Abstract
The concept that the two photosystems of photosynthesis cooperate in series, immortalized in Hill and Bendall's Z scheme, was still a black box that defined neither the structural nor the molecular organization of the thylakoid membrane network into grana and stroma thylakoids. The differentiation of the continuous thylakoid membrane into stacked grana thylakoids interconnected by single stroma thylakoids is a morphological reflection of the non-random distribution of photosystem II/light-harvesting complex of photosystem II, photosystem I and ATP synthase, which became known as lateral heterogeneity.
Keywords: grana, lateral heterogeneity, plant thylakoids, photosystem II, stroma thylakoids
Few phenomena in natural science equal photosynthesis in sweep and grandeur. Martin Kamen [1, p. 1]
1. Introduction
My scientific odyssey with the molecular organization of plant thylakoid membranes began when I came to Canberra in 1961 to work on photosynthesis, appropriately in the Division of Plant Industry, CSIRO. I was inspired by reading Robin Hill and Faye Bendall's famous ‘hypothesis in barest outline’ that is summarized thus: ‘if the cytochrome in the chloroplasts is directly involved in hydrogen (or electron transfer), the system must require more than one light-driven reaction to act in opposition to the thermal gradient’ (figure 1) [2]. Recognition of their hypothesis became the Z scheme that was further refined as additional components were discovered and characterized, but the structure, composition and function of the photosynthetic apparatus were still almost a black box. Neither the Z scheme nor its precursor, the photosynthetic unit, defined either the structural or spatial organization of the photosystems within thylakoids. My challenge then was twofold: (i) to determine whether there were indeed two discrete photosystems as implied by Hill and Bendall's hypothesis and (ii) to ascertain whether these photosystems were associated with the fascinating stacked and/or non-stacked thylakoid membrane domains of higher plants. Explanation of ‘the complicated and visible with the simple and invisible’ is particularly challenging for the thylakoid membranes of higher plant chloroplasts which form an elaborate continuous three-dimensional network comprised of regular cylindrical, tightly stacked membranes, the grana thylakoids that are interconnected with single stroma thylakoids whose outer membrane surface face the stroma.
Figure 1.

Robin and Priscilla Hill in Canberra in 1973.
My first approach to prove the reality of two photosystems was to follow the development during the greening of etiolated bean leaves. Naively, Keith Boardman and I hoped that photosystem I (PSI) might have been formed before PSII during chloroplast development, thereby allowing us to characterize the properties of one photosystem. It was impossible. Following the removal of contaminating mitochondria from isolated bean etioplasts and greening chloroplasts, all photochemical activities were vanishingly low. Although PSI activity appeared before oxygen evolution, we had no idea if this were so in leaves.
Our next strategy involved the non-ionic detergent, digitonin, to fragment spinach chloroplasts derived from hydroponically grown plants from which functional chloroplasts had been obtained overseas. Digitonin fragmentation of isolated thylakoids followed by differential centrifugation yielded a heavy fraction enriched in Chl b and PSII activity, whereas the lighter fraction was highly enriched in PSI activity, P700, cyt b6 and cyt f [3,4]. With the arrival of a Cary model 14R spectrophotometer and a Plant Industry workshop copy of the Bonner type cuvette assembly, I determined the cytochrome difference spectra at 20°C and 77 K in the digitonin fractions. My isolation and characterization of a third cytochrome cyt b type, the enigmatic cyt b559, glimpsed in a hand spectrograph by Lundegårdh [5], was especially pleasing; it was tightly bound within the PSII-enriched fraction and clearly an integral component of PSII, whereas cyt b6 and cyt f were located mainly in the PSI fraction [6]. Fortunately, John Thorne, a former naval radio engineer, built a home-made spectrofluorometer capable of correcting for the wavelength-dependence of instrumental response, so we were able to characterize the fluorescence properties of the PSII-enriched and PSI fragments at 20°C and 77 K. Most of the chlorophyll fluorescence at 20°C was emitted by PSII with Chl b contributing to both PSII-enriched and PSI-enriched fractions [7]. Thus, this first partial separation of the photosystems proved there were indeed two photosystems.
