; the normal area is marked as N. Note the thinning of the mucosa by 25% to 50% compared to the normal area. There is also a loss of basophilic staining in the mucosa and the loss of crypt glands in the treated area.
Abbreviations: HET55, HET Bipolar Ligator at 55°C; T, treated area; N, normal area.
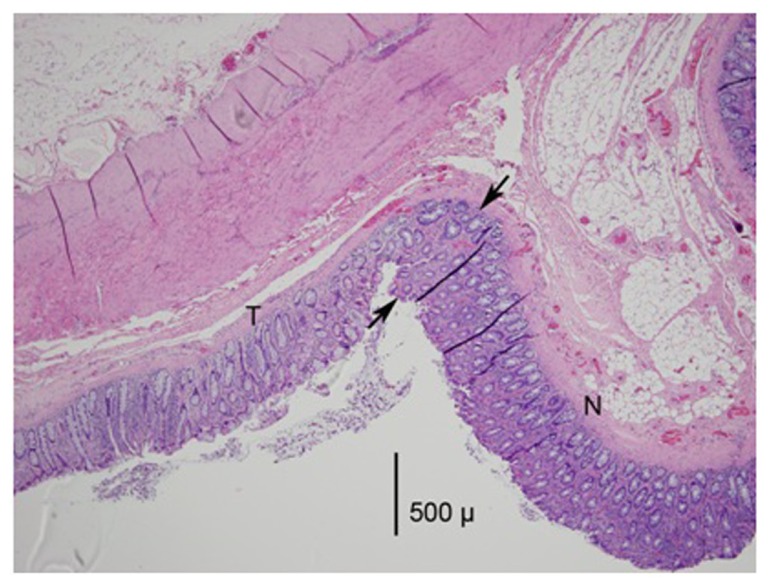
Figure 3.
A photomicrograph (mag 40×) from the HET50 group.
Notes: The treated area is to the left of the arrows and marked as N. Note the thinning of the mucosa by 25% compared to the normal area. There is also a loss of basophilic staining in the mucosa and the loss of crypt glands in the treated area.
Abbreviations: HET50, HET Bipolar Ligator at 50°C; T, treated area; N, normal area.
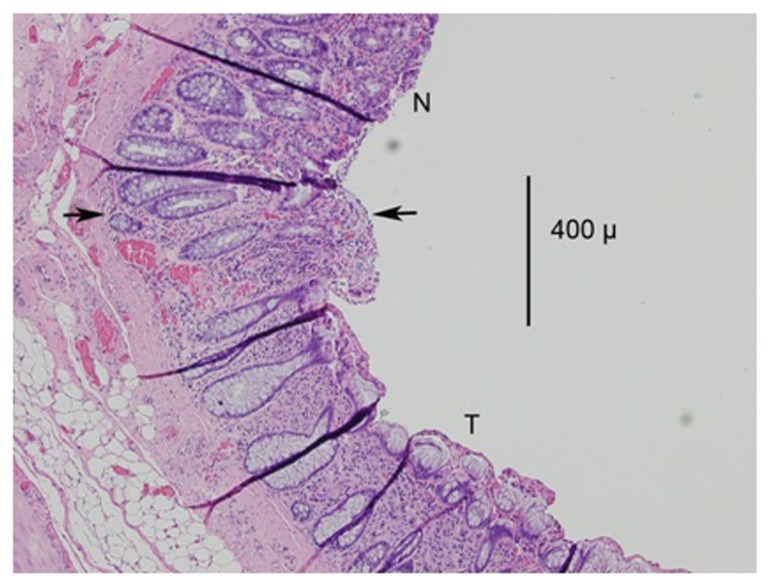
Figure 4.
A photomicrograph (magnification 100×) from the IRC group.
Notes: The treated area is below the arrows and marked as T; the normal area is marked as N. Note the loss of basophilic staining in the mucosa and the loss of crypt glands in the treated area.
Abbreviations: IRC, infrared coagulator; T, treated area; N, normal area.
