Abstract
Photosynthetic membrane sacs (thylakoids) of plants form granal stacks interconnected by non-stacked thylakoids, thereby being able to fine-tune (i) photosynthesis, (ii) photoprotection and (iii) acclimation to the environment. Growth in low light leads to the formation of large grana, which sometimes contain as many as 160 thylakoids. The net surface charge of thylakoid membranes is negative, even in low-light-grown plants; so an attractive force is required to overcome the electrostatic repulsion. The theoretical van der Waals attraction is, however, at least 20-fold too small to play the role. We determined the enthalpy change, in the spontaneous stacking of previously unstacked thylakoids in the dark on addition of Mg2+, to be zero or marginally positive (endothermic). The Gibbs free-energy change for the spontaneous process is necessarily negative, a requirement that can be met only by an increase in entropy for an endothermic process. We conclude that the dominant attractive force in thylakoid stacking is entropy-driven. Several mechanisms for increasing entropy upon stacking of thylakoid membranes in the dark, particularly in low-light plants, are discussed. In the light, which drives the chloroplast far away from equilibrium, granal stacking accelerates non-cyclic photophosphorylation, possibly enhancing the rate at which entropy is produced.
Keywords: depletion attraction, electrostatic repulsion, entropy, grana, LHCII, van der Waals attraction
1. Introduction
Understanding the molecular organization of thylakoid membranes of higher-plant chloroplasts and its relationship to the composition of the membrane components and photosynthetic function has been Jan Anderson's career-long passion [1]. Appropriately, a conference with the short title ‘Why grana?’ was held in Schloss Arnsberg, Germany, in 1998, to acknowledge her contributions to photosynthesis research for over half a century, which were further recognized by a Lifetime Achievement Award of the International Society for Photosynthesis Research in 2007.
Thylakoid membranes in higher-plant chloroplasts differentiate into appressed (stacked) and non-appressed (unstacked) regions. The granal stacks are a prominent feature of chloroplast ultrastructure, visible even under a light microscope [2]. The extent of granal stacking varies considerably with environmental growth conditions. Thus, the ratio of appressed-to-non-appressed membranes is generally higher in plants acclimated to low light than in the same plant species grown in high light, and also higher in obligate shade species than in sun species [3–5]. For example, leaves of Alocasia macrorrhiza plants grown in high light possess few thylakoids per granum, but in low incandescent growth light one granum may contain as many as 160 thylakoids [6].
Although not essential for photosynthesis, granal stacking helps to fine-tune (i) photosynthesis, (ii) photoprotection and (iii) acclimation to ever-changing environments [1,7–13]. Some advantages associated with thylakoid stacking include: (i) an extremely large membrane surface-to-volume ratio to accommodate an optimally high density of light-harvesting pigments [14]; (ii) an extreme spatial separation of the two photosystems [15] that limits excessive spillover of excitation energy from photosystem II (PSII) to PSI [7,8], specifically to keep the rapidly functioning PSI and the relatively slow PSII apart [11]; (iii) enhancement of non-cyclic photophosphorylation [16]; (iv) regulation of non-photochemical dissipation of energy [10]; (v) delay of premature degradation of D1 protein in PSII [17]; (vi) regulation of non-cyclic versus cyclic electron transport and the associated photophosphorylation [18]; and (vii) a potential increase in photosynthetic capacity for a given chloroplast composition in full sunlight [12]. Together, these traits derived from granal stacking may have driven the evolutionary selection for grana in higher plants [12].
In this paper, we examine the attractive force that is required to overcome the repulsion between thylakoid membranes carrying net negative charge, and to bring thylakoids together in granal stacks. We conclude that the predominant attractive force appears to be entropy-driven, and propose several mechanisms whereby entropy is increased to drive the formation of granal stacks in the dark. That is, the order in granal stacks arises out of disorder. In the light, the photosynthetic apparatus is driven far from equilibrium, such that maximization of the rate of entropy increase may be important for structural organization, an area of research that is only beginning to be explored.
2. Acclimation to low light results in less abundance of ATP synthase and less steric hindrance to granal stacking
It is physically and energetically unfavourable for the adenosine triphosphate (ATP) synthase, which has a bulky coupling factor 1 knob protruding into the aqueous stromal phase, to be located within the gap between the appressed membranes of granal stacks. Therefore, it is no surprise that, to avoid steric hindrance, the ATP synthase is found only in non-appressed membranes [19], where its catalytic subunits are also free to rotate. Further, the area of non-appressed membranes required to accommodate the ATP synthase [19] and other protein complexes such as PSI [15] and ferredoxin NADP+ reductase [20] depends on the abundance of these complexes. The abundance of the ATP synthase, in particular, decreases with decrease in growth irradiance [21]. This is confirmed by Chow & Anderson [22], as shown in figure 1, in which the ATP synthase content in pea thylakoids is inferred from its maximum hydrolytic capacity (i), or the capacity for cyclic photophosphorylation driven by saturating irradiance and mediated by phenazine methosulfate (ii). It is seen that ATP synthase-dependent activity is low at low growth irradiance, but increases twofold within 3 days after transfer to higher growth irradiance. Although the activities are expressed on a Chl basis, the chlorophyll (Chl) content per unit pea leaf area was scarcely changed after the transfer [23]. By inference, therefore, the ATP synthase content is low in low growth-light.
Figure 1.
