Abstract
Inhaled corticosteroid (ICS) therapy has become standard in the treatment of asthma. A common local adverse effect of ICS therapy is dysphonia, which has been reported to affect 5% to 58% of patients. Although causes of dysphonia associated with ICS therapy have been underinvestigated, it may result from deposition of an active ICS in the oropharynx during administration, which then causes myopathy or a mucosal effect in the laryngopharynx. Use of ICS should be considered during any evaluation of dysphonia. We recommend using the lowest effective dosage of ICS, administering medication with a spacer, gargling, rinsing the mouth and washing the face after inhalation, and washing the spacer. If dysphonia develops despite these interventions, ICS use should be suspended until symptoms resolve, provided that asthma control is not compromised.
Abbreviations and Acronyms: DPI, dry-powder inhaler; ICS, inhaled corticosteroid; MDI, metered-dose inhaler
CME Activity.
Target Audience: The target audience for Mayo Clinic Proceedings is primarily internal medicine physicians and other clinicians who wish to advance their current knowledge of clinical medicine and who wish to stay abreast of advances in medical research.
Statement of Need: General internists and primary care providers must maintain an extensive knowledge base on a wide variety of topics covering all body systems as well as common and uncommon disorders. Mayo Clinic Proceedings aims to leverage the expertise of its authors to help physicians understand best practices in diagnosis and management of conditions encountered in the clinical setting.
Accreditation: College of Medicine, Mayo Clinic is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Statement: College of Medicine, Mayo Clinic designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s).™ Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Learning Objectives: Educational Objectives. On completion of this article, you should be able to (1) recognize that dysphonia from use of inhaled cortiscosteroids is not rare and should be part of any work-up in the evaluation of dysphonia, (2) describe the most likely mechanism of dysphonia from use of inhaled corticosteroids, and (3) recommend practical advice to decrease the presentation of dysphonia as an adverse effect of using inhaled corticosteroids.
Disclosures: As a provider accredited by ACCME, College of Medicine, Mayo Clinic (Mayo School of Continuous Professional Development) must ensure balance, independence, objectivity, and scientific rigor in its educational activities. Course Director(s), Planning Committee members, Faculty, and all others who are in a position to control the content of this educational activity are required to disclose all relevant financial relationships with any commercial interest related to the subject matter of the educational activity. Safeguards against commercial bias have been put in place. Faculty also will disclose any off-label and/or investigational use of pharmaceuticals or instruments discussed in their presentation. Disclosure of this information will be published in course materials so that those participants in the activity may formulate their own judgments regarding the presentation. In their editorial and administrative roles, William L. Lanier, Jr, MD, Scott C. Litin, MD, Terry L. Jopke, Kimberly D. Sankey, and Nicki M. Smith, MPA, have control of the content of this program but have no relevant financial relationship(s) with industry.
Drs Galván and Guarderas have no potential competing interests to declare.
Method of Participation: In order to claim credit, participants must complete the following:
-
1
Read the activity.
-
2
Complete the online CME Test and Evaluation. Participants must achieve a score of 80% on the CME Test. One retake is allowed.
Participants should locate the link to the activity desired at http://bit.ly/Mb4EBl. Upon successful completion of the online test and evaluation, you can instantly download and print your certificate of credit.
Estimated Time: The estimated time to complete each article is approximately 1 hour.
Hardware/Software: PC or MAC with Internet access.
Date of Release: 9/1/2012
Expiration date: 8/31/2014 (Credit can no longer be offered after it has passed the expiration date.)
Privacy Policy: http://www.mayoclinic.org/global/privacy.html
Questions? Contact letcsupport@mayo.edu.
Dysphonia is a disorder characterized by altered vocal quality, pitch, loudness, or effort that impairs social and professional communication. It can deteriorate quality of life by affecting emotional, physical, social, and work function.1,2
Dysphonia is an important but underrecognized adverse effect of numerous medications, with a lifetime prevalence of 29.9% in adults aged 65 years or younger.3 Women are more frequently affected than men (female to male ratio, 3:2).4 The prevalence of dysphonia is higher in individuals who use their voice extensively in their profession; for example, 31% of telemarketers and 58% of teachers are affected.5 The economic burden of dysphonia is substantial. An estimated 7.2% of the general population misses work for at least 1 day because of voice problems; this represents approximately $2.5 billion in annual work productivity lost in the United States.6 Therefore, dysphonia may be considered a public health challenge.
