Abstract
Major multi-protein photosynthetic complexes, located in thylakoid membranes, are responsible for the capture of light and its conversion into chemical energy in oxygenic photosynthetic organisms. Although the structures and functions of these photosynthetic complexes have been explored, the molecular mechanisms underlying their assembly remain elusive. In this review, we summarize current knowledge of the regulatory components involved in the assembly of thylakoid membrane protein complexes in photosynthetic organisms. Many of the known regulatory factors are conserved between prokaryotes and eukaryotes, whereas others appear to be newly evolved or to have expanded predominantly in eukaryotes. Their specific features and fundamental differences in cyanobacteria, green algae and land plants are discussed.
Keywords: regulatory factors, biogenesis, assembly, thylakoid membrane protein complex, chloroplast
1. Introduction
Thylakoids are flattened vesicles found in cyanobacteria and chloroplasts that probably arose in photosynthetic bacteria, appearing in close correlation with oxygenic photosynthesis [1]. The most advanced and efficient thylakoids are present in the chloroplasts of land plants, in which they are arranged into extensive, highly interconnected networks of grana stacks connected by stroma lamellae [2]. By contrast, the thylakoids in cyanobacteria consist mainly of single layers formed by long lamellae, and extensive stacking of the grana lamellae is not observed [3]. Thus, thylakoid membrane systems evolved in cyanobacteria but became more complex in algae and plants [2], and the ultrastructure and composition differences of the thylakoids in cyanobacteria, algae and plants correspond well with their evolutionary positions [3].
In the chloroplasts of green algae and plants, four photosynthetic protein complexes are embedded in the thylakoid membranes and are functionally connected in a series through a photosynthetic electron transport chain. These complexes include photosystem II (PSII) and photosystem I (PSI) as well as their associated light-harvesting antennae, the cytochrome b6f complex (cyt b6f), and the proton-translocation ATP synthase (ATPase). In cyanobacteria such as Synechocystis PCC6803, cyt b6f is also a part of the respiratory electron transport chain [4]. Three-dimensional structures of several multimeric protein complexes for oxygenic photosynthesis have been reported at atomic resolution [5]. However, our knowledge of the molecular mechanisms involved in the biogenesis of thylakoid membrane protein complexes is still limited. In addition, PSII, PSI and cyt b6f complexes also contain accessory factors such as chlorophylls and xanthophylls as well as haems, quinones and iron–sulphur centres. The processes of pigment synthesis, transport and final incorporation remain largely unknown.
Genetic and biochemical studies have revealed that multistep assembly processes are responsible for the formation of functional photosynthetic protein complexes. Each assembly step is likely to be regulated by a number of factors [6–12]. In the last few decades, analysis of photosynthetic mutants in oxygenic photosynthetic organisms such as Synechocystis, Chlamydomonas reinhardtii, Arabidopsis thaliana, maize and tobacco has identified a considerable number of regulatory proteins involved in these processes and has provided insights into the molecular mechanisms underlying the assembly of thylakoid membrane protein complexes. Some of these factors appear to be newly evolved or to have proliferated predominantly in plants, whereas others are of eubacterial origin, often having gained new functions. In this review, we provide a brief overview of the present knowledge of regulatory factors involved in the assembly of PSII, PSI and cyt b6f complexes in photosynthetic organisms, particularly in three photosynthetic model organisms: the cyanobacterium Synechocystis PCC6803, the unicellular green alga Chlamydomonas and the vascular plant Arabidopsis (table 1).
Table 1.
Regulatory factors for PSII, PSI and cyt b6f assembly. M, thylakoid membrane; L, thylakoid lumen; S, stroma; —, not identified yet.
| Arabidopsis thaliana | Chlamydomonas reinhardtii | Synechocystis PCC6803 | location | protein domain | references |
|---|---|---|---|---|---|
| HCF136 | Chlredraft_112806 | YCF48 | L | BNR repeat | [13–16] |
| LPA1 | REP27 | — | M | TPR | [17,18] |
| PAM68 | Chlredraft_169505 | Sll0933 | M | DUF3464 | [19] |
| LPA2 | — | — | M | unknown | [20] |
| LPA3 | Chlredraft_183275 | — | M, S | DUF1995 | [21] |
| AtAlb3 | CrAlb3.1, CrAlb3.2 | Slr1471 | M | OxaA/YidC | [21–27] |
| LPA19 | — | Slr1645(Psb27) | L | unknown | [28,29] |
| AT4G28660 | — | Sll1398(Psb28) | M, S | unknown | [30,31] |
| AtPsb29 | — | Sll1414(Psb29) | M, S | unknown | [30,32] |
| FKBP-2 | Chlredraft _2101108 | — | L | PPIase | [33] |
| CYP38 | Chlredraft _196558 | — | L | cyclophilin | [34–36] |
| — | — | Slr0286 | M | unknown | [37] |
| — | — | Slr2013 | M | DUF58 | [38] |
| AtYCF3 | CrYCF3 | Slr0823(YCF3) | M | TPR | [39–41] |
| Y3IP1 | — | — | M | unknown | [42] |
| YCF4 | CrYCF4 | Sll0226(YCF4) | M | unknown | [39,43–45] |
| HCF101 | Chlredraft _102098 | — | S | MRP-like | [46,47] |
| APO1 | — | — | S | DUF794 | [47,48] |
| AT1G54500 | — | RubA | M | Rubredoxin | [49,50] |
| AT1G49380 | CCS1 | Slr2087 | M | ResB | [51–53] |
| HCF164 | CCS5 | — | M | Thioredoxin | [54–56] |
| — | CCS4 | — | M | unknown | [57] |
2. Regulatory factors for PSII assembly
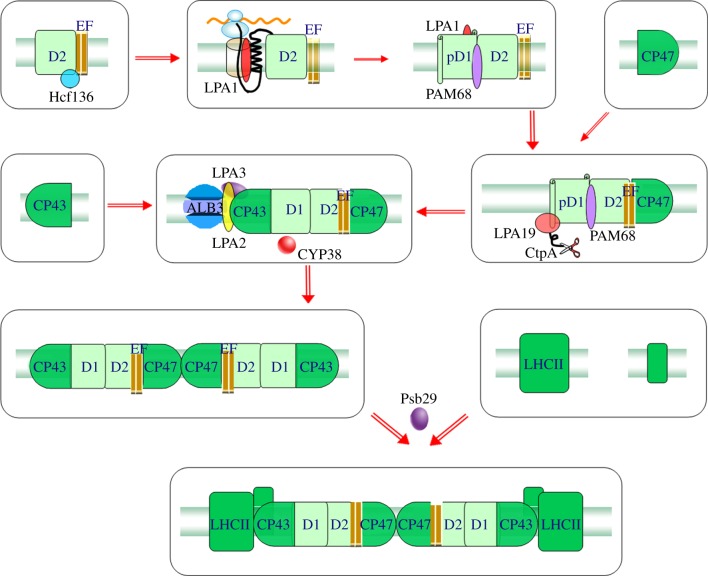
PSII catalyses light-induced electron transfer from water to plastoquinone, with the concomitant production of molecular oxygen. In general, PSII can be described in terms of three major structural domains: (i) the reaction centre consisting of the heterodimeric D1/D2 proteins, the α and β subunits of cytochrome b559 and two low-mass polypeptides PsbI and PsbW; (ii) the inner light-harvesting antenna comprising the chlorophyll-containing proteins CP43 and CP47; and (iii) the oxygen-evolving complexes of extrinsic proteins, including the manganese-stabilizing protein bound on the luminal side of the complex [58,59]. PSII intrinsic membrane proteins are largely conserved between cyanobacteria and higher plants, whereas the extrinsic proteins that form the oxygen-evolving complexes of PSII vary significantly [60]. PSII assembly consists of multiple steps (figure 1), including the formation of an initial complex of the PSII reaction centre, the association of CP47/43 with the PSII reaction centre, the integration of the small subunits and finally the dimerization of PSII monomers [61].
Figure 1.
Model for the assembly of PSII in higher plant. The assembly factors for PSII in this model were shown according to currently reported functions, and their association with PSII is only illustrative. For clarity, the low-molecular-mass subunits and the extrinsic subunits are omitted.