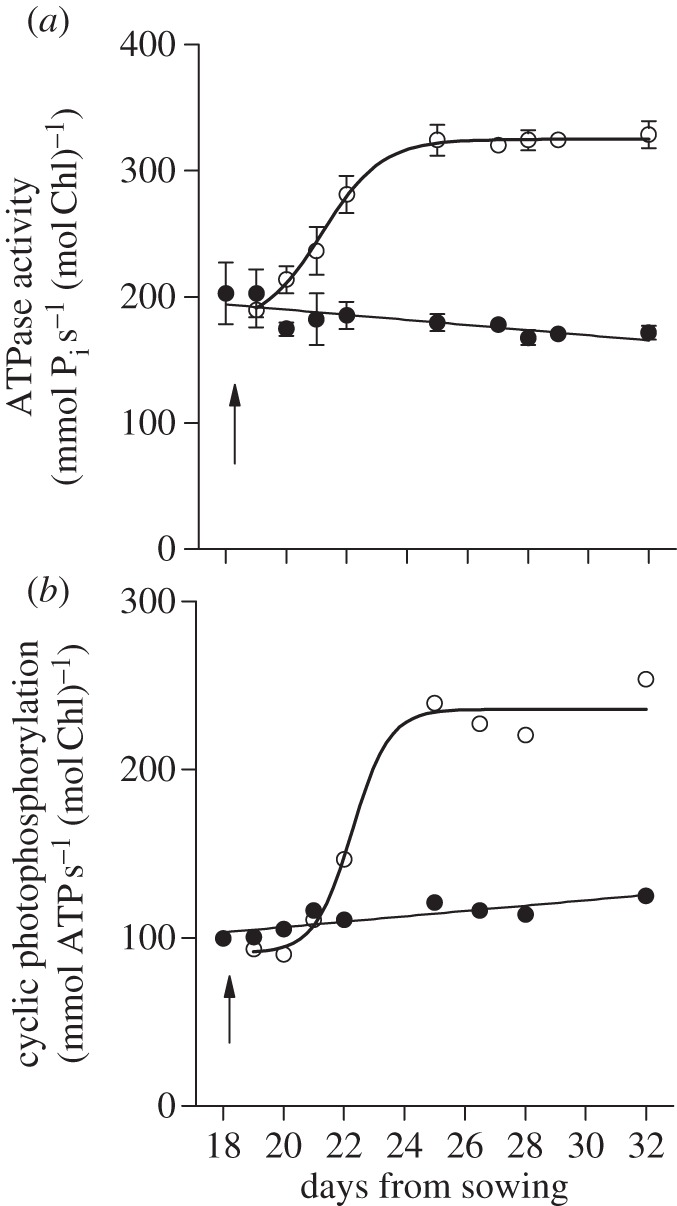
(a) The mean octyl-glucoside-stimulated ATPase activity in three light-transfer experiments ± s.e. (b) Mean phenazine methosulfate-mediated cyclic photophosphorylation rates in two light-transfer experiments. Pea thylakoids were isolated from plants grown in low light (filled circles, 60 μmol photons m−2 s−1) or transferred to high light (open circles, 390 μmol photons m−2 s−1) at the time indicated by the arrow. Re-plotted from fig. 6 of Chow & Anderson [22].
A lower abundance of the ATP synthase at low growth irradiance partly explains the smaller non-appressed membrane area relative to the appressed membrane area. However, it does not explain why net negatively charged thylakoid membranes stack to form grana at all. To elucidate the stacking process, we need to consider the repulsive and attractive forces that operate between thylakoid membranes.
3. Electrostatic repulsion between thylakoids modulates granal stacking
Thylakoid membranes carry net negative charge, as demonstrated by their electrophoretic mobility under an electric field [24], the ability to sequester 9-aminoacridine+ at its diffuse layer adjacent to the membrane surface, and in doing so to bring about concentration quenching of 9-aminoacridine fluorescence [25], and an ability to sequester divalent cations such as 14C-labelled diquat2+ [26] or the non-labelled paraquat2+ (methyl viologen2+, MV) [6]. The value of the surface charge density (σ) obtained in experiments depends on the method used, but a reasonable value is −25 mC m−2 [26,27]. Thus, estimation of σ in relation to growth irradiance, using the release of quenching of 9-aminoacridine fluorescence on separately adding monovalent and divalent cations, and solving two simultaneous equations, yields average values for σ in lettuce thylakoids that increased from −19 to −39 mC m−2 as the growth irradiance increased (table 1, taken from Davies et al. [28]). Analysis of the sequestering of 14C-labelled diquat2+ in the diffuse layer yielded −30 mC m−2 for thylakoids from spinach grown at low irradiance [26]. The use of paraquat2+ gave σ values of approximately −1 mC m−2 for Alocasia grown in low incandescent light and −15 mC m−2 in high fluorescent light [6] (table 1). Because the light-harvesting complex II (LHCII) carries net negative charge on its stromal surface, and is more abundant in plants grown at low irradiance, the lower magnitude of σ in low-light-grown plants is probably due to the lower abundance of the ATP synthase and other membrane components, despite the increase of LHCII.
Table 1.
Surface charge densities (σ) of unstacked thylakoid membranes; values of σ are in mC m−2.
Using 9-aminoacridine+, Kim et al. [29] estimated that σ was −32 mC m−2 in wild-type Arabidopsis thylakoids, and about −22 mC m−2 in two Chl b-less mutants (table 1). Analysis of the sequestering of 14C-labelled diquat2+ yielded −18 mC m−2 for wild-type barley and −10 mC m−2 for Chl b-less barley mutant [26]. The lower magnitude of σ in the Chl b-less mutants of Arabidopsis and barley is almost certainly due to the absence of LHCII, which carries net negative stroma-exposed charge.
In any case, the net surface charge density of thylakoid membranes is negative, implying that the membranes should repel one another; the more negative σ is, the greater is the repulsion. The electrostatic repulsion originates from the formation of a diffuse layer of counterions adjacent to the membrane surface: even though each membrane surface together with its adjacent diffuse layer is electrically neutral, as two membranes approach each other the diffuse layers will be squeezed, resulting in repulsion between two membranes.