2. Lateral heterogeneity of plant thylakoid complexes
(a). Proteins and lipids in lively membranes
Although there was almost immediate recognition of two sequential light reactions, understanding of the structural organization and composition of the components both across and along the thylakoids was very meagre until the 1970s. This was due to ignorance not only of the detailed composition and molecular structure of thylakoid components but, more importantly, of a credible model for membrane architecture. The generalized ‘sandwich membrane model’, proposed in 1925 by Gorter and Grende and widely accepted before 1970 had the phospholipids arranged in a continuous bilayer with their acyl chains occupying the interior membrane core, while the proteins were spread out on both sides of the membranes blanketing the polar phospholipid head groups (reviewed in Singer [8]). Fortunately, the concepts of protein arrangement within lipid bilayers introduced by Singer [8] led Singer & Nicolson [9] to formulate the fluid protein–lipid mosaic model where the membrane consisted of a lipid bilayer into which membrane spanning intrinsic protein complexes are embedded and to which extrinsic proteins are attached.
My greatest serendipitous opportunity coincided with my arrival in Cambridge in 1973. Owing to the coalminers' strike, electricity was severely rationed, and research in Derek Bendall's laboratory became impossible for months. Luckily, I was able to retire to a perfect ivory tower, my attic room in the Pightle, at Newnham College. With the luxury of time to think, and inspired by the brilliant fluid mosaic model of cell membrane structure [9], I dreamt that thylakoid multiprotein complexes spanned the entire thylakoid membrane and danced in the lipid bilayer in the light. My review [10], replete with speculations galore, discussed thylakoid membranes in terms of the fluid protein–lipid mosaic model [9]. It was clear that the astonishing economy of locating molecules asymmetrically across thin membranes imparts order by removing random three-dimensional movement, thereby allowing a spectacular enhancement of effective concentration of the many pigment molecules so exquisitely ordered within their proteins to regulate light harvesting and energy dissipation [10]. ‘This organization allows for rapid changes in the conformation and the distribution of the macromolecular complexes which are essential for the function and structure of the intricate chloroplast membrane’ [10, p. 193].
Although the molecular structure of the thylakoid protein complexes was still very limited, a new era for molecular organization arrived. Trebst [11] focused on the need for vectorial electron and proton transport across the membrane, whereas Anderson [10] assigned chloroplast components within the lipid–protein mosaic membrane model: both emphasized an asymmetry of function along thylakoid membranes. The elegant structural studies of Staehelin and co-workers [12] definitively revealed that ATP synthase with its large head group and possibly PSI were located only in unstacked stroma thylakoids. Slowly, the black box was being opened.
(b). Green gels and aqueous polymer two-phase liquid partition
Photosynthesis researchers have always been intrigued by the way the chlorophylls and carotenoids are arranged within photosynthetic membranes [13]. Although investigators, beginning with Emil Smith in 1941, had used detergents or solvents to disrupt thylakoids and isolate pigment-proteins, the idea still persisted that not all the chlorophyll and carotenoid molecules were non-covalently bound to proteins. In 1966, Phillip Thornber separated two chlorophyll-proteins using SDS–PAGE; by 1975, at least 60–75% of chlorophyll had been resolved as Chl-proteins by green gels [13]. Finally, my extensive struggles to improve green non-denaturing gel procedures were rewarded; over 90 per cent of both chlorophylls and carotenoids were indeed associated with six different Chl-protein complexes [14].
Again, the stage was set for a wonderful surprise and serendipity intervened. Famously, Lawrence of Arabia lost his draft of ‘Seven Pillars of Wisdom’ at the Reading railway station but I found a wonderful colleague there who helped change the way we think about the molecular organization of plant thylakoids. After an otherwise rather dull International Congress of Photosynthesis in 1977, a young Swede, Bertil Andersson bounded along the platform and declared that he was coming to Australia to work with me when he had finished his PhD thesis! Astonished, I declared that I had no money for his support and could offer only laboratory space and facilities; undaunted, Bertil replied he would secure funds. By gaining an EMBO Fellowship, Bertil eventually convinced the EMBO Chairman that his Fellowship had to be held in Canberra which was ‘anyway close to Europe’. Significantly, using the aqueous two-phase polymer partition method of Albertsson [15], Bertil had partitioned a granal fraction into right-side-out and inside-out vesicles derived from non-appressed and appressed membranes, respectively [16]. Although PSII activity was greatly enriched in the appressed granal fraction, quantitation of PSII and PSI was hampered by inactivation of photochemical activities incurred by thylakoid fragmentation [17].