Submucosa layer
Submucosal injury was characterized by thinning and vascular injury. The submucosal thinning (Figure 5) was similar between the IRC and the HET55 groups, while the HET50 group demonstrated significantly less submucosal thinning (P < 0.05). In the HET55 group, 50% of samples displayed a full thickness submucosal injury without muscle involvement, while none of the HET50 samples showed full thickness submucosal thermal injury.
Figure 5.
A micrograph (mag 40×) of a representative sample from the HET55 group.
Note: The treated area is below the arrows and marked as T; the normal area is marked as N. Note the thinning of the submucosa compared to the normal area. There is also hemorrhage in the mucosa, and vascular damage and congestion in the submucosa in the treated area.
Abbreviations: HET55, HET Bipolar Ligator at 55°C; T, treated area; N, normal area.
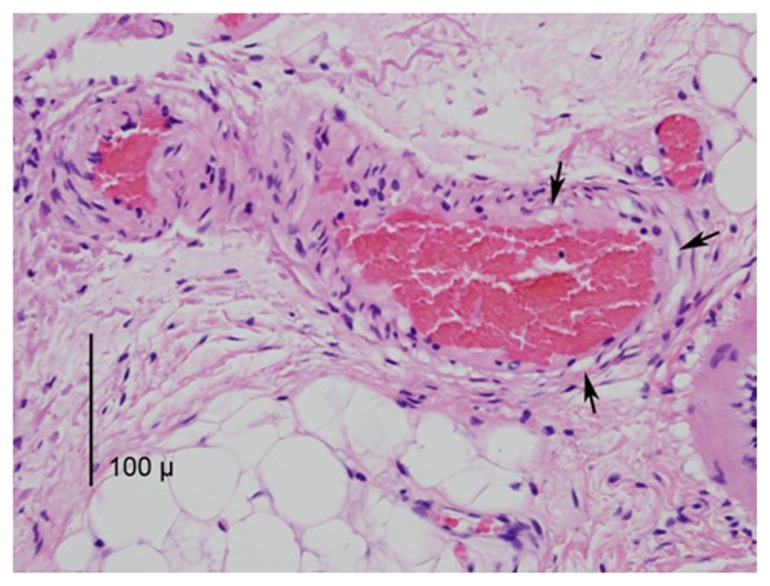
Vascular injury included congestion and small amounts of hemorrhage, and in some cases, the vessel endothelial cells were affected (Figure 6). Small amounts of reactive neutrophilic infiltration were seen in most samples. Vascular changes were similar between the HET55 and HET50 groups.
Figure 6.
A photomicrograph (mag 400×) from the IRC group depicting three vessels in the submucosa with congestion.
Notes: The nuclei in the vessel walls demonstrate thermal damage and are condensed and hyperchromic. The arrows point to several spots of vacuolization caused by heat.
Abbreviation: IRC, infrared coagulator.
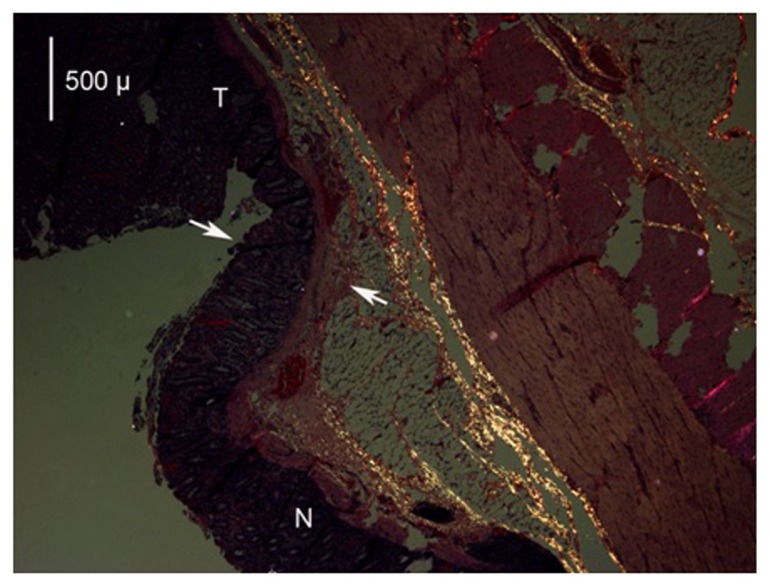
There were signs of collagen denaturation present in all IRC, HET55, and HET50 specimens with all samples showing reduced collagen damage in the areas that were more remote from the source of the heat. This was demonstrated by the polarized photomicrograph showing the normal collagen as bright areas and the denatured as darkened areas (Figure 7).