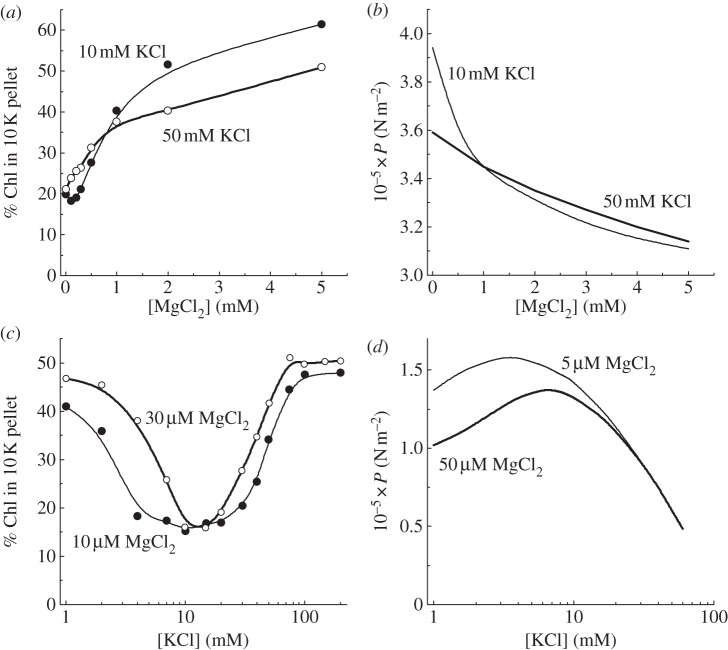
However, the electrostatic repulsion can be modulated by adding different charge-screening cations. To compare the calculated electrostatic repulsion with experiments, we needed a way of conveniently and routinely assaying the extent of granal formation in a large number of envelope-free chloroplasts. For this purpose, we adopted a digitonin treatment of such chloroplasts, with the rationale that digitonin breaks up the stromal lamellae, which form small vesicles that remain in the supernatant upon centrifugation, while the granal stacks are sedimented at 10 000g to form a ‘10 K pellet’ [30]. The Chl content in the pellet (aided by the Chl a/Chl b ratio) gives a good indication of the extent of granal formation. Figure 2a depicts the variation of the extent of stacking with MgCl2 added to a buffer medium with two background KCl concentrations, as reported by Rubin et al. [31]. In the absence of MgCl2, the membranes were unstacked at each background KCl concentration, as indicated by a low Chl content in the 10 K pellet. At 5 mM MgCl2, stacking was maximal. Interestingly, the two curves for the two KCl concentrations crossed each other at a [MgCl2] slightly below 1 mM. The calculated electrostatic repulsion per area of membrane surface (P) decreased with increase in [MgCl2] (figure 2b); the two curves corresponding to two background KCl concentrations also crossed each other at a [MgCl2] similarly slightly below 1 mM. Thus, the extent of granal stacking could be modified according to the modulation of the electrostatic repulsion by screening cations.
Figure 2.
(a,c) Experimental determination of the extent of stacking of pea thylakoids as a function of ionic composition of the suspension medium, and (b,d) the corresponding electrostatic repulsion calculated for each particular ionic medium. Either the monovalent or divalent cation concentration was varied while the other was kept constant. Re-drawn from figs. 2, 3 and 5 of Rubin et al. [31] in which the assumed membrane separation was 1 nm in (b) and 4 nm in (d).
In a low-salt buffer medium with a monovalent cation concentration less than or equal to 1 mM and a low divalent cation concentration in the micromolar range, previously stacked thylakoid membranes remain stacked [32]. When the monovalent cation concentration is increased to several millimolar, the membranes unstack; on increasing the monovalent concentration further to about 100 mM, the membranes stack again (figure 2c). This interesting ‘dip’ was first reported by Gross & Prasher [32]. Duniec et al. [33] and later Rubin et al. [31] calculated the electrostatic repulsion (figure 2d) as a function of monovalent cation concentration at low background concentrations of a divalent cation, obtaining repulsive forces that were qualitatively the mirror image of the extent of stacking, i.e. the peak repulsion (figure 2d) corresponds to the minimum in granal stacking (figure 2c). Further, the peak position shifted to a higher [KCl] when the background [MgCl2] was higher, in approximate correspondence to the shift in the trough in granal stacking. Overall, the results demonstrate that electrostatic repulsion is the main force opposing the formation of grana.
4. The attractive van der Waals force is vastly exceeded by electrostatic repulsion
Given the electrostatic repulsion between thylakoid membranes that carry net negative charge, an attractive force is required to overcome the repulsion and bring about granal stacking. The attractive force could potentially be van der Waals attraction, which is dominated by the London dispersion force among instantaneously induced dipoles [34]. Figure 3 (in which the van der Waals attraction is taken from fig. 3 of Sculley et al. [35]) has been replotted on a linear scale for a better visual comparison between the magnitudes of the electrostatic repulsion and the van der Waals attraction as functions of membrane separation. The van der Waals attraction was calculated for two volume fractions of protein in the membrane, 0.6 (curve b) and 0.4 (curve c). The electrostatic repulsion curve a (at constant surface potential and σ = −25 mC m−2) was calculated by Rubin et al. [30] for a stacking ionic medium containing 5 mM Mg2+ and 10 mM K+. A recent estimate of the inter-membrane distance yielded 3.2 nm [36]. At 3.2 nm membrane separation (vertical line), the calculated van der Waals attraction is at least an order of magnitude too small to match the electrostatic repulsion. It seems unlikely that even if a higher protein volume fraction were assumed, the van der Waals attraction will ever be large enough to match the electrostatic repulsion. Thus, we need to search elsewhere for the predominant component of the attractive force that brings about granal stacking.
Figure 3.