(c). Partitioning photosystem II and photosystem I
Bertil's and my joint aim was to compare the content of the main chlorophyll-protein complexes of the photosynthetic apparatus resolved by my improved ‘green gel’ method, using the membrane fractionation method of Albertsson [15]. Having no equipment to shake the two-phase fractions in Canberra Bertil Andersson spent many hours in the Canberra cold room (figure 2). Following Yeda-press fragmentation of thylakoids, stroma thylakoids (Y-100) were separated from granal stacks (Y-40) by differential centrifugation, and enriched inside-out vesicles (B3) were isolated by aqueous polymer two-phase partition of the granal fraction (Y-40 fraction). Stroma thylakoid fractions were highly enriched in PSI complex together with some 10–20% of PSII and light-harvesting complex of photosystem II (LHCII) complexes [18]. By contrast, the grana-appressed vesicles were substantially depleted in PSI complex and enriched in PSII and LHCII (figure 3) [18]. Allowing for the contamination of some right-side-out vesicles in the appressed inside-out vesicles, we proposed that PSI is exclusively restricted to non-appressed granal domains, comprising the grana end membranes, grana margins and stroma thylakoids [18]. Thus, a lateral heterogeneity of distribution of the photosystems exists with PSI exclusively in stroma-exposed thylakoid domains and PSII/LHCII supercomplexes mainly, but not exclusively located in appressed membranes [18]. We were delighted that our paper was speedily accepted for Biochimica et Biophysica Acta, then the most prestigious journal for photosynthesis research [18]. Also, molecular organization was included for the first time at the splendid Fifth International Photosynthesis Congress at Halkadiki, 1980, attracting over 60 posters. To our astonishment and frustration, however, our concept of lateral heterogeneity presented by a symposium lecture and poster was met with horror or ignored. Neither Bertil nor I were allowed to speak at two relevant Discussion series: an English chairman introduced one session by stating that there were two crazy ideas, fit only for the waste paper basket which would not be discussed: Andersson & Anderson's lateral heterogeneity [18] and Dan Arnon's concept [19] of the photosystems operating in parallel rather than in series, with PSII alone driving linear electron transport from water to ferredoxin and NADP, without the collaboration of PSI, whose role was limited to cyclic electron transport and cyclic phosphorylation: Dan adopted his new concept for life [19]. In 1980, Barber [20] recognized that a lateral separation of some PSI from PSII in the grana would explain his observations on the relationship between salt-induced changes in spillover from PSII to PSI; his proposed extent of lateral heterogeneity of the photosystems was not as extreme as we had demonstrated [18].
Figure 2.

Bertil Andersson as usual in the Plant Industry cold room in 1979.
Figure 3.
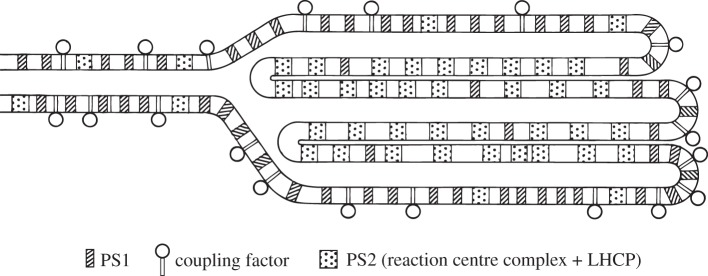
The original schematic of the lateral heterogeneity in the distribution of PSII, PSI and ATP synthase between appressed and non-appressed thylakoid of plant chloroplasts [18].
Our figure for the lateral distribution of the thylakoid protein complexes (figure 3) should have been termed a schematic model or cartoon; no mention was made of it in the text as a possible three-dimensional model of the thylakoid membrane network and that was not intended [18]. Cytochrome b6f complex was not placed in the original model [18]. Separate proof of the uniform distribution of cyt b6f complexes in both stacked and unstacked regions followed extensive quantitation of cyts f and b6 [21,22]. We assigned the task of long-range transport of electrons between PSII and PSI to plastocyanin rather than plastoquinone (figure 4) [23].
Figure 4.