Warfarin sodium, thrombolytic agents, and phosphodiesterase-5 inhibitors can induce dysphonia by causing vocal fold hematomas7; biphosphonates can cause chemical laryngitis; and angiotensin-converting enzyme inhibitors can produce cough followed by dysphonia. Antihistamine, diuretic, and anticholinergic agents have a drying effect on the laryngopharyngeal mucosa.8 Danazol and testosterone can alter sex hormone production or use, or both, causing dysphonia. Of importance, the widely used classes of inhaled medications, in particular inhaled corticosteroids (ICS), can cause dysphonia, most likely via a myopathy or mucosal effect on the laryngopharynx (Figure).
FIGURE.
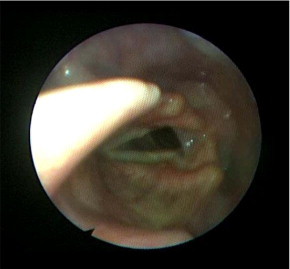
Laryngoscopic image in a patient with asthma who recently started inhaled corticosteroid therapy and reported dysphonia. Note posterior bowing of the vocal cords. After performing the work-up for dysphonia, we concluded that this change was due to inhaled corticosteroid therapy.
It is well known that ICS therapy is the cornerstone of long-term treatment of asthma. However, ICS can cause systemic and local adverse effects. In addition to the widely studied systemic adverse effects of corticosteroids, dysphonia, oropharyngeal candidiasis, and pharyngitis are the most common local adverse effects related to ICS therapy. Therefore, clinicians must consider the risks and benefits of this treatment.
In this article, we review the literature pertaining to the incidence of dysphonia associated with ICS therapy, the potential mechanisms of the disorder, and relationships between dysphonia development and ICS dosage or administration devices. In addition, we provide some practical recommendations for patients using ICS.
Incidence of Dysphonia
Inhaled corticosteroid therapy has become the mainstay treatment of bronchial asthma.9 One of the most common local adverse effects of ICS therapy is dysphonia, with an incidence that reportedly varies from 5% to 58%. In 1974, Williams et al10 described an incidence of transient dysphonia in 55% of patients who received treatment using triamcinolone acetonide aerosol. In 1993, Dong et al11 reported that 8 of 100 patients with asthma who received beclomethasone propionate had dysphonia. In 1995, Williamson et al12 reported dysphonia in 58% of patients using pressurized aerosol ICS preparations, compared with 13% of controls. In 2000, Lavy et al13 confirmed dysphonia via videostrobolaryngoscopy in 58% of patients who were receiving ICS therapy. More recently, Rachelefsky et al14 analyzed data from 23 studies published from 1966 through 2004 and determined that, compared with placebo, ICS at all dosages was associated with a 5.2-fold greater risk of dysphonia.
The wide range of incidence rates in these studies likely was due to methodologic factors, dosage variability, and how the diagnosis was made, which included self-reported questionnaires, telephone surveys, clinical diagnoses, endoscopic examinations, and histologic analyses of biopsy samples. Bearing in mind the limitations of the described studies, we believe that dysphonia caused by ICS therapy is not rare and should be considered a frequent cause of dysphonia.
Mechanisms of Dysphonia
Causes of dysphonia associated with ICS therapy have been poorly investigated, and the origins of dysphonia may have multiple confounding factors. Patients with asthma tend to have longer pauses between speech segments, pronounce fewer syllables per breath, and require more time in nonspeech ventilatory activity.15 In 1983, Williams et al15 identified bowing in the vocal folds and proposed a possible association between dysphonia and ICS therapy. Subsequently, they postulated that this bowing was due to a bilateral adductor myopathy induced by local deposition of topical corticosteroids. Although Babu and Samuel16 in 1988 reported similar findings as those of Williams et al,15 in other studies, vocal cord bowing was rarely found.17
Lavy et al13 described 22 patients with ICS-associated dysphonia who underwent videostrobolaryngoscopy and an objective acoustic analysis. Seventeen of the 22 were affected by dysphonia on a daily basis: 9 had some evidence of poor vocal fold apposition, and supraglottic hyperfunction was identified in 8. Mucosal quality abnormalities were identified in 13 patients. The mucosal wave was difficult to evaluate; however, the authors identified 2 patients with mucosal wave asynchrony. Insofar as speech evaluation, they noted cycle-to-cycle irregularity of 39% (mean, range between 2%-94%), and the maximum phonation time was reduced in 16 patients. The individual analysis found no correlation between the degree of vocal cord apposition and the speech evaluation findings. The authors suggested that atrophy with bowing was not likely the primary cause of dysphonia. Although they noted that the primary cause was difficult to establish because of the various findings, they maintained that corticosteroids had a direct effect on the mucosa or on the mucus-secreting glands of the ventricles or the trachea.