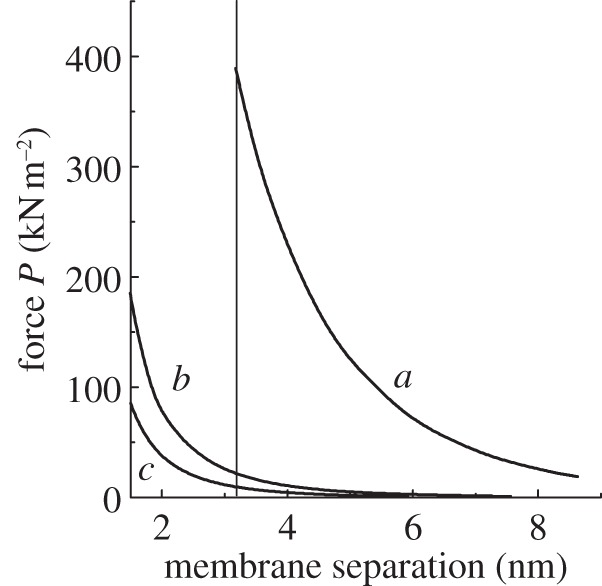
Theoretical calculations of the contributions to the force per unit area, P, between two membranes as a function of separation, re-plotted on a linear scale from Rubin et al. [31]. The curve a represents the electrostatic repulsion in a stacking ionic medium (5 mM Mg2+ together with 10 mM K+), calculated at constant surface potential and σ = −25 mC m−2. Curves b and c (taken from fig. 3 of Sculley et al. [35]) denote the van der Waals attraction, corresponding to dielectric constants of 3 and 2.5, and volume fractions of protein in the membrane of 0.6 and 0.4, respectively. The vertical line indicates the membrane separation recently determined by Daum et al. [36].
5. In the dark, the predominant attractive force that counteracts electrostatic repulsion is entropy-driven
Granal stacking of thylakoid membranes in the dark is an equilibrium process. On adding cations such as MgCl2 to a suspension of previously unstacked thylakoids, the membranes spontaneously stack, reaching a new equilibrium in which the Gibbs free energy (ΔG) is lower than before. That is, the change in ΔG is negative for a spontaneous process. We used the sensitive technique of isothermal titration calorimetry to obtain the enthalpy change (ΔH, heat gained or lost at constant pressure) of granal stacking on adding MgCl2. After accounting for a large heat component unrelated to stacking, we obtained a ΔH value for stacking of 0.04 ± 0.35 kJ (mol Chl)−1 (J. Jia, J. R. Liggins and W. S. Chow 2012, unpublished data). That is, the ΔH for stacking was close to zero; if anything, it was slightly positive (endothermic). Therefore, for ΔG = ΔH−TΔS to be negative, where T is the absolute temperature and ΔS is the entropy change, ΔS has to be positive. That is, the overall entropy of the system is increased upon stacking of thylakoid membranes. This may seem counterintuitive, as granal stacks are ordered membrane structures, associated with which is a laterally heterogeneous (non-random) distribution of membrane protein complexes. However, there are at least five mechanisms by which entropy can be increased upon stacking of thylakoid membranes; these mechanisms operate in chloroplasts in vivo in general, but the first four described below could be particularly facilitated during acclimation to low light owing to the increased abundance of LHCII.
(i) LHCII trimers exist in thylakoid membranes, with discrete domains of negative and positive charge [37], but with an overall negative surface charge. Stacking two surfaces, each with net negative charge, requires a force exerted over a certain distance to reach the final separation between the membranes. That is, work has to be done to bring two membrane surfaces closer, and an input of energy is required in the process. Given that the van der Waals attraction is so small, the net enthalpy change ΔH will be positive (endothermic), and will not help to make ΔG negative, but quite the opposite. However, it has been proposed that during stacking, domains of opposite charge approach each other in a non-specific ‘Velcro’ manner [37]. When two domains of opposite charge come together, the counterions in their adjacent diffuse layers could be released in pairs of oppositely charged ions, each ion eventually able to diffuse freely in the bulk solution, thereby increasing entropy [38] and helping to make ΔG negative. This effect should be accentuated in low-light-acclimated plants because of the abundance of LHCII.
(ii) Similarly, as two surfaces with net negative charge approach each other, such that two domains of opposite charge approach each other in a non-specific Velcro manner, water molecules loosely bound to one domain could be released because the ends of water dipoles pointing away from the domain are somewhat attracted by an approaching domain of opposite charge. Released water molecules will obviously contribute to an increase in entropy. This effect also depends on the abundance of the LHCII that provides the Velcro effect, and is particularly prominent in plants acclimated to a low-light environment.
(iii) Adjacent to each domain of one type of charge, the magnitude of the surface charge density may be much greater than that of the net surface charge density σ on the membrane surface overall; in that case, the electric field strength E near the surface may be strong enough to align water dipoles. Our unpublished calculation of E according to the Gouy–Chapman theory [39] for an unstacking buffer medium gave an E value at the surface such that a water dipole placed in the electric field has an energy of about −2kT, where k is Boltzmann's constant. On the other hand, in a stacking buffer medium such as one containing MgCl2 (which is abundant in the chloroplast stroma), E is fourfold smaller, so that a loss of alignment of water dipoles is expected. The loss of alignment of water dipoles increases orientational freedom and, therefore, the entropy of the system. The increase in entropy is dependent on the number of domains, and therefore the abundance of LHCII. Thus, more abundant LHCII in low-light-acclimated plants enhances the increase in entropy upon stacking of thylakoid membranes.
-
(iv) In addition to the above mechanisms arising from small molecules alone, there is an entropy-driven attraction (depletion attraction) arising from a mixture of small and large particles/macromolecules in chloroplasts [40]. In depletion attraction, large particles are pushed together so as to free up volume for diffusion of the smaller particles when both types of particles are present at a high concentration. Depletion attraction in physics, whereby order is generated from an overall increase in disorder (entropy), is also called macromolecular crowding in biology.