Figure 7.
A photomicrograph (mag 40×) of the application of the HET device at 55°C viewed with polarized light.
Notes: The treated area is above the arrows and marked as T; the normal area is marked as N. As collagen is heated, the molecule loses its isotropic conformation and becomes anisotropic; when viewed under polarized light the isotropic collagen appears bright and denatured collagen is dark. The normal isotropic collagen molecule appears bright and is represented in the lower area as several bands; however, closer to the treatment application and in the treated area, there is denaturation of collagen (loss of brightness).
Abbreviations: HET, HET Bipolar Ligator; T, treated area; N, normal area.
The maximal tissue damage was observed immediately adjacent to the ligation electrodes in the HET55 and HET50 groups. While hemorrhage and occasional thinning were observed in the mucosa, the thermal effects were prevalent in the submucosa and presented as vascular injury including congestion and hemorrhage. Evaluation of folds of tissue between the ligation electrodes demonstrated no difference in vascular damage between the two HET groups (Table 3).
Table 3.
Comparison of submucosal changes between HET55 and HET50 groups in the treated tissue folds beyond immediate contact with the ligation electrodes
| HET55 (Avg ± SE) | HET50 (Avg ± SE) | |
|---|---|---|
| Number of samples | 16 | 16 |
| Submucosa vascular damage | 1.75 ± 0.11 | 1.5 ± 0.13 |
Note: Graded from 0 to 4; avg ± SD.
Abbreviations: HET55, HET Bipolar Ligator at 55°C; HET50, HET Bipolar Ligator at 50°C; SE, standard error.
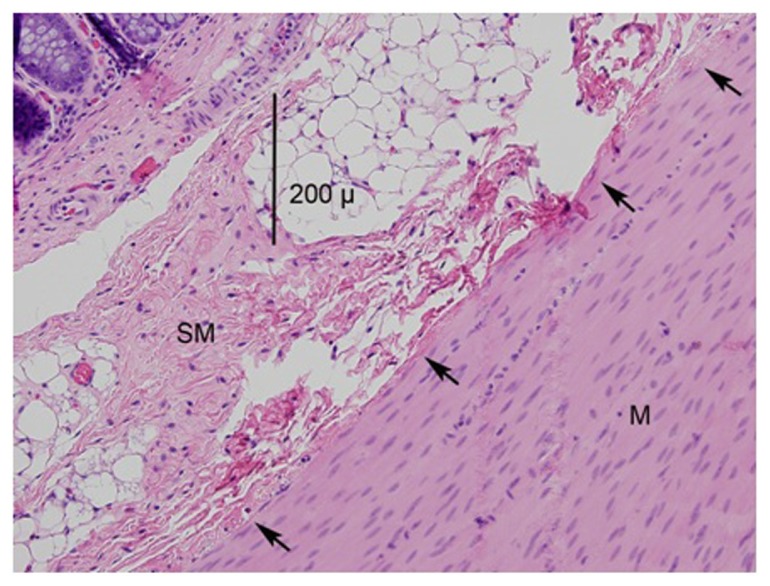
Muscularis layer
All samples from the IRC group displayed similar thermal damage to the muscularis layer, but this damage was scant and isolated. In the HET50 and HET55 groups, 75% of samples had no muscular injury, while 25% of the samples presented only with superficial muscularis injury (Figure 8). Lateral thermal spread from the application site into normal tissue was minimal in all samples.
Figure 8.
A photomicrograph (mag 200×) of the application of the HET device at 55°C.
Notes: The submucosa is marked SM and the muscularis is marked M. The arrows point to the slight muscle damage, estimated to be a few cell layers thick.
Abbreviations: SM, submucosa; M, muscularis.