Some studies have found that ICS use predisposes to development of an inflammatory infiltrate; however, this does not necessarily have a clinical correlate. In a prospective observational study of 50 patients,18 more inflammatory infiltrate was identified in ICS users compared with nonusers; however, pharyngeal erythema was not correlated with an inflammatory infiltrate.
Dysphonia associated with ICS use likely results from deposition of active ICS in the oropharynx during administration of the medication. However, taking into account the studies discussed earlier, we believe that a specific cause or mechanism of this disorder has not yet been elucidated.
Dosage and Delivery of ICS
Budesonide, beclomethasone, and fluticasone are associated with similar rates of dysphonia as an adverse effect, although initial reports have suggested a higher risk of dysphonia with fluticasone propionate than with beclomethasone19 or budesonide.20 In another study, treatment with 200 μg of mometasone administered using a metered-dose inhaler (MDI) was associated with fewer instances of dysphonia than was 168 μg of MDI-administered budesonide.21 However, a more recent meta-analysis of randomized controlled trials demonstrated that budesonide was associated with the highest risk of dysphonia when compared with beclomethasone and fluticasone. When comparing ICSs at high dosages, budesonide was associated with the greatest risk of dysphonia.14 The novel synthetic ICS ciclesonide has demonstrated no large differences in occurrence of dysphonia as an adverse effect when compared with placebo. However, further research is needed to establish the probably lower risk of dysphonia between ICSs.22
Dysphonia may be affected by the method used to administer medication. Selroos et al23 described 154 patients who received ICS therapy for 2 years via an MDI and then switched to administration via a dry-powder inhaler (DPI). They noted that the frequency of dysphonia decreased from 21% to 6%. This change may be attributable to differences in vocal cord positioning when using a DPI compared with an MDI. The meta-analysis of randomized controlled trials by Rachelefsky et al14 found that ICS MDI devices were associated with a 5-fold greater risk of dysphonia when compared with placebo MDI devices, whereas the ICS DPI devices had a 3-fold greater risk vs placebo DPI devices. Insofar as dysphonia in patients receiving combined corticosteroid and bronchodilator therapy, Mirza et al24 described voice and laryngeal changes in 5 patients who switched from corticosteroid and bronchodilator therapy administered separately to concurrent administration. Most patients had areas of hyperemia and a plaque pattern on the surface mucosa. The combination therapy was stopped to assess reversibility of the mucosal lesions. Twelve weeks after stopping the combination therapy, patients underwent a laryngeal examination; 3 showed substantial improvement in lesions, and 2 seemed to have complete recovery. More recently, a systematic review of randomized controlled trials by Frois et al25 found no differences in dysphonia or other local adverse effects when comparing fluticasone or budesonide combined with long-term bronchodilator therapy.
Conclusion and Recommendations
Inhaled corticosteroids are associated with an increased occurrence of dysphonia. Any evaluation of dysphonia should consider use of ICS or other medications as an adverse effect, include a thorough examination of the larynx, and rule out vocal cord nodules, posttussive trauma, and gastroesophageal reflux. Well-designed studies, particularly prospective studies, must be conducted to identify clinical, endoscopic, functional, and histopathologic correlates of ICS-associated dysphonia.