Depletion attraction in thylakoid membranes could drive the initial approach of two PSII monomers in the formation of a PSII dimer [41,42], prior to any interaction of specific subunits. Within the thylakoid membrane, dimerization of membrane protein complexes (‘large particles’) would allow more volume for free diffusion of smaller molecules (‘small particles’) such as plastoquinone and lipids in the plane of the thylakoid membrane. If one views a membrane protein complex along the perpendicular to the membrane, it is approximately a large circle. Similarly, a small molecule is viewed approximately as a small circle. The centre of a small circle cannot be any closer to the large circle than the radius of the small circle. Therefore, surrounding each large circle is an exclusion zone that the centre of a small circle cannot reach. However, when two large circles touch, the exclusion zone is less than the sum of the two separate exclusion zones [40]. Therefore, the formation of a dimer of protein complexes (large circles) provides more space for free diffusion of the small molecules. Formation of trimers of LHCII would similarly increase the volume for diffusion of small molecules, as would the attachment of LHCII trimers to the PSII core. This mechanism may be particularly important in low-light-acclimated plants in which there is an abundance of LHCII.
Not only is the diffusion of small molecules in the plane of the thylakoid membrane increased by aggregation of larger complexes within the thylakoid membrane, but the flexing of the highly unsaturated acyl chains (with bending due to cis-double bonds) of the lipids is also enhanced, thereby increasing entropy. Thus, in this mechanism, the tendency to maximize entropy by maximizing the flexing of acyl chains also drives the aggregation of membrane protein complexes LHCII and PSII to form protein-rich domains; in turn, the aggregated complexes on opposite membrane surfaces position themselves for the Velcro effect mentioned earlier. Conversely, saturated acyl chains are more straight and their flexing more limited, with less entropy to be gained from aggregation of membrane complexes; thus, stacking should be less pronounced. In this regard, it is interesting to note that in a mutant of Arabidopsis deficient in lipid desaturation the amount of appressed membranes was decreased by 48 per cent [43]. Further, during in vitro ageing of isolated thylakoids, the fluidity of the thylakoid membrane was decreased, as demonstrated by an increase in the polarization of 1,6-diphenyl-1,3,5-hexatriene fluorescence; stacking was also impaired, as was the salt-induced increase in the maximum Chl fluorescence yield, although the time courses did not coincide exactly [44].
-
(v) A second example of depletion attraction in chloroplasts concerns a mixture of thylakoid membranes (‘large particles’) and stromal macromolecular complexes (small particles) such as Rubisco (ribulose 1,5-bisphosphate carboxylase/oxygenase) [40]. The centre of the Rubisco complex cannot be closer to the thylakoid membrane than the radius of the complex (approx. 6 nm). Therefore, surrounding the thylakoid surface is an exclusion zone that the centre of the Rubisco complex cannot reach. However, upon stacking of two or more thylakoids, the total exclusion zone becomes smaller, allowing more volume for free diffusion of Rubisco and other macromolecular complexes in the stroma [40]. Because Rubisco is present at a very high concentration, an entropy-driven mechanism that allows freer diffusion of the complex will be favoured.
This mechanism is applicable in general to the chloroplast in vivo, though it may be enhanced in plants acclimated to high light, rather than to low light, because Rubisco and other stromal enzyme complexes are much more abundant in high growth light. Nevertheless, it should contribute to the attractive force even in low-light plants. Experimental support for this mechanism comes from adding macromolecules such as the negatively charged bovine serum albumin or the neutral dextran polymer to a suspension of unstacked thylakoids; stacking was increased in the presence of the added macromolecules [45].
The mechanisms mentioned above point to the maximization of entropy in an equilibrium system as being a predominant factor that underpins the attractive force between thylakoid membranes in the dark. Additionally, the increase in entropy associated with the above mechanisms would offset the decrease in entropy as a result of the steric segregation of PSI and the ATP synthase (both with bulky protrusions facing the stroma) to non-stacked membranes.
For the Velcro effect to operate, however, the membranes need to be brought to a sufficiently close distance between them. Because the required long-range attraction seems unlikely to be sufficiently provided by van der Waals interaction, other possibilities need to be considered. One possibility is that the depletion attraction between thylakoid membranes, owing to the need to maximize the diffusion of macromolecules such as Rubisco in the chloroplast stroma, can be long range in the sense that Rubisco is distributed throughout the stroma at a very high concentration. Another way to bring unstacked membranes into sufficiently close proximity for the Velcro effect to occur is through their ‘wobbling’ motion. Just by chance, some protein-rich domain will occasionally come into the vicinity of another protein-rich domain on an opposite membrane; once the Velcro effect occurs locally, the increase in entropy may lower the Gibbs energy for the local stacking to propagate.
6. In the light, granal stacking accelerates non-cyclic ATP synthesis and may increase entropy production
The discussion presented so far concerns thylakoids in equilibrium in darkness in which maximization of entropy drives the main attractive force between stacked membranes. In the light, by contrast, chloroplasts are driven far from equilibrium by an input of energy. A system far from equilibrium maximizes its entropy production (i.e. maximizes the rate of entropy increase, rather than maximizing entropy), subject to the prevailing constraints, because its state of maximum entropy production (MaxEP) is the most probable sum of its microscopic parts [46]. It has been concluded from theoretical calculations coupled with experimental measurements that the ATP synthase may have been optimized during evolution for MaxEP [47], subject to constraints. At present, it is not clear whether granal stacking has evolved to increase entropy production. To begin to address this possibility, we revisit some experimental findings concerning the rate of ATP synthesis in stacked versus unstacked thylakoids in envelope-free chloroplasts [16].