Discussion
The goal of therapeutic intervention is to effectively target the pathologic lesion while minimizing injury to collateral tissues. Using energy to treat gastrointestinal lesions, including hemorrhoids, is not new. Electrocoagulation as a treatment modality was utilized as early as 1867 and explained by Dr WE Kessey in 1934.23 Over the past several decades, new developments and techniques to treat hemorrhoids in a less invasive manner have targeted the superior hemorrhoidal vasculature and the associated submucosa above the dentate line in order to minimize patient pain, discomfort, and tenesmuses.24–28
Both the IRC device and the HET™ Bipolar Ligator intend to produce histologic changes proximal to the internal hemorrhoid, which would lead to an occlusion of the superior hemorrhoid blood supply and formation of moderate scarring in the associated submucosa. To minimize potential collateral tissue damage, a precise capture of the target tissue proximal to the internal hemorrhoid, and predictable energy delivery to both the submucosal vessels and the surrounding connective tissue, are thus highly desirable.
In this evaluation there was greater variability in the temperature at the tissue treatment site in the IRC group than in the HET groups, and the temperature reached in the IRC treatment group was significantly higher. With the IRC device, the procedure was driven by the manufacturer recommended time setting and operator variability in compression pressure.21 Both inevitably impacted the amount of energy delivered to the target site. In contrast, when the HET™ Bipolar Ligator was used, temperature at the treatment site was monitored continuously, and energy was delivered consistently until the target tissue temperature of 50°C–55°C was achieved. The time of energy delivery during tissue ligation using the HET™ Bipolar Ligator is a derivative of the targeted tissue temperature. Additionally, the device is designed to provide constant consistent compression, which further minimized variability in both the energy delivery to the tissue and consequent tissue changes.29
Tissue compression
Tissue compression is a known factor affecting energy delivery to targeted tissue.30,31 Variability in the tissue compression, therefore, would lead to variability in the energy delivery to the tissue. This was demonstrated in this investigation given that the consistently compressed tissue (among the HET groups) required much less temperature than non-compressed tissue (among the IRC group) to cause similar histologic effects.
The IRC device does not provide a mechanism for predictable or measurable tissue compression, meaning that the tissue compression was operator-dependent. Contrary and advantageously, the minimal variability observed within the HET™ treatment groups was a function of the unique design of the HET™ Bipolar Ligator device.
Tissue temperature
The amount of energy delivered to the tissue correlates with the histological changes.32 The tissue temperature monitoring, therefore, may serve as a useful guideline during the energy delivery to the tissue.
The IRC device did not offer a mechanism for temperature monitoring and relied strictly on the time of energy delivery for tissue treatment.17 As information on temperature generated by the IRC probe was not found in the literature, it was measured directly in this study. The IRC generated 149.9°C ± 11.1°C during 1.5 seconds of activation in this hemorrhoid treatment model. In contrast, the HET™ Bipolar Ligator was designed to allow temperature monitoring of the treated tissue. This affords standardization of the treatment by specifically exposing the target tissue to a constant 55°C temperature to achieve the desired therapeutic effect.
Both devices created similar histological changes in the submucosal vessels and submucosa layer of the target tissues. The IRC achieved these changes in 1.5 seconds at much higher temperature, while the HET™ Bipolar Ligator achieved the desired tissue changes using temperatures that were 3.0 and 2.7 times lower in HET50 and HET55 groups, respectively. It appears that the constant tissue compression accompanying the tissue ligation using the HET Bipolar Ligator reduced the amount of energy required to produce similar histological effects. Recent discovery of targeted metal nanoparticles in selective tissue hyperthermia and cytotoxicity may play an interesting role in facilitating a minimally-invasive chemical ligation of the targeted small vessels.33–35
Tissue desiccation
In the IRC and both HET groups, signs of tissue desiccation were observed, including some loss of intercellular water and associated pyknotic and hyperchromic changes in the nuclei. Changes such as loss of normal tissue architecture and complete cellular protein denaturation were not observed in any of the samples.