The multi-step formation of functional PSII is likely to be assisted by many nuclear-encoded regulatory proteins. HCF136 (high chlorophyll fluorescence 136), which encodes a hydrophilic protein localized in the lumen of stroma thylakoids, was first identified as being involved in PSII assembly in Arabidopsis through isolation and characterization of high chlorophyll fluorescence mutants [13]. In the absence of HCF136, PSII proteins are synthesized normally but do not assemble into a stable PSII complex [13]. Under standard illumination, the amounts of both PSII and PSI are reduced, suggesting that HCF136 may be required for photosystem biogenesis in general [13]. However, under low light HCF136 selectively regulates the biogenesis of PSII. HCF136 associates with a PSII pre-complex containing at least D2 and cytochrome b559 and is essential for the assembly of the PSII reaction centre [14]. An HCF136 homologue, YCF48 (hypothetical chloroplast open reading frame 48), was identified in the cyanobacterium Synechocystis PCC6803 [15]. Disruption of ycf48 slows the formation of PSII complexes and causes a decrease in the final level of PSII core complexes, similar to the effects of the hcf136 mutation [15]. In addition, the absence of YCF48 leads to a dramatic decrease in the levels of precursor and partially processed D1 protein, and only low levels of unassembled mature D1 are detected. YCF48 is also important for the repair of PSII in Synechocystis [15]. Interestingly, a recessive allele of the maize HCF136 homologue causes a lack of PSII complexes and grana thylakoids in mesophyll chloroplasts, which is consistent with the previously defined function of its Arabidopsis counterpart. However, maize hcf136 is also defective in processing the full-length psbB-psbT-psbH-petB-petD polycistronic RNA specifically in bundle sheath cells [16]. Microarray analysis revealed that mesophyll and bundle sheath cell transcript pools are altered in the hcf136 mutant, but the reason for this has not yet been determined [16].
LPA1 (low PSII accmulation 1) appears to be an integral membrane chaperone that is required for the efficient assembly of PSII reaction centres [17]. In lpa1 Arabidopsis mutants, the assembly of newly synthesized PSII is less efficient; in addition, the turnover rates of the PSII core proteins CP47, CP43, D1 and D2 are higher in lpa1 mutants compared with wild-type plants [17]. LPA1 encodes a chloroplast protein that contains two TPR (tetratricopeptide repeat) domains and interacts with D1, but not with D2, cyt b6 or Alb3 (ALBINO3, see below for details) [17]. LPA1 homologues are present in Ostreococcus and in Chlamydomonas, in which REP27 (repair-aberrant mutant of PSII) is the LPA1 homologue. In contrast to the inactivation of LPA1, inactivation of REP27 does not affect the accumulation of PSII subunits other than the D1 protein [18]. REP27 is specifically required for efficient D1 turnover but is not essential for de novo biogenesis and assembly of PSII [18]. Another PSII assembly factor, PAM68 (photosynthesis affected mutant 68), is associated with an early intermediate complex in Arabidopsis that may contain D1 and LPA1 [19]. PAM68 is a thylakoid membrane protein and, similar to HCF136 and Alb3, it is conserved in cyanobacteria and photosynthetic eukaryotes. This protein acts at the level of D1 maturation and stability, promoting the transition from the reaction centre assembly intermediate to the larger PSII assembly complexes in Arabidopsis [19]. However, removal of PAM68 results in different compensatory mechanisms in plants and cyanobacteria: in Synechocystis the transition from the reaction centre to later PSII intermediates appears to be accelerated when PAM68 is absent [19]. The higher-plant-specific HCF243 chloroplast protein has been implicated in maintaining the stability of the D1 protein and promoting subsequent PSII assembly [62].
LPA2 and LPA3 (low PSII accmulation 2 and 3), two other thylakoid membrane proteins, have overlapping functions in assisting CP43 assembly, and the cooperation between LPA2 and LPA3 is essential for PSII assembly [20,21]. LPA2 encodes an integral membrane protein containing two transmembrane domains. Homologues of LPA2 have been identified in other plants, but not in cyanobacteria or Chlamydomonas [20]. LPA3 does not have any transmembrane domains, and highly conserved LPA3 sequences have been found in plants, Chlamydomonas and Ostreococcus [21]. LPA2 may have evolved after the divergence of the land plant lineage or may have been lost in other lineages. Both LPA2 and LPA3 interact with Alb3. This interaction with Alb3 suggests that the function of Alb3 in some PSII assembly processes is most probably mediated through interactions with LPA2 and LPA3 [21].
Alb3 is related to the Oxa1p and YidC proteins from E. coli. Such proteins are essential components for insertion and assembly of multi-subunit membrane protein complexes. Two Alb3 proteins (Alb3.1 and Alb3.2) are present in Chlamydomonas, whereas only one Alb3 protein is present in Arabidopsis. Alb3.1 of Chlamydomonas is involved mainly in the insertion of the light-harvesting complex [22], and it is required for the insertion of D1 into functional PSII [23]. Alb3.2 interacts with Alb3.1 and the reaction centre polypeptides of PSI and PSII. The amounts of both PSI and PSII were reduced in Alb3.2 RNA interference lines, indicating that the level of Alb3.2 is limiting for the assembly and/or maintenance of these complexes [24]. Thus, it appears that Alb3 is a general assembly factor rather than a PSII-specific assembly factor in Chlamydomonas. Indeed, Alb3 interacts with PSII subunits D1, D2 and CP47, as well as with PSI reaction centre PsaA and ATP synthase subunit CF0III [23–25]. Synechocystis contains one Alb3-like protein, Slr1471, which shares 39% identity with the Arabidopsis ALB3 protein [26]. One study demonstrated that the thylakoid membrane structure of the merodiploid slr1471 mutant cell is disorganized, in accordance with the phenotypes of the corresponding mutants in Arabidopsis and Chlamydomonas [26]. Another study suggested that Slr1471 is important for the de novo assembly of the D1 precursor protein into the PSII reaction centre [27].
During PSII assembly, the D1 protein is synthesized in most organisms as a precursor with a C-terminal extension that must be cleaved to allow insertion of the mature D1 protein. A specific endoprotease cTPA (carboxy-terminal processing proteinase) and the TPR protein Prat from Synechocystis are involved in the processing of the C-terminal extension of the D1 protein [28,63–65]. Indeed, the Prat protein interacts with the C-terminal region of the precursor D1 protein in yeast two-hybrid assays [65]. Further study revealed that PratA is a Mn2+-binding protein and that PratA is required for efficient delivery of Mn2+ to PSII in vivo [66]. Interestingly, a significantly higher amount of Psb27 is associated with PSII in a cyanobacterial mutant lacking CtpA, suggesting an important role for Psb27 in precursor D1 processing [28]. In Arabidopsis, the Psb27 homologue LPA19 (low PSII accumulation 19) plays a role in facilitating the C-terminal processing of precursor D1 [29]. Chloroplast protein labelling assays indicated that the C-terminal processing of D1 protein is impaired in lpa19 mutants. The LPA19 protein specifically interacts with the soluble C terminal region present in the precursor and mature D1 [29]. A second psb27 homologue in Arabidopsis is required for efficient repair of photodamaged PSII, but not for PSII accumulation [67]. However, in Synechocystis, Psb27 has been implicated in the assembly of the Mn4Ca cluster and the stabilization of unassembled CP43 [68–70].
Along with Psb27, Psb28 and Psb29 proteins were also identified as substoichiometric components of His-tagged CP47 preparations isolated from Synechocystis PCC6803 [30]. In Synechocystis PCC6803, Psb28 is not a component of the fully assembled dimeric PSII core complex, but it is preferentially bound to PSII assembly intermediates containing the inner antenna CP47, probably by attachment to the cytoplasmic side of CP47 [31]. In psb28 null mutants, the functional properties of PSII are not affected but synthesis of CP47 and PSI subunits PsaA and PsaB is reduced. In addition, inactivation of Psb28 results in inhibition of chlorophyll synthesis at the cyclization step, which suggests that Psb28 functions in regulating the synthesis of chlorophylls [31]. A Psb28 homologue is also found in the Arabidopsis genome but its role remains elusive. Arabidopsis and Synechocystis psb29 mutants show increased light sensitivity and an increase in the proportion of uncoupled antenna with increasing light intensities [32]. It is supposed that Psb29 is required for proper assembly of PSII supercomplexes or efficient disassembly of photodamaged PSII complexes [30,32].