To compare the rate of ATP synthesis in stacked versus unstacked thylakoids, it is necessary to devise a common reaction buffer, so that differences in rates cannot be attributed to different ionic media. ATP synthesis requires Mg2+, which normally induces stacking. To maintain the unstacked state in a medium containing Mg2+ for investigating ATP synthesis, Chow [16] modified an earlier reaction buffer [48] used to study only electron transport in stacked versus unstacked thylakoids. The search for an appropriate assay medium is based on the idea that, owing to the activation energy that has to be overcome in going from one structural state to the other, stacked thylakoids in an appropriate medium will remain stacked, and unstacked thylakoids will remain unstacked in the same medium [48]. Figure 4a shows the response of the maximum relative Chl fluorescence yield as a function of [Mg2+] in a basic medium containing 100 mM sorbitol, 3 mM adenosine diphosphate (ADP), 2 mM K2HPO4 and 0.1–0.5 mM tricine buffer (pH 8.0), supplemented by 0.1 mM MV, 0.5 mM NaN3 and 10 μM 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU). The maximum Chl fluorescence yield (Fm) is a convenient indicator of stacking, being correlated with the extent of granal stacking obtained using the digitonin method [31]. Thylakoids that had been previously unstacked maintained a low Fm (largely unstacked) at 3.5 mM Mg2+ (vertical line), while pre-stacked thylakoids maintained a high Fm (largely stacked) in the same buffer medium (figure 4a). Evidently, most of the Mg2+ had been complexed by ADP, so that the chosen ionic medium was intermediate between a stacking medium and an unstacking medium, allowing thylakoids to retain their prior structural state. Therefore, this particular ionic composition was chosen for assaying non-cyclic ATP synthesis [16].
Figure 4.
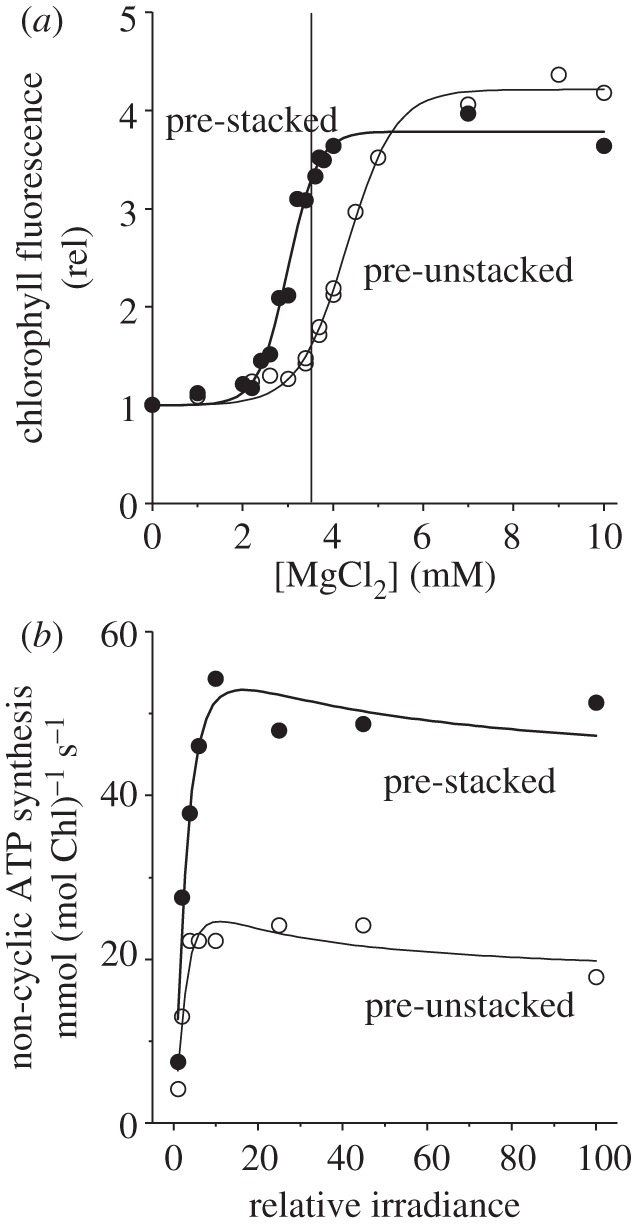
(a) Room-temperature Chl fluorescence (686 nm, excited by blue-green light at about 22 μmol photons m−2 s−1) from pre-stacked or pre-unstacked lettuce thylakoids suspended in a basic medium containing 100 mM sorbitol, 3 mM ADP, 2 mM K2HPO4 and 0.1–0.5 mM tricine buffer (pH 8.0), supplemented with 0.1 mM MV, 0.5 mM NaN3 and 10 μM DCMU with varying [MgCl2]. The vertical line indicates the selected [MgCl2], which allowed pre-stacked or pre-unstacked thylakoids to remain largely in their prior structural state. (b) Steady-state rates of non-cyclic ATP synthesis of pre-stacked or pre-unstacked thylakoids suspended in the same basic medium supplemented with 3.5 mM MgCl2, 0.1 mM MV and 0.5 mM NaN3. The maximum irradiance was 2800 μmol photons m−2 s−1. Re-drawn from figs 1 and 3 of Chow [16].
The non-cyclic rate of ATP synthesis of stacked thylakoids at the highest irradiance was approximately 2.5-fold as high as that of unstacked thylakoids (figure 4b, replotted from fig. 3 of Chow [16]). This experiment was conducted with thylakoids isolated from lettuce plants grown in moderate light (80 W m−2 ≈ 350 µmol photons m−2 s−1). In a separate experiment, the growth irradiance was varied from 20 to 80 to 150 W m−2; the ratio of light-saturated non-cyclic ATP synthesis of stacked membranes to that of unstacked membranes was 1.7, 2.6 and 3.2, respectively, when the assays were done in a single medium [28]. Thus, at all growth irradiances, granal stacking enhanced non-cyclic ATP synthesis, particularly at high growth irradiance but still substantially at low growth irradiance. Differential uncoupling between stacked and unstacked membranes was not large enough to account for the difference in non-cyclic ATP synthesis rate, because the ratio of the photophosphorylation rate to linear electron transport rate (‘P/2e’) was only slightly lower in unstacked membranes [28]. Chow [40] speculated that granal stacking hastens the proton circuit that drives non-cyclic ATP synthesis.