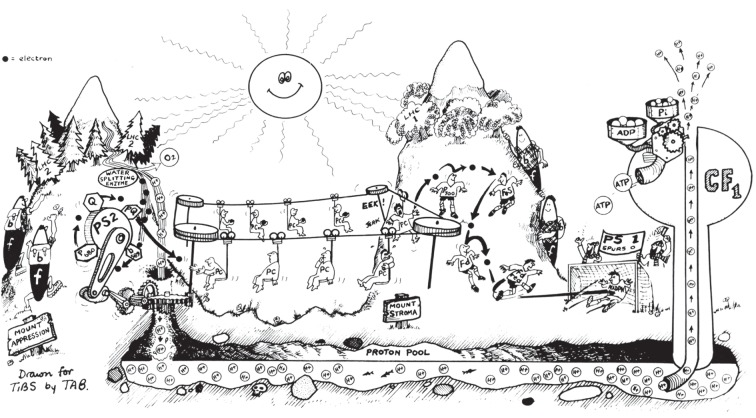
Cartoon drawn by TAB of Trends Biochem. Sci. (inspired by Bertil Andersson after visiting Queenstown, New Zealand, where J.M.A. grew up) depicting the lateral separation of PSII/LHCII on Mt Appression from PSI of Mount Stroma, while mountaineering cyt b6f complexes receive electrons from PSII and laterally transport them to PSI then return back empty-handed for more [23].
(d). Why was the concept of lateral heterogeneity such a heretical idea?
Prior to the 1980s, it was assumed that most PSII and LHCII were located in granal stacks, yet evidence that PSI was excluded from the granal-stacked domain was initially a heretical idea. Two extreme models for the regulation of light excitation energy distribution between the photosystems were in vogue in the 1970s: continuous array versus separate packages. Continuous array models were strongly favoured with a common light-harvesting antenna (LHCII) being shared between PSII and PSI (earlier studies [24,25] contain references for earlier models), whereas Boardman et al. [26] proposed a separate package model with limited interaction between the two photosystems to distribute some excitation energy between them. Part of the difficulty of accepting lateral heterogeneity of the photosystems was the notion that PSI did not have its own Chl b. However, noting that the LHCP/PSII ratios were similar in appressed and non-appressed subchloroplast fragments, Andersson & Anderson [18] suggested that it was more reasonable to assume a close structural linkage of LHCII with PSII rather than it being shared between the photosystems. Mullet et al. [27] first showed that ‘native’ PSI had its own antenna Chl-proteins, although they suggested that the low Chl b content present was due to contamination with LHCII. Later Chl b was shown to be an integral component of PSI, when a Chl a/b-protein was first isolated from Chlamydomonas [28] and then several Chl a/b-proteins were isolated from spinach [29].
Challenged by early scepticism about the lateral heterogeneity of thylakoid protein complexes for the functional and structural consequences of excluding PSI and ATP synthase from stacked granal domains that store the large PSII/LHCII supercomplexes, I focused early attention on the significance of grana stacking [23,30–34]. Fortunately, many others joined the challenge and the 1980s heralded a decade of excitement concerning ‘why grana?’ which is still continuing.
Acknowledgements
This paper is dedicated to my wonderful colleague and friend, Bertil Andersson, whose boundless curiosity and passion has brightened my ‘why grana?’ journey. I greatly appreciate the splendid meeting at the Royal Society at Chicheley Hall, hosted by my friends, Jim Barber and Peter Horton, which gave me the opportunity to meet so many colleagues and friends. I also thank Keith Boardman, my early mentor.