Several luminal immunophilin proteins have been found to be associated with PSII assembly. The immunophilin family includes FKBP (FK-506 binding protein) and cyclophilin proteins originally identified as receptors for immunosuppressive drugs (FK506 and cyclosporin A) [71,72]. Most immunophilin proteins have PPIase (peptidyl–prolyl cis–trans isomerase) activity that catalyses the cis–trans conversion of X-Pro peptide bonds, a rate-limiting step in protein folding [71,72]. This association with protein-folding capability suggests that these enzymes play a central role in the biogenesis of protein complexes, probably including PSII complexes. Indeed, Lima et al. [33] found that a redox-active FKBP-type immunophilin FKBP-2 functions in the accumulation of the PSII supercomplex in Arabidopsis, although its mechanism remains undetermined [33]. TLP40 (thylakoid lumen PPIase of 40 kDa), a cyclophilin-type immunophilin identified in spinach, is associated with and regulates the activity of a PSII-specific protein phosphatase within the thylakoid membrane [73,74]. Cyclophilin 38 (CYP38), the Arabidopsis orthologue of the TLP40 protein, was shown to be important for assembly and maintenance of PSII, particularly the supercomplexes [34]. CYP38 assists in the proper folding and insertion of D1 and CP43 into PSII and the correct assembly of the water-splitting Mn4–Ca cluster [35]. However, CYP38 was shown not to possess PPIase activity, although it can interact with the E-loop of CP47 through its cyclophilin domain [36]. Crystal structural analysis of CYP38 revealed the presence of N-terminal helical bundle and C-terminal cyclophilin β-sheet domains. This study also uncovered a unique and previously uncharacterized domain, which may provide further understanding of the auto-inhibition mechanism of CYP38 function [36]. There are more than 50 gene models in Chlamydomonas with similarity to immunophilin proteins [75]. However, their possible functions in PSII assembly await further investigation.
At least two PSII assembly factors specific to cyanobacteria have been described. One is Slr0286 of Synechocystis, which appears to facilitate functional assembly and stability of the water splitting system of PSII [37]. It lacks known motifs and no homologues have been found in other organisms, not even in other cyanobacteria [37]. The other is Slr2013, which is involved in functional assembly of PSII by regulating the folding of D2 protein [38]. Slr2013 is annotated as a hypothetical protein with a DUF58 (domain of unknown function 58) domain, and its homologues are found in other cyanobacteria but not in eukaryotes [38]. In addition, a chloroplast-localized Deg1 protease has been shown to assist in PSII assembly in Arabidopsis, probably through interaction with the D2 protein [76].
3. Regulatory factors for PSI assembly
PSI mediates light-induced electron transfer from plastocyanin on the luminal side of thylakoids to ferredoxin on the stromal side. The central components and structures of PSI complexes from cyanobacteria and chloroplasts are remarkably similar. Nevertheless, some differences do exist between them. The PSI complex of chloroplasts exists as a monomer and is associated with membrane-bound light-harvesting complexes [77], whereas that of cyanobacteria is present as a trimer and is connected to phycobilisomes on top of the thylakoid membrane as additional antenna systems [78]. The PSI complex of cyanobacteria consists of 12 proteins per monomer, whereas that of plants is composed of at least 15 subunits plus at least four light-harvesting proteins [8]. Among these 15 subunits, four (PsaG, PsaH, PsaN and PsaO) are newly evolved in chloroplasts, whereas one cyanobacterial subunit (PsaM) was lost in higher plants [8].
The assembly of the PSI complex initiates with the co-translational membrane insertion of PsaA and PsaB. Following the binding of co-factors to the two reaction centre proteins, the PsaA/B reaction centre heterodimer is formed [79]. Subsequently, relatively small subunits with a maximum molecular mass of 18 kDa are attached to the heterodimer [80]. During this step, three PSI subunits (PsaC, D and E), which are positioned on the stromal side, form the ferredoxin-binding site. These three PSI subunits do not contain any transmembrane domains and constitute the so-called ‘stromal ridge’ of PSI, which can form a large ternary complex with ferredoxin and the FNR (ferredoxin-NADP(+) reductase) enzyme on top of PSI [81]. The final formation of the mature PSI complex through the binding of LHCI (light harvesting complex I) and the PsaG, PsaK, PsaL, PsaN, PsaO and PsaP subunits appears to be a slow process; however, the sequence of events is currently unclear [8]. Several regulatory factors involved in PSI assembly have been identified using genetic approaches.
YCF3 (hypothetical chloroplast open reading frame 3), a small chloroplast-encoded protein with three TPR domains, was first identified in tobacco and Chlamydomonas, and its role in PSI assembly was subsequently investigated in Chlamydomonas using a reverse genetics approach [39]. Using random and site-directed mutagenesis of ycf3, Naver et al. [40] demonstrated that YCF3 is required for PSI assembly, but not for its stability. YCF3 interacts directly with the PSI subunits PsaA and PsaD, suggesting that YCF3 may function in the assembly of the stromal ridge of the PSI complex [40]. In a temperature-sensitive barley mutant in which ycf3 mRNA is impaired at high temperature, PSI accumulation is also affected. In this barley mutant, the reduction in PSI is related to the decrease in YCF3 protein content at different temperatures [41]. Thus, it appears that YCF3 may mediate a rate-limiting step in PSI assembly.
Y3IP1 (YCF3-interacting protein 1), which specifically interacts with the YCF3 protein, was identified by co-immunoprecipitation with a FLAG-tagged YCF3 in transplastomic tobacco [42]. Subsequent reverse genetics analysis of Y3IP1 function in tobacco and Arabidopsis revealed that the knockdown of Y3IP1 leads to a specific deficiency in PSI but does not result in loss of YCF3. Y3IP1 is loosely associated with thylakoid membranes and exists in two distinct protein complexes [42]. Unlike all other PSI assembly factors known to date, Y3IP1 appears to be newly evolved in photosynthetic eukaryotes. This observation suggests that it may function in a step of PSI assembly that differs between cyanobacteria and chloroplasts. Such a difference could be in the formation of the stromal ridge, as this step requires import and sorting of PsaD and PsaE into the thylakoid membrane from the cytosol in chloroplasts, a step that is not required in cyanobacteria [8]. Y3IP1 most probably functions as a receptor for (one of) these proteins before their integration into the PSI complex [8].
YCF4 (hypothetical chloroplast open reading frame 4) a 22-kDa protein with two putative transmembrane domains, is another chloroplast-encoded assembly chaperone for PSI. Compared with YCF3, it is more stably associated with the thylakoid membrane and accumulates more abundantly [39]. A tandem affinity purification tagged version of YCF4 was used to purify a stable YCF4-containing complex of approximately 1500 kDa from Chlamydomonas, which also contained the opsin-related COP2 and the PSI subunits PsaA, PsaB, PsaC, PsaD, PsaE and PsaF [43]. Pulse-chase protein labelling revealed that the PSI proteins associated with the YCF4-containing complex were newly synthesized and partially assembled as an intermediate assembly subcomplex, indicating that the YCF4 complex may act as a scaffold for PSI assembly [43]. YCF4 is highly conserved amongst photosynthetic organisms from cyanobacteria to higher plants. It appears to be essential for PSI complex assembly in Chlamydomonas, whereas a cyanobacterial mutant deficient in YCF4 is still able to assemble the PSI complex, although at a reduced rate [44]. It was recently found that YCF4 may function as a non-essential assembly factor for PSI also in higher plants, based on results from tobacco [45].
PSI contains three [4Fe–4S] clusters that are associated with the PsaA/B heterodimer and PsaC and that are directly involved in electron transfer. HCF101 (high chlorophyll fluorescence 101) and APO1 (accumulation of PSI 1), two chloroplast proteins, have been genetically identified as essential and specific factors for the assembly of [4Fe–4S]-cluster-containing protein complexes, including PSI complexes [46,82]. HCF101 was identified from the seeding-lethal Arabidopsis mutant hcf101 in which PSI activity is abolished [46]. The hcf101 plants not only fail to accumulate mature PSI but also have reduced levels of the soluble [4Fe-4S]-cluster-containing complex ferredoxin–thioredoxin reductase in the stroma [46]. HCF101 belongs to an ancient and universally conserved family of P-loop ATPases previously designated as the ‘MRP’ (metG related protein) family. Schwenkert et al. [47] demonstrated that HCF101 might serve as a chloroplast scaffold protein that specifically assembles [4Fe–4S] clusters and transfers them to the chloroplast membrane and soluble target proteins [47]. By contrast, APO1 is conserved only among higher plants. In the apo1 Arabidopsis mutant, levels of the PSI core subunits as well as the intrinsic and peripheral PSI subunits are reduced [82]. APO1 had been proposed to be required for the accumulation of [4Fe–4S]-cluster-containing chloroplast complexes and antenna proteins. However, a recent study suggested that APO1 promotes the splicing of several chloroplast group II introns, and these splicing defects can account for the loss of photosynthetic complexes in the apo1 mutant [48].