Histologically, cells showed lighter hematoxylin and eosin staining due to the loss of cell nuclei and intracellular protein; such cells are sometimes referred to as ghost cells. White cell infiltration and capillary growth into the damaged area will occur from the surrounding normal tissue. This regenerative process will also lead to fibrosis.28 In contrast, a more significant heat-related injury typically produces more significant volume of tissue damage, and the adjacent tissue would be unable to regenerate, usually leading to ulceration.36,37
Comparing HET™ bipolar ligator at 55°C and 50°C
While the overall histological changes created by the tissue ligation using the HET Bipolar Ligator at 50°C (HET50) appeared to be milder than the ligation at 55°C (HET55), the submucosal changes in the treated folds of tissue between the ligation electrodes were substantially equivalent between the groups.
The observed vascular injury was consistent with vascular occlusion in both groups. This finding suggests that the efficacy of the HET™ device operating at 50°C and 55°C is similar. However, from a clinical standpoint, patients exposed to a lower temperature (50°C) may experience less discomfort.
The thermal injury in all HET™ specimens was limited to the areas compressed between the bipolar electrodes with no or minimal collateral tissue damage outside of the tissue that was in contact with the electrodes. Since the operator controls the position of the electrodes, the tissue compression within the unique HET™ clamp is constant, and therefore the tissue is treated to the defined temperature. The energy delivery to the target tissue by the HET™ Bipolar Ligator is highly consistent and predictable, minimizing collateral tissue damage. The demonstrated recognizable architecture, the lack of full thickness damage, the intact tunica muscularis, and the minimally damaged blood supply suggest that little ulceration would occur in tissues treated with the HET™ Bipolar Ligator at 55°C or 50°C. The intact residual blood supply would thus promote routine fibroblast infiltration and active fibrosis in the area of the thermal desiccation.36–38
Conclusion
The combination of constant tissue compression and temperature-guided energy delivery achieves desirable histological changes between 50°C and 55°C.
The unique design of the HET™ Bipolar Ligator provides precise delivery of energy to the target tissue, as well as real-time temperature monitoring and well-gauged, constant tissue compression. These mechanisms facilitate accurate therapeutic energy delivery to the target tissues between 50°C and 55°C while minimizing the collateral muscular damage.
In this comparative histological study, while both devices achieved similar results, the HET™ Bipolar Ligator achieved the desired histologic changes with less muscular damage, using focused delivery of radio frequency energy at a much lower temperature than IRC. Therefore, the use of the HET™ Bipolar Ligator could provide significant clinical advantages, potentially enhance patients’ tolerance of the ligation procedure, and minimize the chance of heat-related complications and collateral tissue damage. The conclusions of this study need to be considered with caution due to the anatomical differences between humans and pigs. Specifically, the differences in colonic layer thickness, tissue metabolism, and reactions to heat between human and pig tissue could potentially influence the study results. To make clinically meaningful conclusions, it is important to continue studying these new devices in a well-designed, outcome-based clinical study. Comparative clinical evaluations of the devices are planned to confirm these outcomes.
Footnotes
Disclosure
Gregory Piskun, MD is an inventor of HET™ Bipolar Ligator and founder of HET Systems. Robert Tucker reports no conflicts of interest in this work.