References
- 1.Kamen M. K. 1963. Primary-processes in photosynthesis. New York, NY: Academic Press [Google Scholar]
- 2.Hill R., Bendall F. 1960. Function of the two cytochrome components in chloroplasts: a working hypothesis. Nature 186, 136–137 10.1038/186136a0 (doi:10.1038/186136a0) [DOI] [Google Scholar]
- 3.Boardman N. K., Anderson J. M. 1964. Isolation from spinach chloroplasts of particles containing different proportions of chlorophyll a and chlorophyll b and their possible role in the light reactions of photosynthesis. Nature 203, 166–167 10.1038/203166a0 (doi:10.1038/203166a0) [DOI] [Google Scholar]
- 4.Anderson J. M., Boardman N. K. 1966. Fractionation of photochemical systems of photosynthesis. I. Chlorophyll contents and photochemical activities of particles isoltated from spinach chloroplasts. Biochim. Biophys. Acta 112, 403–421 10.1016/0926-6585(66)90244-5 (doi:10.1016/0926-6585(66)90244-5) [DOI] [PubMed] [Google Scholar]
- 5.Lundegårdh H. 1961. Response of chloroplast cytochromes to light and substrates. Nature 192, 243–248 10.1038/192243a0 (doi:10.1038/192243a0) [DOI] [PubMed] [Google Scholar]
- 6.Boardman N. K., Anderson J. M. 1967. Fractionation of photochemical systems of photosynthesis II. Cytochrome and carotenoid contents of particles isolated from spinach chloroplasts. Biochim. Biophys. Acta 143, 187–203 10.1016/0005-2728(67)90120-X (doi:10.1016/0005-2728(67)90120-X) [DOI] [PubMed] [Google Scholar]
- 7.Boardman N. K., Thorne S. W., Anderson J. M. 1966. Fluorescence properties of particles obtained by digitonin fragmentation of spinach chloroplasts. Proc. Natl Acad. Sci. USA 56, 586–593 10.1073/pnas.56.2.586 (doi:10.1073/pnas.56.2.586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer S. J. 1971. The molecular organization of biological membranes. In Structure and function of biological membranes (ed. L. I. Rothfield). New York, NY: Academic Press [Google Scholar]
- 9.Singer S. J., Nicolson G. L. 1972. Fluid mosaic model of structure of cell membranes. Science 175, 720–731 10.1126/science.175.4023.720 (doi:10.1126/science.175.4023.720) [DOI] [PubMed] [Google Scholar]
- 10.Anderson J. M. 1975. Molecular organization of chloroplast thylakoids. Biochim. Biophys. Acta 416, 191–235 10.1016/0304-4173(75)90007-5 (doi:10.1016/0304-4173(75)90007-5) [DOI] [PubMed] [Google Scholar]
- 11.Trebst A. 1974. Energy conservation in photosynthetic electron-transport of chloroplasts. Annu. Rev. Plant Physiol. 25, 423–458 10.1146/annurev.pp.25.060174.002231 (doi:10.1146/annurev.pp.25.060174.002231) [DOI] [Google Scholar]
- 12.Miller K. R., Staehelin L. A. 1976. Analysis of the thylakoid outer surface. Coupling factor is limited to unstacked membrane regions. J. Cell Biol. 68, 30–47 10.1083/jcb.68.1.30 (doi:10.1083/jcb.68.1.30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thornber J. P. 1975. Chlorophyll proteins: light-harvesting and reaction center components of plants. Annu. Rev. Plant Physiol. 26, 127–158 10.1146/annurev.pp.26.060175.001015 (doi:10.1146/annurev.pp.26.060175.001015) [DOI] [Google Scholar]
- 14.Anderson J. M., Waldron J. C., Thorne S. W. 1978. Chlorophyll-protein complexes of spinach and barley thylakoids: spectral characterization of 6 complexes resolved by an improved electrophoretic procedure. FEBS Lett. 92, 227–233 10.1016/0014-5793(78)80760-1 (doi:10.1016/0014-5793(78)80760-1) [DOI] [Google Scholar]
- 15.Albertsson P.-Å. 1971. Partition of cell particles and macromolecules. New York, NY: Wiley-Interscience [Google Scholar]
- 16.Andersson B. 1978. Separation of spinach chloroplast lamellae fragments by phase partition including the isolation of inside-out thylakoids. PhD thesis, Lund University, Sweden [Google Scholar]
- 17.Åckerlund H. E., Andersson B., Albertsson P.-Å. 1976. Isolation of photosystem II enriched membrane vesicles from spinach chloroplasts by phase partition. Biochim. Biophys. Acta 449, 525–535 10.1016/0005-2728(76)90161-4 (doi:10.1016/0005-2728(76)90161-4) [DOI] [PubMed] [Google Scholar]