In cyanobacteria, a membrane-associated rubredoxin denoted RubA is required for assembly of functional PSI complexes [49]. RubA mutants produce trimeric PSI complexes that are inactive in electron transport to flavodoxin or the artificial acceptor methyl viologen. The PSI complexes from RubA mutants lack the stromal subunits PsaC, PsaD and PsaE but contain all of the intrinsic membrane subunits [49]. However, the chloroplast-localized rubredoxin is associated with PSII, but not PSI [50]. Further analysis of the roles of chloroplast rubredoxin is required.
4. Regulatory factors for cytochrome b6f complex assembly
The cyt b6f complex of thylakoid membranes functions as a plastoquinol–plastocyanin oxidoreductase that links PSI and PSII. This complex is also involved in cyclic electron transport around PSI and acts as a proton translocase. In its native form, the cyt b6f complex exists as a dimer with a molecular weight of 310 kDa that may be converted into a 140-kDa monomer with increasing detergent concentrations [83,84]. In plants, the complex is composed of four major subunits (cyt f (encoded by petA), cyt b6 (petB), the Rieske-FeS protein (petC) and PetD) and four small subunits (PetG, PetL, PetM and PetN) [85]. In addition to these subunits, additional proteins may transiently interact with the cyt b6f complex. In higher plants, cyt b6f has been co-isolated with FNR [86], and the functional coupling of a small phosphoprotein PetO to cyt b6f has been reported [87]. PetP has been proposed as a new cyanobacterial cyt b6f subunit that might be analogous to PetO [88].
The assembly process and the roles of cyt b6f subunits have been studied in Chlamydomonas and plants. The assembly initiates with the formation of a mildly protease-resistant subcomplex consisting of cyt b6 and PetD, which acts as a scaffold for the subsequent assembly of the cyt f and PetG proteins [58,89]. The PetC and PetL proteins then participate in the assembly of the functional dimer [58,89]. Although the roles of the cyt bf subunits in the assembly, stability, and dimerization of the cyt bf complex have been examined in several studies [89–93], the assembly mechanism of the cyt bf complex remains largely unknown.
In the cyt b6f complex, two haem prosthetic groups are covalently bound to the protein moieties: haem c of cytochrome f and haem ci attached to cytochrome b6 in the quinone-binding site Qi [94,95]. Covalent haem-binding plays a major role during the assembly of cyt b6f. Two maturation pathways for haem attachment exist in chloroplasts. System II, a maturation pathway, was originally discovered in Chlamydomonas through genetic studies of ccs (cytochrome c synthesis) mutants that exhibited a photosynthetic deficiency and failed to accumulate holo forms of both cytochrome f and c6. At least six factors, chloroplast-encoded CCSA and nuclear-encoded CCS1 to CCS5, are required for haem attachment to the apo forms of cyt f and cyt b6 on the lumen side of the thylakoid membrane [51–53].
In bacteria, a thioldisulphide membrane transporter of the CcdA/DsbD (disulfide bond D) family and a membrane-anchored, periplasm-facing thioredoxin-like protein CcsX are postulated to act sequentially to reduce the disulphide-bonded CXXCH in apocytochrome c before the haem ligation [96], and their orthologues have been identified and studied in chloroplasts. In Arabidopsis, the loss of CcdA and HCF164 (high chlorophyll fluorescence 164, an orthologue of CcSX) impairs photosynthesis and results in a cyt b6f assembly defect, suggesting that these proteins are required for cyt b6f biogenesis in plant chloroplasts [54,55]. The role of HCF164 was further investigated through a study of CCS5, its orthologue in Chlamydomonas [56]. CCS5 interacted with apocytochrome f and c6 in a yeast two-hybrid analysis and reduced a disulphide in the CXXCH haem-binding site of apocytochrome f. Moreover, the ccs5 mutant was rescued by exogenous thiols [56]. These results indicate that CCS5/HCF164 is a component of a trans-thylakoid redox pathway and operates by reducing the CXXCH haem-binding site of apocytochrome c before the haem ligation reaction [56].
Recently, the small novel protein CCS4 was identified as a third component of the system II pathway in Chlamydomonas. Although CCS4 does not display sequence motifs suggestive of redox- or haem-binding function, biochemical and genetic complementation experiments suggest a role in the disulphide-reducing pathway required for haem attachment to apo forms of cyt c [57]. CCS4 might function in stabilizing CCDA or regulating its activity [57].
System IV maturation pathways, namely the assembly of co-factors on complex C subunit B (CCB), are responsible for the attachment of haem ci to apocytochrome b6 on the stromal side of the thylakoid membrane [97]. At least four protein factors, CCB1–CCB4, are necessary for this maturation pathway [98]. The CCB proteins of Chlamydomonas are conserved amongst all oxygenic photosynthetic organisms [99]. The ccb1, ccb2 and ccb4 Arabidopsis mutants all are deficient in the accumulation of cytochrome b6f complex subunits and lack haem covalently bound to cytochrome b6 [99,100]. Photosynthesis is impaired in the cyanobacteria ccb1 and ccb3 mutants [4]. The cyanobacterial cyt b6f complex is essential because it is involved in the respiratory and photosynthetic electron transfer chains. It is expected that a cyt b6f complex lacking haem ci would accumulate to higher levels in cyanobacteria than in Chlamydomonas because of lower quality control, as observed for crippled PSII complexes [4].
5. Conclusion and perspective
Many regulatory factors involved in the biogenesis of thylakoid membrane protein complexes have been identified and functionally characterized in recent years through a combination of genetic, transcriptomic and proteomic approaches. However, thylakoid membrane protein complex biogenesis is more complex than originally anticipated. It is expected that the regulatory factors identified thus far represent only a fraction of those that are involved in these processes; thus, the identification of the remaining regulators represents a goal for future work. In addition, thylakoid membrane protein complexes are dynamic and flexible in response to developmental and environmental inputs. Thus, one of the focuses in this field is to understand how these regulatory factors respond to environmental cues, especially light quality and quantity, and developmental states. Current challenges include elucidating the integration of the actions of different regulatory factors and identifying the signal transduction pathways that link these regulatory factors to internal and external signals.
Some of the known regulatory factors, such as Alb3, seem to have a general function in several steps of thylakoid membrane protein complex biogenesis; in addition, each assembly step may require the participation of many factors that act simultaneously. It appears that there is a dynamic regulatory network, in which the biogenesis of thylakoid membrane protein complexes is mediated by numerous regulatory proteins at different levels. Thus, another challenge is to understand how these factors are coordinated. Some of these factors, such as LPA1 and PAM68, that function in the same step, may form a protein complex mediated by protein–protein interactions [19]; however, the relationships among most of the known factors remain unknown.
To understand the mechanisms of these regulatory factors in more depth, novel techniques must be developed to overcome current obstacles. For instance, a major obstacle to elucidating the assembly of thylakoid membrane protein complexes is tracking of the assembly process in vivo and reconstituting it in vitro. Most of the assembly intermediates occur only transiently or are unstable and are therefore difficult to dissect using ordinary separation techniques, such as native electrophoresis or density gradient centrifugation. Various types of advanced fluorescent labelling techniques, coupled with the structural information available, may allow the assembly of thylakoid membrane protein complexes to be visualized in real time [101].
Acknowledgements
The work in our laboratory was supported by the National Natural Science Foundation of China (grant no. 31070213) and the Major State Basic Research Development Program (grant no. 2009CB118500).