Because ICS therapy is the cornerstone of asthma treatment, we recommend using the lowest effective dosage of ICS and administering it with a spacer to decrease oropharyngeal deposition of inhaled aerosols. After using a spacer, it must be washed with tap water and allowed to air dry. Patients should be instructed to rinse the mouth, gargle, and wash the face after inhalation. Instructions should be provided on the proper method of inhalation. Some of these recommendations are pragmatic expert opinions that need future research (Table). If dysphonia develops despite these interventions, ICS use should be suspended until symptoms resolve, provided that asthma control is not compromised.
TABLE.
Practical Recommendations to Reduce Dysphonia Caused by Inhaled Corticosteroids
| Use the lowest dosage of inhaled corticosteroid that maintains asthma control |
| Use a spacer, wash it with tap water, and allow it to air dry after use |
| Rinse the mouth, gargle, and wash the face after inhalation |
| Instruct patients in the proper method of inhalation |
References
- 1.Schwartz S.R., Cohen S.M., Dailey S.H. Clinical practice guideline: hoarseness (dysphonia) Otolaryngol Head Neck Surg. 2009;141(3, suppl 2):S1–S31. doi: 10.1016/j.otohns.2009.06.744. [DOI] [PubMed] [Google Scholar]
- 2.Angelillo N., Di Costanzo B., Angelillo M., Costa G., Barillari M.R., Barillari U. Epidemiological study on vocal disorders in paediatric age. J Prev Med Hyg. 2008;49(1):1–5. [PubMed] [Google Scholar]
- 3.Roy N., Merrill R.M., Gray S.D., Smith E.M. Voice disorders in the general population: prevalence, risk factors, and occupational impact. Laryngoscope. 2005;115(11):1988–1995. doi: 10.1097/01.mlg.0000179174.32345.41. [DOI] [PubMed] [Google Scholar]
- 4.Titze I.R., Lemke J., Montequin D. Populations in the U.S. workforce who rely on voice as a primary tool of trade: a preliminary report. J Voice. 1997;11(3):254–259. doi: 10.1016/s0892-1997(97)80002-1. [DOI] [PubMed] [Google Scholar]
- 5.Jones K., Sigmon J., Hock L., Nelson E., Sullivan M., Ogren F. Prevalence and risk factors for voice problems among telemarketers. Arch Otolaryngol Head Neck Surg. 2002;128(5):571–577. doi: 10.1001/archotol.128.5.571. [DOI] [PubMed] [Google Scholar]
- 6.Ramig L.O., Verdolini K. Treatment efficacy: voice disorders. J Speech Lang Hear Res. 1998;41(1):S101–S116. doi: 10.1044/jslhr.4101.s101. [DOI] [PubMed] [Google Scholar]
- 7.Kerr H.D., Kwaselow A. Vocal cord hematomas complicating anticoagulant therapy. Ann Emerg Med. 1984;13(7):552–553. doi: 10.1016/s0196-0644(84)80530-2. [DOI] [PubMed] [Google Scholar]
- 8.Verdolini K., Titze I.R., Fennell A. Dependence of phonatory effort on hydration level. J Speech Hear Res. 1994;37(5):1001–1007. doi: 10.1044/jshr.3705.1001. [DOI] [PubMed] [Google Scholar]
- 9.Adams N.P., Bestall J.C., Lasserson T.J., Jones P., Cates C.J. Fluticasone versus placebo for chronic asthma in adults and children [update of Cochrane Database Syst Rev. 2005;(4):CD003135] Cochrane Database Syst Rev. 2008;(4) doi: 10.1002/14651858.CD003135.pub3. CD003534. [DOI] [PubMed] [Google Scholar]
- 10.Williams M.L.T., Jr, Kane C., Shim C.S. Treatment of asthma with triamcinolone acetonide delivered by aerosol. Am Rev Respir Dis. 1974;109(5):538–543. doi: 10.1164/arrd.1974.109.5.538. [DOI] [PubMed] [Google Scholar]
- 11.Dong J.C., Shen Z.Y., Wang W.J. The investigation on 100 bronchial asthma and asthmatic bronchitis cases treated with high dose beclomethasone dipropionate aerosol [in Chinese] Zonghua Jie He He Hu Xi Za Zhi. 1993;16(1):33–35. 63. [PubMed] [Google Scholar]