References
- 1.Hardwick RH, Durdey P. Should rubber band ligation of haemorrhoids be performed at the initial outpatient visit? Ann R Coll Surg Engl. 1994;76(3):185–187. [PMC free article] [PubMed] [Google Scholar]
- 2.Walker AJ, Leicester RJ, Nicholls RJ. A prospective study of infrared coagulation, injection and rubber band ligation in the treatment of haemorrhoids. Int J Colorectal Dis. 1990;5(2):113–116. doi: 10.1007/BF00298482. [DOI] [PubMed] [Google Scholar]
- 3.Bat L, Melzer E, Koler M, Dreznick Z, Shemesh E. Complications of rubber band ligation of symptomatic internal hemorrhoids. Dis Colon Rectum. 1993;36(3):287–290. doi: 10.1007/BF02053512. [DOI] [PubMed] [Google Scholar]
- 4.Poen AC, Felt-Bersma RJ, Cuesta MA, Devillé W, Meuwissen SG. A randomized controlled trial of rubber band ligation versus infrared coagulation in the treatment of internal haemorrhoids. Eur J Gastroenterol Hepatol. 2000;12(5):535–539. doi: 10.1097/00042737-200012050-00010. [DOI] [PubMed] [Google Scholar]
- 5.Marques CF, Nahas SC, Nahas CS, Sobrado CW, Jr, Habr-Gama A, Kiss DR. Early results of the treatment of internal hemorrhoid disease by infrared coagulation and elastic banding: a prospective randomized cross-over trial. Tech Coloproctol. 2006;10(4):312–317. doi: 10.1007/s10151-006-0299-5. [DOI] [PubMed] [Google Scholar]
- 6.Ricci MP, Matos D, Saad SS. Rubber band ligation and infrared photocoagulation for the outpatient treatment of hemorrhoidal disease. Acta Cir Bras. 2008;23(1):102–106. doi: 10.1590/s0102-86502008000100016. [DOI] [PubMed] [Google Scholar]
- 7.Russell TR, Donahue JH. Hemorrhoidal banding: A warning. Dis Colon Rectum. 1985;28(5):291–293. doi: 10.1007/BF02560424. [DOI] [PubMed] [Google Scholar]
- 8.Wechter DG, Luna GK. An unusual complication of rubber band ligation of hemorrhoids. Dis Colon Rectum. 1987;30(2):137–140. doi: 10.1007/BF02554954. [DOI] [PubMed] [Google Scholar]
- 9.Shemesh EI, Kodner IJ, Fry RD, Neufeld DM. Severe complication of rubber band ligation of internal hemorrhoids. Dis Colon Rectum. 1987;30(3):199–200. doi: 10.1007/BF02554339. [DOI] [PubMed] [Google Scholar]
- 10.Quevedo-Bonilla G, Farkas AM, Abcarian H, Hambrick E, Orsay CP. Septic complications of hemorrhoidal banding. Arch Surg. 1988;123(5):650–651. doi: 10.1001/archsurg.1988.01400290136024. [DOI] [PubMed] [Google Scholar]
- 11.Clay LD, 3rd, White JJ, Jr, Davidson JT, Chandler JJ. Early recognition and successful management of pelvic cellulitis following hemorrhoidal banding. Dis Colon Rectum. 1986;29(9):579–581. doi: 10.1007/BF02554261. [DOI] [PubMed] [Google Scholar]
- 12.Scarpa FJ, Hillis W, Sabetta JR. Pelvic cellulitis: a life-threatening complication of hemorrhoidal banding. Surgery. 1988;103(3):383–385. [PubMed] [Google Scholar]
- 13.Odelowo OO, Mekasha G, Johnson MA. Massive life-threatening lower gastrointestinal hemorrhage following hemorrhoidal rubber band ligation. J Natl Med Assoc. 2002;94(12):1089–1092. [PMC free article] [PubMed] [Google Scholar]
- 14.Longman RJ, Thomson WH. A prospective study of outcome from rubber band ligation of piles. Colorectal Dis. 2006;8(2):145–148. doi: 10.1111/j.1463-1318.2005.00873.x. [DOI] [PubMed] [Google Scholar]
- 15.Johanson JF, Rimm A. Optimal nonsurgical treatment of hemorrhoids: a comparative analysis of infrared coagulation, rubber band ligation, and injection sclerotherapy. Am J Gastroenterol. 1992;87(11):1600–1606. [PubMed] [Google Scholar]
- 16.Antonelli A. Infrared coagulation of hemorrhoids. Endosc Rev. 1989 Jan-Feb;:75–76. [Google Scholar]
- 17.Nevah EI. The outpatient management of internal hemorrhoids by infrared photocoagulation. Rev Med Panama. 1993;18(3):166–170. [PubMed] [Google Scholar]
- 18.O’Holleran TP. Infrared photocoagulation of hemorrhoids. Nebr Med J. 1990;75(11):307–308. [PubMed] [Google Scholar]