References
- 1.Xiong J., Fischer W. M., Inoue K., Nakahara M., Bauer C. E. 2000. Molecular evidence for the early evolution of photosynthesis. Science 289, 1724–1730 10.1126/science.289.5485.1724 (doi:10.1126/science.289.5485.1724) [DOI] [PubMed] [Google Scholar]
- 2.Adam Z., Charuvi D., Tsabari O., Knopf R. R., Reich Z. 2011. Biogenesis of thylakoid networks in angiosperms: knowns and unknowns. Plant Mol. Biol. 76, 221–234 10.1007/s11103-010-9693-5 (doi:10.1007/s11103-010-9693-5) [DOI] [PubMed] [Google Scholar]
- 3.Vothknecht U. C., Westhoff P. 2001. Biogenesis and origin of thylakoid membranes. Biochim. Biophys. Acta 1541, 91–101 10.1016/j.bbr.2011.03.031 (doi:10.1016/j.bbr.2011.03.031) [DOI] [PubMed] [Google Scholar]
- 4.de Vitry C. 2011. Cytochrome c maturation system on the negative side of bioenergetic membranes: CCB or System IV. FEBS J. 278, 4189–4197 10.1111/j.1742-4658.2011.08373.x. (doi:10.1111/j.1742-4658.2011.08373.x.) [DOI] [PubMed] [Google Scholar]
- 5.Nelson N., Ben-Shem A. 2004. The complex architecture of oxygenic photosynthesis. Nat. Rev. Mol. Cell Biol. 5, 971–982 10.1038/nrm1525 (doi:10.1038/nrm1525) [DOI] [PubMed] [Google Scholar]
- 6.Rochaix J. D. 2011. Assembly of the photosynthetic apparatus. Plant Physiol. 155, 1493–1500 10.1104/pp.110.169839 (doi:10.1104/pp.110.169839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulo P., Sirpio S., Suorsa M., Aro E. M. 2008. Auxiliary proteins involved in the assembly and sustenance of photosystem II. Photosynth. Res. 98, 489–501 10.1007/s11120-008-9320-3 (doi:10.1007/s11120-008-9320-3) [DOI] [PubMed] [Google Scholar]
- 8.Schöttler M. A., Albus C. A., Bock R. 2011. Photosystem I: its biogenesis and function in higher plants. J. Plant Physiol. 168, 1452–1461 10.1016/j.jplph.2010.12.009 (doi:10.1016/j.jplph.2010.12.009) [DOI] [PubMed] [Google Scholar]
- 9.Nakamoto S. S., Hamel P., Merchant S. 2000. Assembly of chloroplast cytochromes b and c. Biochimie 82, 603–614 10.1016/S0300-9084(00)00605-2 (doi:10.1016/S0300-9084(00)00605-2) [DOI] [PubMed] [Google Scholar]
- 10.Komenda J., Sobotka R., Nixon P. J. 2012. Assembling and maintaining the photosystem II complex in chloroplasts and cyanobacteria. Curr. Opin. Plant Biol. 15, 245–251 10.1016/j.pbi.2012.01.017 (doi:10.1016/j.pbi.2012.01.017) [DOI] [PubMed] [Google Scholar]
- 11.Chi W., Sun X., Zhang L. 2012. The roles of chloroplast proteases in the biogenesis and maintenance of photosystem II. Biochim. Biophys. Acta 1817, 239–246 10.1016/j.bbabio.2011.05.014. (doi:10.1016/j.bbabio.2011.05.014.) [DOI] [PubMed] [Google Scholar]
- 12.Nixon P. J., Michoux F., Yu J. F., Boehm M., Komenda J. 2010. Recent advances in understanding the assembly and repair of photosystem II. Ann. Bot. 106, 1–16 10.1093/aob/mcq059 (doi:10.1093/aob/mcq059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meurer J., Plucken H., Kowallik K. V., Westhoff P. 1998. A nuclear encoded protein of prokaryotic origin is essential for the stability of photosystem II in Arabidopsis thaliana. EMBO J. 17, 5286–5297 10.1093/emboj/17.18.5286 (doi:10.1093/emboj/17.18.5286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plucken H., Muller B., Grohmann D., Westhoff P., Eichacker L. A. 2002. The HCF136 protein is essential for assembly of the photosystem II reaction center in Arabidopsis thaliana. FEBS Lett. 532, 85–90 10.1016/S0014-5793(02)03634-7 (doi:10.1016/S0014-5793(02)03634-7) [DOI] [PubMed] [Google Scholar]
- 15.Komenda J., Nickelsen J., Tichy M., Prasil O., Eichacker L. A., Nixon P. J. 2008. The cyanobacterial homologue of HCF136/YCF48 is a component of an early photosystem II assembly complex and is important for both the efficient assembly and repair of photosystem II in Synechocystis sp PCC 6803. J. Biol. Chem. 283,22 390–22 399 10.1074/jbc.M801917200 (doi:10.1074/jbc.M801917200) [DOI] [PubMed] [Google Scholar]
- 16.Covshoff S., Majeran W., Liu P., Kolkman J. M., van Wijk K. J., Brutnell T. P. 2008. Deregulation of maize C4 photosynthetic development in a mesophyll cell-defective mutant. Plant Physiol. 146, 1469–1481 10.1104/pp.107.113423 (doi:10.1104/pp.107.113423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng L., Ma J., Chi W., Guo J., Zhu S., Lu Q., Lu C., Zhang L. 2006. LOW PSII ACCUMULATION1 is involved in efficient assembly of photosystem II in Arabidopsis thaliana. Plant Cell 18, 955–969 10.1105/tpc.105.037689 (doi:10.1105/tpc.105.037689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park S., Khamai P., Garcia-Cerdan J. G., Melis A. 2007. REP27, a tetratricopeptide repeat nuclear-encoded and chloroplast-localized protein, functions in D1/32-kDa reaction center protein turnover and photosystem II repair from photodamage. Plant Physiol. 143, 1547–1560 10.1104/pp.107.096396 (doi:10.1104/pp.107.096396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armbruster U., et al. 2010. The Arabidopsis thylakoid protein PAM68 is required for efficient D1 biogenesis and photosystem II assembly. Plant Cell 22, 3439–3460 10.1105/tpc.110.077453 (doi:10.1105/tpc.110.077453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma J., Peng L., Guo J., Lu Q., Lu C., Zhang L. 2007. LPA2 is required for efficient assembly of photosystem II in Arabidopsis thaliana. Plant Cell 19, 1980–1993 10.1105/tpc.107.050526 (doi:10.1105/tpc.107.050526) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Cai W., Ma J., Chi W., Zou M., Guo J., Lu C., Zhang L. 2010. Cooperation of LPA3 and LPA2 is essential for photosystem II assembly in Arabidopsis. Plant Physiol. 154, 109–120 10.1104/pp.110.159558 (doi:10.1104/pp.110.159558) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Bellafiore S., Ferris P., Naver H., Göhre V., Rochaix J. D. 2002. Loss of Albino3 leads to the specific depletion of the light-harvesting system. Plant Cell 14, 2303–2314 10.1105/tpc.003442 (doi:10.1105/tpc.003442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ossenbuhl F., Gohre V., Meurer J., Krieger-Liszkay A., Rochaix J. D., Eichacker L. A. 2004. Efficient assembly of photosystem II in Chlamydomonas reinhardtii requires Alb3.1p, a homolog of Arabidopsis ALBINO3. Plant Cell 16, 1790–1800 10.1105/tpc.023226 (doi:10.1105/tpc.023226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gohre V., Ossenbuhl F., Crevecoeur M., Eichacker L. A., Rochaix J. D. 2006. One of two alb3 proteins is essential for the assembly of the photosystems and for cell survival in Chlamydomonas. Plant Cell 18, 1454–1466 10.1105/tpc.105.038695 (doi:10.1105/tpc.105.038695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasch J. C., Nickelsen J., Schunemann D. 2005. The yeast split-ubiquitin system to study chloroplast membrane protein interactions. Appl. Microbiol. Biotechnol. 69, 440–447 10.1007/s00253-005-0029-3 (doi:10.1007/s00253-005-0029-3) [DOI] [PubMed] [Google Scholar]
- 26.Spence E., Bailey S., Nenninger A., Moller S. G., Robinson C. 2004. A homolog of Albino3/OxaI is essential for thylakoid biogenesis in the cyanobacterium Synechocystis sp. PCC6803. J. Biol. Chem. 279, 55 792–55 800 10.1074/jbc.M411041200 (doi:10.1074/jbc.M411041200) [DOI] [PubMed] [Google Scholar]