Regardless of the exact mechanism of enhancement of ATP synthesis due to granal stacking, because the ATP synthase is optimized for MaxEP for a given set of constraints [47], granal stacking appears to alter one or more of the constraints, thereby allowing the ATP synthase to produce entropy at a greater rate than it would in the absence of granal stacking.
7. Concluding remarks
While energy conversion and storage are obviously important in photosynthesis, entropy seems to provide a unifying concept for understanding the diverse phenomena of the generation of order in the chloroplast ultrastructure, the aggregation of thylakoid membrane protein complexes, the high degree of unsaturation of thylakoid lipids, the accommodation of a high [Rubisco] in the highly crowded chloroplast stroma and the common occurrence of large grana in low-light-acclimated plants. For plants in the light, driven far from equilibrium, the role of entropy production in photosynthesis has only just begun to be explored.
Acknowledgements
We thank Jan Anderson FRS, FAA, FDhc for helpful comments on this manuscript. Fred Chow expresses his deep gratitude to Jan on the occasion of her 80th birthday, for her formative role as postdoctoral supervisor, colleague, mentor and family friend for more than three decades. This work was partly supported consecutively by Australian Research Council grants (no. DP0664719 and DP1093927).
References
- 1.Anderson J. M. 1999. Insights into the consequences of grana stacking of thylakoid membranes in vascular plants: a personal perspective. Aust. J. Plant Physiol. 26, 625–639 10.1071/PP99070 (doi:10.1071/PP99070) [DOI] [Google Scholar]
- 2.Spencer D., Wildman S. 1962. Observations on the grana-containing chloroplasts and a proposed model of chloroplast structure. Aust. J. Biol. Sci. 15, 599–610 [Google Scholar]
- 3.Boardman N. K. 1977. Comparative photosynthesis of sun and shade plants. Annu. Rev. Plant Physiol. 28, 355–377 10.1146/annurev.pp.28.060177.002035 (doi:10.1146/annurev.pp.28.060177.002035) [DOI] [Google Scholar]
- 4.Lichtenthaler H. K., Buschmann C., Döll M., Fietz H.-J., Bach T., Kozel U., Meier D., Rahmsdorf U. 1981. Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth. Res. 2, 115–141 10.1007/BF00028752 (doi:10.1007/BF00028752) [DOI] [PubMed] [Google Scholar]
- 5.Anderson J. M. 1986. Photoregulation of the composition, function, and structure of thylakoid membranes. Annu. Rev. Plant Physiol. 37, 93–136 10.1146/annurev.pp.37.060186.000521 (doi:10.1146/annurev.pp.37.060186.000521) [DOI] [Google Scholar]
- 6.Chow W. S., Qian L., Goodchild D. J., Anderson J. M. 1988. Photosynthetic acclimation of Alocasia macrorrhiza (L.) G. Don to growth irradiance: structure, function and composition of chloroplasts. Aust. J. Plant Physiol. 15, 107–122 10.1071/PP9880107 (doi:10.1071/PP9880107) [DOI] [Google Scholar]
- 7.Anderson J. M. 1981. Consequences of spatial separation of Photosystem 1 and 2 in thylakoid membranes of higher plant chloroplasts. FEBS Lett. 124, 1–10 10.1016/0014-5793(81)80041-5 (doi:10.1016/0014-5793(81)80041-5) [DOI] [Google Scholar]
- 8.Anderson J. M. 1982. The significance of grana stacking in chlorophyll b-containing chloroplasts. Photobiochem. Photobiophys. 3, 225–241 [Google Scholar]
- 9.Miller K. R., Lyon M. K. 1985. Do we really know why chloroplast membranes stack? Trends Biochem. Sci. 10, 219–222 10.1016/0968-0004(85)90132-X (doi:10.1016/0968-0004(85)90132-X) [DOI] [Google Scholar]
- 10.Horton P. 1999. Are grana necessary for regulation of light harvesting? Aust. J. Plant Physiol. 26, 659–669 10.1071/PP99095 (doi:10.1071/PP99095) [DOI] [Google Scholar]
- 11.Trissl H.-W., Wilhelm C. 1993. Why do thylakoid membranes from higher plants form grana stacks? Trends Biochem. Sci. 18, 415–419 10.1016/0968-0004(93)90136-B (doi:10.1016/0968-0004(93)90136-B) [DOI] [PubMed] [Google Scholar]
- 12.Chow W. S., Kim E.-H., Horton P., Anderson J. M. 2005. Granal stacking of thylakoid membranes in higher plant chloroplasts: the physicochemical forces at work and the functional consequences that ensue. Photochem. Photobiol. Sci. 4, 1081–1099 10.1039/b507310n (doi:10.1039/b507310n) [DOI] [PubMed] [Google Scholar]
- 13.Nevo R., Charuvi D., Tsabari O., Reich Z. 2012. Composition, architecture and dynamics of the photosynthetic apparatus in higher plants. Plant J. 70, 157–176 10.1111/j.1365-313X.2011.04876.x (doi:10.1111/j.1365-313X.2011.04876.x) [DOI] [PubMed] [Google Scholar]
- 14.Barber J. 1980. Membrane surface charges and potentials in relation to photosynthesis. Biochim. Biophys. Acta 594, 253–308 10.1016/0304-4173(80)90003-8 (doi:10.1016/0304-4173(80)90003-8) [DOI] [PubMed] [Google Scholar]