- 19.Gupta PJ. Infra red photocoagulation of early grades of hemorrhoids – 5-year follow-up study. Bratisl Lek Listy. 2007;108(4–5):223–226. [PubMed] [Google Scholar]
- 20.HET Bipolar Ligator System User’s Manual. Northvale, NJ: HET Systems; 2012. [Google Scholar]
- 21.Redfield IRC 2100 Infrared Coagulator Operating and Maintenance Manual. Redfield Corporation; Rochelle Park, NJ, USA: [Google Scholar]
- 22.Weast RC, editor. CRC Handbook of Probability and Statistics. Cleveland: The Chemical Rubber Co; 1968. pp. 282–292. [Google Scholar]
- 23.Keesey WE. Obliteration of hemorrhoids with negative galvanism. Archives of Physical Therapy, X-ray, Radium. 1934;1:533–546. [Google Scholar]
- 24.Infantino A, Altomare DF, Bottini C, et al. Prospective randomized multicentre study comparing stapler haemorrhoidopexy with Dopplerguided transanal haemorrhoid dearterialization for third-degree haemorrhoids. Colorectal Dis. 2012;14(2):205–211. doi: 10.1111/j.1463-1318.2011.02628.x. [DOI] [PubMed] [Google Scholar]
- 25.Nunoo-Mensah JW, Kaiser AM. Stapled hemorrhoidectomy. Am J Surg. 2005;190(1):127–130. doi: 10.1016/j.amjsurg.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 26.Aigner F, Bodner G, Gruber H, et al. The vascular nature of hemorrhoids. J Gastrointest Surg. 2006;10(7):1044–1050. doi: 10.1016/j.gassur.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Giamundo P, Cecchetti W, Esercizio L, et al. Doppler-guided hemorrhoidal laser procedure for the treatment of symptomatic hemorrhoids: experimental background and short-term clinical results of a new miniinvasive treatment. Surg Endosc. 2011;25(5):1369–1375. doi: 10.1007/s00464-010-1370-x. [DOI] [PubMed] [Google Scholar]
- 28.Yilmaz I, Sücüllü I, Karakaş DÖ, Özdemir Y, Yücel E, Akin ML. Doppler-guided hemorrhoidal artery ligation: experience with 2 years follow-up. Am Surg. 2012;78(3):344–348. [PubMed] [Google Scholar]
- 29.Piskun G. New technology for minimally-invasive treatment of symptomatic grade I and II hemorrhoids: pilot study with 1-year follow-up. Abstract presented at SAGES 2012 Annual Meeting; 2012 March, 7–10; San Diego, CA. [Google Scholar]
- 30.Laine L. Determination of the optimal technique for bipolar electrocoagulation treatment. An experimental evaluation of the BICAP and Gold probes. Gastroenterology. 1991;100(1):107–112. doi: 10.1016/0016-5085(91)90589-d. [DOI] [PubMed] [Google Scholar]
- 31.Dilley AV, Friend MA, Morris DL. An experimental study of optimal parameters for bipolar electrocoagulation. Gastrointest Endosc. 1995;42(1):27–30. [PubMed] [Google Scholar]
- 32.Goulet CJ, Disario JA, Emerson L, Hilden K, Holubkov R, Fang JC. In vivo evaluation of argon plasma coagulation in a porcine model. Gastrointest Endosc. 2007;65(3):457–462. doi: 10.1016/j.gie.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128(6):2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 34.Cherukuri P, Glazer ES, Curley SA. Targeted hyperthermia using metal nanoparticles. Adv Drug Deliv Rev. 2010;62(3):339–345. doi: 10.1016/j.addr.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain S, Hirst DG, O’Sullivan JM. Gold nanoparticles as novel agents for cancer therapy. Br J Radiol. 2012;85(1010):101–113. doi: 10.1259/bjr/59448833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLean AJ. Characteristics of adequate electrosurgical current. Am J Surg. 1932;18:417–441. [Google Scholar]
- 37.Tucker RD, Platz CE, Landas SK. Histological characteristics of electrosurgical injuries. J Am Assoc Gynecol Laparosc. 1997;4(2):201–206. doi: 10.1016/s1074-3804(97)80010-2. [DOI] [PubMed] [Google Scholar]
- 38.Kumar V, Abbas AK, Fausto N, Mitchell RN, editors. Robbins Basic Pathology. 8th ed. Philadelphia: Saunders; 2007. Tissue repair: regeneration, healing and fibrosis; pp. 59–79. [Google Scholar]