- 27.Fulgosi H., Gerdes L., Westphal S., Glockmann C., Soll J. 2002. Cell and chloroplast division requires ARTEMIS. Proc. Natl Acad. Sci. USA 99, 11 501–11 506 10.1073/pnas.172032599 (doi:10.1073/pnas.172032599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anbudurai P. R., Mor T. S., Ohad I., Shestakov S. V., Pakrasi H. B. 1994. The ctpA gene encodes the C-terminal processing protease for the D1 protein of the photosystem II reaction center complex. Proc. Natl Acad. Sci. USA 91, 8082–8086 10.1073/pnas.91.17.8082 (doi:10.1073/pnas.91.17.8082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei L., Guo J., Ouyang M., Sun X., Ma J., Chi W., Lu C., Zhang L. 2010. LPA19, a Psb27 homolog in Arabidopsis thaliana, facilitates D1 protein precursor processing during PSII biogenesis. J. Biol. Chem. 285, 21 391–21 398 10.1074/jbc.M110.105064 (doi:10.1074/jbc.M110.105064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kashino Y., Lauber W. M., Carroll J. A., Wang Q., Whitmarsh J., Satoh K., Pakrasi H. B. 2002. Proteomic analysis of a highly active photosystem II preparation from the cyanobacterium Synechocystis sp PCC 6803 reveals the presence of novel polypeptides . Biochemistry 41, 8004–8012 10.1021/bi026012 (doi:10.1021/bi026012) [DOI] [PubMed] [Google Scholar]
- 31.Dobakova M., Sobotka R., Tichy M., Komenda J. 2009. Psb28 protein is involved in the biogenesis of the photosystem II inner antenna CP47 (PsbB) in the cyanobacterium Synechocystis sp PCC 6803. Plant Physiol. 149, 1076–1086 10.1104/pp.108.130039 (doi:10.1104/pp.108.130039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keren N., Ohkawa H., Welsh E. A., Liberton M., Pakrasi H. B. 2005. Psb29, a conserved 22-kDa protein, functions in the biogenesis of photosystem II complexes in Synechocystis and Arabidopsis. Plant Cell 17, 2768–2781 10.1105/tpc.105.035048 (doi:10.1105/tpc.105.035048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lima A., Lima S., Wong J. H., Phillips R. S., Buchanan B. B., Luan S. 2006. A redox-active FKBP-type immunophilin functions in accumulation of the photosystem II supercomplex in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 103, 12 631–12 636 10.1073/pnas.0605452103 (doi:10.1073/pnas.0605452103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu A., He Z., Cho H. S., Lima A., Buchanan B. B., Luan S. 2007. A chloroplast cyclophilin functions in the assembly and maintenance of photosystem II in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 104, 15 947–15 952 10.1073/pnas.0707851104 (doi:10.1073/pnas.0707851104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sirpiö S., et al. 2008. AtCYP38 ensures early biogenesis, correct assembly and sustenance of photosystem II. Plant J. 55, 639–651 10.1111/j.1365-313X.2008.03532.x (doi:10.1111/j.1365-313X.2008.03532.x) [DOI] [PubMed] [Google Scholar]
- 36.Fu A., He Z., Cho H. S., Lima A., Buchanan B. B., Luan S. 2012. Crystal structure of Arabidopsis cyclophilin38 reveals a previously uncharacterized immunophilin fold and a possible autoinhibitory mechanism. Plant Cell. 24, 2666–2674 10.1105/tpc.111.093781 (doi:10.1105/tpc.111.093781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kufryk G. I., Vermaas W. F. J. 2001. A novel protein involved in the functional assembly of the oxygen-evolving complex of photosystem II in Synechocystis sp PCC 6803. Biochemistry 40, 9247–9255 10.1021/bi0026526 (doi:10.1021/bi0026526) [DOI] [PubMed] [Google Scholar]
- 38.Kufryk G. I., Vermaas W. F. J. 2003. Slr2013 is a novel protein regulating functional assembly of photosystem II in Synechocystis sp strain PCC 6803. J. Bacteriol. 185, 6615–6623 10.1128/JB.185.22.6615-6623.2003 (doi:10.1128/JB.185.22.6615-6623.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boudreau E., Takahashi Y., Lemieux C., Turmel M., Rochaix J. D. 1997. The chloroplast ycf3 and ycf4 open reading frames of Chlamydomonas reinhardtii are required for the accumulation of the photosystem I complex. EMBO J. 16, 6095–6104 10.1093/emboj/16.20.6095 (doi:10.1093/emboj/16.20.6095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naver H., Boudreau E., Rochaix J. D. 2001. Functional studies of Ycf3: its role in assembly of photosystem I and interactions with some of its subunits. Plant Cell 13, 2731–2745 10.1105/tpc.010253 (doi:10.1105/tpc.010253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landau A. M., Lokstein H., Scheller H. V., Lainez V., Maldonado S., Prina A. R. 2009. A cytoplasmically inherited barley mutant is defective in photosystem I assembly due to a temperature-sensitive defect in ycf3 splicing. Plant Physiol. 151, 1802–1811 10.1104/pp.109.147843 (doi:10.1104/pp.109.147843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albus C. A., Ruf S., Schöttler M. A., Lein W., Kehr J., Bock R. 2010. Y3IP1, a nucleus-encoded thylakoid protein, cooperates with the plastid-encoded Ycf3 protein in photosystem I assembly of tobacco and Arabidopsis. Plant Cell 22, 2838–2855 10.1105/tpc.110.073908 (doi:10.1105/tpc.110.073908) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozawa S., Nield J., Terao A., Stauber E. J., Hippler M., Koike H., Rochaix J. D., Takahashi Y. 2009. Biochemcial and structural studies of the large Ycf4-photosystem I assembly complex of the green alga Chlamydomonas reinhardtii. Plant Cell 21, 2424–2442 10.1105/tpc.108.063313 (doi:10.1105/tpc.108.063313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilde A., Hartel H., Hübschmann T., Hoffmann P., Shestakov S. V., Borner T. 1995. Inactivation of a Synechocystis sp strain PCC-6803 gene with homology to conserved chloroplast open reading frame 184 increases the photosystem II-to-photosystem I ratio. Plant Cell 7, 649–658 10.1105/tpc.7.5.649 (doi:10.1105/tpc.7.5.649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krech K. et al. 2012. The plastid genome-encoded ycf4 protein functions as a nonessential assembly factor for photosystem I in higher plants. Plant Physiol. 159, 579–591 10.1104/pp.112.196642 (doi:10.1104/pp.112.196642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lezhneva L., Amann K., Meurer J. 2004. The universally conserved Hcf101 protein is involved in the assembly of [4Fe–4S] cluster containing complexes in Arabidopsis thaliana chloroplasts. Plant J. 37, 174–185 10.1046/j.1365-313X.2003.01952.x (doi:10.1046/j.1365-313X.2003.01952.x) [DOI] [PubMed] [Google Scholar]
- 47.Schwenkert S., Netz D. J., Frazzon J., Pierek A. J., Bill E., Gross J., Lill R., Meurer J. 2010. Chloroplast Hcf101 protein is a scaffold protein for [4Fe–4S] cluster assembly. Biochem. J. 425, 207–214 10.1042/BJ20091290 (doi:10.1042/BJ20091290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watkins K. P., Rojas M., Friso G., van Wijk K. J., Meurer J., Barkan A. 2011. APO1 promotes the splicing of chloroplast group II introns and harbors a plant-specific zinc-dependent RNA binding domain. Plant Cell 23, 1082–1092 10.1105/tpc.111.084335 (doi:10.1105/tpc.111.084335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen G., Zhao J., Reimer S. K., Antonkine M. L., Cao Q., Weiland S. M., Golbeck C. H., Bryant D. A. 2002. Assembly of photosystem I. I. Inactivation of the rubA gene encoding a membrane-associated rubredoxin in the cyanobacterium Synechococcus sp. PCC7002 causes a loss of photosystem I activity. J. Biol. Chem. 277, 20 343–20 354 10.1074/jbc.M201103200 (doi:10.1074/jbc.M201103200) [DOI] [PubMed] [Google Scholar]