- 12.Williamson I.J., Matusiewicz S.P., Brown P.H., Greening A.P., Crompton G.K. Frequency of voice problems and cough in patients using pressurized aerosol inhaled steroid preparations. Eur Respir J. 1995;8(4):590–592. [PubMed] [Google Scholar]
- 13.Lavy J.A., Wood G., Rubin J.S., Harries M. Dysphonia associated with inhaled steroids. J Voice. 2000;14(4):581–588. doi: 10.1016/s0892-1997(00)80014-4. [DOI] [PubMed] [Google Scholar]
- 14.Rachelefsky G.S., Liao Y., Faruqi R. Impact of inhaled corticosteroid–induced oropharyngeal adverse events: results from a meta-analysis. Ann Allergy Asthma Immunol. 2007;98(3):225–238. doi: 10.1016/S1081-1206(10)60711-9. [DOI] [PubMed] [Google Scholar]
- 15.Williams A.J., Baghat M.S., Stableforth D.E., Cayton R.M., Shenoi P.M., Skinner C. Dysphonia caused by inhaled steroids: recognition of a characteristic laryngeal abnormality. Thorax. 1983;38(11):813–821. doi: 10.1136/thx.38.11.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babu S., Samuel P. The effect of inhaled steroids on the upper respiratory tract. J Laryngol Otol. 1988;102(7):592–594. doi: 10.1017/s0022215100105808. [DOI] [PubMed] [Google Scholar]
- 17.Crompton G.K., Sanderson R., Dewar M.H. Comparison of Pulmicort pMDI plus Nebuhaler and Pulmicort Turbuhaler in asthmatic patients with dysphonia. Respir Med. 2000;94(5):448–453. doi: 10.1053/rmed.1999.0762. [DOI] [PubMed] [Google Scholar]
- 18.Bhalla R.K., Taylor W., Jones A.S., Roland N.J. The inflammation produced by corticosteroid inhalers in the pharynx in asthmatics. Clin Otolaryngol. 2008;33(6):581–586. doi: 10.1111/j.1749-4486.2008.01837.x. [DOI] [PubMed] [Google Scholar]
- 19.Fabbri L., Burge P.S., Croonenborgh L., International Study Group Comparison of fluticasone propionate with beclomethasone dipropionate in moderate to severe asthma treated for one year. Thorax. 1993;48(8):817–823. doi: 10.1136/thx.48.8.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayres J.G., Bateman E.D., Lundbäck B., Harris T.A., International Study Group High dose fluticasone propionate, 1 mg daily, versus fluticasone propionate, 2 mg daily, or budesonide, 1.6 mg daily, in patients with chronic severe asthma. Eur Respir J. 1995;8(4):579–586. [PubMed] [Google Scholar]
- 21.Chervinsky P., Nelson H.S., Bernstein D.I., Berkowitz R.A., Siegel S.C. Comparison of mometasone furoate administered by metered dose inhaler with beclomethasone dipropionate. Int J Clin Pract. 2002;56(6):419–425. [PubMed] [Google Scholar]
- 22.Adachi M., Ishihara K., Inohue H. Efficacy and safety of once-daily inhaled ciclesonide in adults with mild to moderate asthma: a double-blind, placebo-controlled study. Respirology. 2007;12(4):566–572. doi: 10.1111/j.1440-1843.2007.01111.x. [DOI] [PubMed] [Google Scholar]
- 23.Selroos O., Backman R., Forsén K.O. Local side-effects during 4-year treatment with inhaled corticosteroids: a comparison between pressurized metered-dose inhalers and Turbuhaler. Allergy. 1994;49(10):888–890. doi: 10.1111/j.1398-9995.1994.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 24.Mirza N., Kasper Schwartz S., Antin-Oserkis D. Laryngeal findings in users of combination corticosteroid and bronchodilator therapy. Laryngoscope. 2004;114(9):1566–1569. doi: 10.1097/00005537-200409000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Frois C., Wu E.Q., Ray S., Colice G.L. Inhaled corticosteroids or long-acting beta-agonists alone or in fixed-dose combinations in asthma treatment: a systematic review of fluticasone/budesonide and formoterol/salmeterol. Clin Ther. 2009;31(12):2779–2803. doi: 10.1016/j.clinthera.2009.12.021. [DOI] [PubMed] [Google Scholar]