- 18.Andersson B., Anderson J. M. 1980. Lateral heterogeneity in the distribution of chlorophyll-protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim. Biophys. Acta 593, 427–440 10.1016/0005-2728(80)90078-X (doi:10.1016/0005-2728(80)90078-X) [DOI] [PubMed] [Google Scholar]
- 19.Arnon D. I., Tsujimoto H. Y., Tang G. M. S. 1981. Proton transport in photo-oxidation of water: a new perspective on photosynthesis. Proc. Natl Acad. Sci. USA 78, 2942–2946 10.1073/pnas.78.5.2942 (doi:10.1073/pnas.78.5.2942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barber J. 1980. An explanation for the relationship between salt-induced thylakoid stacking and the chlorophyll fluorescence changes in spillover of energy from photosystem I to photosystem II. FEBS Lett. 118, 1–10 10.1016/0014-5793(80)81207-5 (doi:10.1016/0014-5793(80)81207-5) [DOI] [Google Scholar]
- 21.Cox R. P., Andersson B. 1981. Lateral and transverse organisation of cytochromes in the chloroplast thylakoid membrane. Biochem. Biophys. Res. Commun. 103, 1336–1342 10.1016/0006-291X(81)90269-2 (doi:10.1016/0006-291X(81)90269-2) [DOI] [PubMed] [Google Scholar]
- 22.Anderson J. M. 1982. Distribution of the cytochromes of spinach chloroplasts between the appressed membranes of grana stacks and stroma-exposed thylakoid regions. FEBS Lett. 138, 62–66 10.1016/0014-5793(82)80395-5 (doi:10.1016/0014-5793(82)80395-5) [DOI] [Google Scholar]
- 23.Anderson J. M., Andersson B. 1982. The architecture of photosynthetic membranes: lateral and transverse organization. Trends Biochem. Sci. 7, 288–292 10.1016/0968-0004(82)90014-7 (doi:10.1016/0968-0004(82)90014-7) [DOI] [Google Scholar]
- 24.Thornber J. P., Alberte R. S., Hunter F. A., Shiozawa J. A., Kan K.-S. 1976. The organization of chlorophyll in the plant photosynthetic unit. Brookhaven Symp. Biol. 28, 132–148 [PubMed] [Google Scholar]
- 25.Butler W. L. 1977. Energy distribution in the photosynthetic apparatus of plants. Brookhaven Symp. Biol. 28, 338–346 [PubMed] [Google Scholar]
- 26.Boardman N. K., Anderson J. M., Goodchild D. J. 1978. Chlorophyll–protein complexes and structure of mature and developing chloroplasts. In Current topics in bioenergetics (eds Sanadi D. R., Vernon L. P.), pp. 35–109 New York, NY: Academic Press [Google Scholar]
- 27.Mullet J. E., Burke J. J., Arntzen C. J. 1980. Chlorophyll proteins of photosystem I. Plant Physiol. 65, 814–822 10.1104/pp.65.5.814 (doi:10.1104/pp.65.5.814) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wollman F. A., Bennoun P. 1982. A new chlorophyll-protein complex related to photosystem I in Chlamydomonas reinhardii. Biochim. Biophys. Acta 680, 352–360 10.1016/0005-2728(82)90149-9 (doi:10.1016/0005-2728(82)90149-9) [DOI] [Google Scholar]
- 29.Anderson J. M., Brown J. S., Lam E., Malkin R. 1983. Chlorophyll b: an integral component of photosystem I of higher plant chloroplasts. Photochem. Photobiol. 38, 205–210 10.1111/j.1751-1097.1983.tb03863.x (doi:10.1111/j.1751-1097.1983.tb03863.x) [DOI] [Google Scholar]
- 30.Anderson J. M. 1981. Consequences of spatial separation of photosystem I and photosystem II in the thylakoid membranes of higher plant chloroplasts. FEBS Lett. 124, 1–10 10.1016/0014-5793(81)80041-5 (doi:10.1016/0014-5793(81)80041-5) [DOI] [Google Scholar]
- 31.Anderson J. M. 1982. The significance of grana stacking in chlorophyll b-containing chloroplasts. Photobiochem. Photobiophys. 3, 225–241 [Google Scholar]
- 32.Anderson J. M. 1982. The role of chlorophyll–protein complexes in the function and structure of chloroplast thylakoids. Mol. Cell. Biochem. 46, 161–172 10.1007/BF00239665 (doi:10.1007/BF00239665) [DOI] [PubMed] [Google Scholar]
- 33.Anderson J. M., Andersson B. 1988. The dynamic photosynthetic membrane and regulation of solar energy conversion. Trends Biochem. Sci. 13, 351–355 10.1016/0968-0004(88)90106-5 (doi:10.1016/0968-0004(88)90106-5) [DOI] [PubMed] [Google Scholar]
- 34.Anderson J. M. 1999. Insights into the consequences of grana stacking of thylakoid membranes in vascular plants: a personal perspective. Aust. J. Plant Physiol. 26, 625–329 10.1071/PP99070 (doi:10.1071/PP99070) [DOI] [Google Scholar]