- 50.Friso G., Giacomelli L., Ytterberg A. J., Peltier J. B., Rudella A., Sun Q., van Wijk K. J. 2004. In depth analysis of the thylakoid membrane proteome of Arabidopsis thaliana chloroplasts: new proteins, new functions, and a plastid proteome database. Plant Cell 16, 478–499 10.1105/tpc.017814 (doi:10.1105/tpc.017814) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howe G., Merchant S. 1992. The biosynthesis of membrane and soluble plastidic c-type cytochromes of Chlamydomonas reinhardtii is dependent on multiple common gene products. EMBO J. 11, 2789–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howe G., Mets L., Merchant S. 1995. Biosynthesis of cytochrome f in Chlamydomonas reinhardtii: analysis of the pathway in gabaculine-treated cells and in the heme attachment mutant B6. Mol. Gen. Genet. 246, 156–165 10.1007/BF00294678 (doi:10.1007/BF00294678) [DOI] [PubMed] [Google Scholar]
- 53.Xie Z., Culler D., Dreyfuss B. W., Kuras R., Wollman F. A., Merchant S. 1998. Genetic analysis of chloroplast c-type cytochrome assembly in Chlamydomonas reinhardtii: one chloroplast locus and at least four nuclear loci are required for heme attachment. Genetics 148, 681–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Page M. L., et al. 2004. A homolog of prokaryotic thiol disulfide transporter CcdA is required for the assembly of the cytochrome b6f complex in Arabidopsis chloroplasts. J. Biol. Chem. 279, 32 474–32 482 10.1074/jbc.M404285200 (doi:10.1074/jbc.M404285200) [DOI] [PubMed] [Google Scholar]
- 55.Lennartz K., Plücken H., Seidler A., Westhoff P., Bechtold N., Meierhoff K. 2001. HCF164 encodes a thioredoxin-like protein involved in the biogenesis of the cytochrome b6f complex in Arabidopsis. Plant Cell 13, 2539–2551 10.1105/tpc.010245 (doi:10.1105/tpc.010245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gabilly S. T., Dreyfuss B. W., Karamoko M., Corvest V., Kropat J., Page M. D., Merchant S. S., Hamel P. P. 2010. CCS5, a thioredoxin-like protein involved in the assembly of plastid c-type cytochromes. J. Biol. Chem. 285, 29 738–29 749 10.1074/jbc.M109.099069 (doi:10.1074/jbc.M109.099069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gabilly S. T., Kropat J., Karamoko M., Page M. D., Nakamoto S. S., Merchant S. S., Hamel P. P. 2011. A novel component of the disulfide-reducing pathway required for cytochrome c assembly in plastids. Genetics 187, 793–802 10.1534/genetics.110.125369 (doi:10.1534/genetics.110.125369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wollman F. A., Minai L., Nechushtai R. 1999. The biogenesis and assembly of photosynthetic proteins in thylakoid membranes. Biochim. Biophys. Acta 1141, 21–85 [DOI] [PubMed] [Google Scholar]
- 59.Nelson N., Yocum C. F. 2006. Structure and function of photosystem I and II. Annu. Rev. Plant Biol. 57, 521–565 10.1146/annurev.arplant.57.032905.105350 (doi:10.1146/annurev.arplant.57.032905.105350) [DOI] [PubMed] [Google Scholar]
- 60.Enami I., Okumura A., Nagao R., Suzuki T., Iwai M., Shen J. R. 2008. Structures and functions of the extrinsic proteins of photosystem II from different species. Photosynth. Res. 98, 349–363 10.1007/s11120-008-9343-9 (doi:10.1007/s11120-008-9343-9) [DOI] [PubMed] [Google Scholar]
- 61.Rochaix J. D. 2001. Assembly, function, and dynamics of the photosynthetic machinery in Chlamydomonas reinhardtii. Plant Physiol. 127, 1394–1398 10.1104/pp.010628 (doi:10.1104/pp.010628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang D., et al. 2011. HCF243 encodes a chloroplast-localized protein involved in the D1 protein stability of the Arabidopsis photosystem II complex. Plant Physiol. 157, 608–619 10.1104/pp.111.183301 (doi:10.1104/pp.111.183301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shestakov S. V., Anbudurai P. R., Stanbekova G. E., Gadzhiev A., Lind L. K., Pakrasi H. B. 1994. Molecular cloning and characterization of the ctpA gene encoding a carboxyl terminal processing protease. Analysis of a spontaneous photosystem II-deficient mutant strain of the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 269, 19 354–19 359 [PubMed] [Google Scholar]
- 64.Klinkert B., Ossenbuhl F., Sikorski M., Berry S., Eichacker L., Nickelsen J. 2004. PratA, a periplasmic tetratricopeptide repeat protein involved in biogenesis of photosystem II in Synechocystis sp. PCC 6803. J. Biol. Chem. 279, 44 639–44 644 10.1074/jbc.M405393200 (doi:10.1074/jbc.M405393200) [DOI] [PubMed] [Google Scholar]
- 65.Schottkowski M., Gkalympoudis S., Tzekova N., Stelljes C., Schünemann D., Ankele E., Nickelsen J. 2008. Interaction of the periplasmic PratA factor and the PsbA (D1) protein during biogenesis of photosystem II in Synechocystis sp. PCC 6803. J. Biol. Chem. 284, 1813–1819 10.1074/jbc.M806116200 (doi:10.1074/jbc.M806116200) [DOI] [PubMed] [Google Scholar]
- 66.Stengel A., Gügel I. L., Hilger D., Rengstl B., Jung H., Nickelsen J. 2012. Initial steps of photosystem II de novo assembly and preloading with manganese take place in biogenesis centers in Synechocystis. Plant Cell 24, 660–675 10.1105/tpc.111.093914 (doi:10.1105/tpc.111.093914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen H., Zhang D., Guo J., Wu H., Jin M., Lu Q., Lu C., Zhang L. 2006. A Psb27 homologue in Arabidopsis thaliana is required for efficient repair of photodamaged photosystem II. Plant Mol. Biol. 61, 567–575 10.1007/s11103-006-0031-x (doi:10.1007/s11103-006-0031-x) [DOI] [PubMed] [Google Scholar]
- 68.Roose J. L., Pakrasi H. B. 2008. The Psb27 protein facilitates manganese cluster assembly in photosystem II. J. Biol. Chem. 283, 4044–4050 10.1074/jbc.M708960200 (doi:10.1074/jbc.M708960200) [DOI] [PubMed] [Google Scholar]
- 69.Komenda J., Knoppová J., Kopečná J., Sobotka R., Halada P., Yu J., Nickelsen J., Boehm M., Nixon P. J. 2012. The Psb27 assembly factor binds to the CP43 complex of photosystem II in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 158, 476–486 10.1104/pp.111 (doi:10.1104/pp.111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu H., Huang R. Y. C., Chen J., Gross M. L., Pakrasi H. B. 2011. Psb27, a transiently associated protein, binds to the chlorophyll-binding protein in photosystem II assembly intermediates. Proc. Natl Acad. Sci. USA 108, 18 536–18 541 10.1073/pnas.1111597108 (doi:10.1073/pnas.1111597108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lücke C., Weiwad M. 2011. Insights into immunophilin structure and function. Curr. Med. Chem. 18, 5333–5354 [DOI] [PubMed] [Google Scholar]
- 72.Geisler M., Bailly A. 2007. Tete-a-tete: the function of FKBPs in plant development. Trends Plant Sci. 12, 465–473 10.1016/j.tplants.2007.08.015 (doi:10.1016/j.tplants.2007.08.015) [DOI] [PubMed] [Google Scholar]
- 73.Fulgosi H., Vener A. V., Altschmied L., Herrmann R. H., Andersson B. 1998. A novel multi-functional chloroplast protein: identification of a 40 kDa immunophilin-like protein located in the thylakoid lumen. EMBO J. 17, 1577–1587 10.1093/emboj/17.6.1577 (doi:10.1093/emboj/17.6.1577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rokka A., Aro E. M., Herrmann R. G., Andersson B., Vener A. V. 2000. Dephosphorylation of photosystem II reaction center proteins in plant photosynthetic membranes as an immediate response to abrupt elevation of temperature. Plant Physiol. 123, 1525–1536 10.1104/pp.123.4.1525 (doi:10.1104/pp.123.4.1525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vallon O. 2005. Chlamydomonas immunophilins and parvulins: survey and critical assessment of gene models. Eukaryotic Cell 4, 230–241 10.1128/EC.4.2.230-241.2005 (doi:10.1128/EC.4.2.230-241.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun X., Ouyang M., Guo J., Ma J., Lu C., Adam Z., Zhang L. 2010. The thylakoid protease Deg1 is involved in photosystem II assembly in Arabidopsis thaliana. Plant J. 62, 240–249 10.1111/j.1365-313X.2010.04140.x (doi:10.1111/j.1365-313X.2010.04140.x) [DOI] [PubMed] [Google Scholar]
- 77.Amunts A., Drory O., Nelson N. 2007. The structure of a photosystem I supercomplex at 3.4 Å resolution. Nature 447, 58–63 10.1038/nature05687 (doi:10.1038/nature05687) [DOI] [PubMed] [Google Scholar]
- 78.Jordan P., Fromme P., Witt H. T., Klukas O., Saenger W., Krauß N. 2001. Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature 411, 9–17 10.1038/35082000 (doi:10.1038/35082000) [DOI] [PubMed] [Google Scholar]
- 79.Ozawa S. I., Onishi T., Takahashi Y. 2010. Identification and characterization of an assembly intermediate subcomplex of photosystem I in the green alga Chlamydomonas reinhardtii. J. Biol. Chem. 285, 20 072–20 079 10.1074/jbc.M109.098954 (doi:10.1074/jbc.M109.098954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jagannathan B., Golbeck J. H. 2009. Understanding of the binding interface between PsaC and the PsaA/PsaB heterodimer in photosystem I. Biochemistry 48, 5405–5416 10.1021/bi900243f (doi:10.1021/bi900243f) [DOI] [PubMed] [Google Scholar]
- 81.Antonkine M. L., Jordan P., Fromme P., Krauß N., Golbeck J. H., Stehlik D. 2003. Assembly of protein subunits within the stromal ridge of photosystem I: structural changes between unbound and sequentially PSI-bound polypeptides and correlated changes of the magnetic properties of the terminal iron sulphur clusters. J. Mol. Biol. 327, 671–697 10.1016/S0022-2836(03)00145-1 (doi:10.1016/S0022-2836(03)00145-1) [DOI] [PubMed] [Google Scholar]
- 82.Amann K., Lezhneva L., Wanner G., Herrmann R. G., Meurer J. 2004. Accumulation of photosystem 1, a member of a novel gene family, is required for accumulation of [4Fe–4S] cluster-containing chloroplast complexes and antenna proteins. Plant Cell 16, 3084–3089 10.1105/tpc.104.024935 (doi:10.1105/tpc.104.024935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mosser G., Breyton C., Olofsson A., Popot J. L., Rigaud J. L. 1997. Projection map of cytochrome b6f complex at 8Å resolution. J. Biol. Chem. 272, 20 263–20 268 10.1074/jbc.272.32.20263 (doi:10.1074/jbc.272.32.20263) [DOI] [PubMed] [Google Scholar]
- 84.Baniulis D., Yamashita E., Whitelegge J. P., Zatsman A. I., Hendrich M. P., Hasan S. S., Ryan C. M., Cramer W. A. 2009. Structure-function, stability, and chemical modification of the cyanobacterial cytochrome b6f complex from Nostoc sp. PCC 7120. J. Biol. Chem. 284, 9861–9869 10.1074/jbc.M809196200 (doi:10.1074/jbc.M809196200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wollman F. A. 2004. The structure, function and biogenesis of cytochrome b6f complexes. Adv. Photosyn. Res. 7, 459–476 10.1007/0-306-48204-5-24 (doi:10.1007/0-306-48204-5-24) [DOI] [Google Scholar]
- 86.Zhang H. M., Whitelegge J. P., Cramer W. A. 2001. Ferredoxin: NADP(+) oxidoreductase is a subunit of the chloroplast cytochrome b(6)f complex. J. Biol. Chem. 276, 38 159–38 165 10.1074/jbc.M105454200 (doi:10.1074/jbc.M105454200) [DOI] [PubMed] [Google Scholar]
- 87.Hamel P., Olive J., Pierre Y., Wollman F. A., de Vitry C. 2000. A new subunit of cytochrome b(6)f complex undergoes reversible phosphorylation upon state transition. J. Biol. Chem. 275, 17 072–17 079 10.1074/jbc.M001468200 (doi:10.1074/jbc.M001468200) [DOI] [PubMed] [Google Scholar]
- 88.Volkmer T., Schneider D., Bernat G., Kirchhoff H., Wenk S. O., Rogner M. 2007. Ssr2998 of Synechocystis sp PCC 6803 is involved in regulation of cyanobacterial electron transport and associated with the cytochrome b(6)f complex. J. Biol. Chem. 282, 3730–3737 10.1074/jbc.M604948200 (doi:10.1074/jbc.M604948200) [DOI] [PubMed] [Google Scholar]
- 89.Schwenkert S., Legen J., Takami T., Shikanai T., Herrmann R. G., Meurer J. 2007. Role of the low-molecular-weight subunits PetL, PetG, and PetN in assembly, stability, and dimerization of the cytochrome b6f complex in tobacco. Plant Physiol. 144, 1924–1935 10.1104/pp.107.100131 (doi:10.1104/pp.107.100131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herrmann R. G., Westhoff P., Alt J., Tittgen J., Nelson N. 1985. Thylakoid membrane proteins and their genes. In Molecular form and function of the plant genome (eds Vloten-Doting L., Groot G., Hall T.), pp. 233–256 New York, NY: Plenum Publishing [Google Scholar]
- 91.Kuras R., Wollman F. A. 1994. The assembly of cytochrome b6f complexes: an approach using genetic transformation of the green alga Chlamydomonas reinhardtii. EMBO J. 13, 1019–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Monde R. A., Zito F., Olive J., Wollman F. A., Stern D. B. 2000. Post-transcriptional defects in tobacco chloroplast mutants lacking the cytochrome b6/f complex. Plant J. 21, 61–72 10.1046/j.1365-313x.2000.00653.x (doi:10.1046/j.1365-313x.2000.00653.x) [DOI] [PubMed] [Google Scholar]
- 93.Schneider D., Berry S., Rich P., Seidler A., Rögner M. 2001. A regulatory role of the PetM subunit in a cyanobacterial cytochrome b6f complex. J. Biol. Chem. 276, 16 780–16 785 10.1074/jbc.M009503200 (doi:10.1074/jbc.M009503200) [DOI] [PubMed] [Google Scholar]
- 94.Stroebel D., Choquet Y., Popot J. L., Picot D. 2003. An atypical haem in the cytochrome b(6)f complex. Nature 426, 413–418 10.1038/nature02155 (doi:10.1038/nature02155) [DOI] [PubMed] [Google Scholar]
- 95.Kurisu G., Zhang H., Smith J. L., Cramer W. A. 2003. Structure of the cytochrome b6f complex of oxygenic photosynthesis: tuning the cavity. Science 302, 1009–1014 10.1126/science.1090165 (doi:10.1126/science.1090165) [DOI] [PubMed] [Google Scholar]
- 96.Mapller M., Hederstedt L. 2006. Role of membrane-bound thiol-disulfide oxidoreductases in endospore-forming bacteria. Antioxid. Redox Signal. 8, 823–833 10.1089/ars.2006.8.823. (doi:10.1089/ars.2006.8.823.) [DOI] [PubMed] [Google Scholar]
- 97.Kuras R., de Vitry C., Choquet Y., Girard-Bascou J., Culler D., Buschlen S., Merchant S., Wollman F. A. 1997. Molecular genetic identification of a pathway for heme binding to cytochrome b6. J. Biol. Chem. 272, 32 427–32 435 10.1074/jbc.272.51.32427 (doi:10.1074/jbc.272.51.32427) [DOI] [PubMed] [Google Scholar]
- 98.Kuras R., Saint-Marcoux D., Wollman F. A., de Vitry C. 2007. A specific c-type cytochrome maturation system is required for oxygenic photosynthesis. Proc. Natl Acad. Sci. USA 104, 9906–9910 10.1073/pnas.0702340104 (doi:10.1073/pnas.0702340104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lezhneva L., Kuras R., Ephritikhine G., de Vitry C. 2008. A novel pathway of cytochrome c biogenesis is involved in the assembly of the cytochrome b6f complex in Arabidopsis chloroplasts. J. Biol. Chem. 283, 24 608–24 616 10.1074/jbc.M803869200 (doi:10.1074/jbc.M803869200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lyska D., Paradies S., Meierhoff K., Westhoff P. 2007. HCF208, a homolog of Chlamydomonas CCB2, is required for accumulation of native cytochrome b6 in Arabidopsis thaliana. Plant Cell Physiol. 48, 1737–1746 10.1093/pcp/pcm146 (doi:10.1093/pcp/pcm146) [DOI] [PubMed] [Google Scholar]
- 101.Vermaas W. F., Timlim J. A., Jones H. D., Sinclair M. B., Nieman L. T., Hamad S. W., Melgaard D. K., Haaland D. M. 2008. In vivo hyperspectral confocal fluorescence imaging to determine pigment localization and distribution in cyanobacterial cells. Proc. Natl Acad. Sci. USA 105, 4050–4055 10.1073/pnas.0708090105 (doi:10.1073/pnas.0708090105) [DOI] [PMC free article] [PubMed] [Google Scholar]