- 15.Andersson B., Anderson J. M. 1980. Lateral heterogeneity in the distribution of chlorophyll–protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim. Biophys. Acta 593, 427–440 10.1016/0005-2728(80)90078-X (doi:10.1016/0005-2728(80)90078-X) [DOI] [PubMed] [Google Scholar]
- 16.Chow W. S. 1984. Electron transport, photophosphorylation and thylakoid stacking. In Advances in photosynthesis research, vol. III (ed. Sybesma C.), pp. 83–86 The Hague, The Netherlands: Dr W. Junk Publishers [Google Scholar]
- 17.Anderson J. M., Aro E.-M. 1994. Grana stacking and the protection of photosystem II membranes of higher plant leaves under sustained high irradiance: an hypothesis. Photosynth. Res. 41, 315–326 10.1007/BF00019409 (doi:10.1007/BF00019409) [DOI] [PubMed] [Google Scholar]
- 18.Joliot P., Joliot A. 2002. Cyclic electron transfer in plant leaf. Proc. Natl Acad. Sci. USA 99, 10 209–10 214 10.1073/pnas.102306999 (doi:10.1073/pnas.102306999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller K. R., Staehelin L. A. 1976. Analysis of the thylakoid outer surface: coupling factor is limited to unstacked membrane regions. Cell Biol. 68, 30–47 10.1083/jcb.68.1.30 (doi:10.1083/jcb.68.1.30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jennings R. C., Garlaschi F. M., Gerola P. D., Forti G. 1979. Partition zone penetration by chymotrypsin, and the localization of the chloroplast flavoprotein and photosystem II. Biochim. Biophys. Acta 546, 207–219 10.1016/0005-2728(79)90040-9 (doi:10.1016/0005-2728(79)90040-9) [DOI] [PubMed] [Google Scholar]
- 21.Berzborn R. J., Müller D., Roos P., Andersson B. 1981. Significance of different quantitative determinations of photosynthetic ATP-synthase CF1 for heterogeneous CF1 distribution and grana formation. In Proc. 5th Int. Congress on photosynthesis, vol. III (ed. Akoyunoglou G.), pp. 107–120 Philadelphia, PA: Balaban International Science Services [Google Scholar]
- 22.Chow W. S., Anderson J. M. 1987. Photosynthetic responses of Pisum sativum to an increase in irradiance during growth. II. Thylakoid membrane components. Aust. J. Plant Physiol. 14, 9–19 10.1071/PP9870009 (doi:10.1071/PP9870009) [DOI] [Google Scholar]
- 23.Chow W. S., Anderson J. M. 1987. Photosynthetic responses of Pisum sativum to an increase in irradiance during growth. I. Photosynthetic activities. Aust. J. Plant Physiol. 14, 1–8 10.1071/PP9870001 (doi:10.1071/PP9870001) [DOI] [Google Scholar]
- 24.Nakatani H. Y., Barber J., Forrester J. A. 1978. Surface charges on chloroplast membranes as studied by particle electrophoresis. Biochim. Biophys. Acta 504, 215–225 10.1016/0005-2728(78)90019-1 (doi:10.1016/0005-2728(78)90019-1) [DOI] [PubMed] [Google Scholar]
- 25.Chow W. S., Barber J. 1980. Salt-dependent changes of 9-aminoacridine fluorescence as a measure of charge densities of membrane surfaces. J. Biochem. Biophys. Methods 3, 173–185 10.1016/0165-022X(80)90016-0 (doi:10.1016/0165-022X(80)90016-0) [DOI] [PubMed] [Google Scholar]
- 26.Chow W. S., Miller C., Anderson J. M. 1991. Surface charges, the heterogeneous lateral distribution of the two photosystems, and thylakoid stacking. Biochim. Biophys. Acta 1057, 69–77 10.1016/S0005-2728(05)80085-4 (doi:10.1016/S0005-2728(05)80085-4) [DOI] [Google Scholar]
- 27.Barber J. 1982. Influence of surface charges on thylakoid structure and function. Annu. Rev. Plant Physiol. 33, 261–295 10.1146/annurev.pp.33.060182.001401 (doi:10.1146/annurev.pp.33.060182.001401) [DOI] [Google Scholar]
- 28.Davies E. C., Chow W. S., Jordan B. R. 1986. A study of factors which regulate the membrane appression of lettuce thylakoids in relation to irradiance. Photosynth. Res. 9, 359–370 10.1007/BF00029800 (doi:10.1007/BF00029800) [DOI] [PubMed] [Google Scholar]
- 29.Kim E.-H., Li X.-P., Razeghifard R., Anderson J. M., Niyogi K. K., Pogson B. J., Chow W. S. 2009. The multiple roles of light-harvesting chlorophyll a/b-protein complexes define structure and optimize function of Arabidopsis chloroplasts: a study using two chlorophyll b-less mutants. Biochim. Biophys. Acta 1787, 973–984 10.1016/j.bbabio.2009.04.009 (doi:10.1016/j.bbabio.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 30.Chow W. S., Thorne S. W., Duniec J. T., Sculley M. J., Boardman N. K. 1980. Stacking of chloroplast thylakoids. Effects of cation screening and cation binding, studied by the digitonin method. Arch. Biochem. Biophys. 201, 347–355 10.1016/0003-9861(80)90520-2 (doi:10.1016/0003-9861(80)90520-2) [DOI] [PubMed] [Google Scholar]
- 31.Rubin B. T., Chow W. S., Barber J. 1981. Experimental and theoretical considerations of mechanisms controlling cation effects on thylakoid membrane stacking and chlorophyll fluorescence. Biochim. Biophys. Acta 634, 174–190 10.1016/0005-2728(81)90137-7 (doi:10.1016/0005-2728(81)90137-7) [DOI] [PubMed] [Google Scholar]
- 32.Gross E. L., Prasher S. H. 1974. Correlation between monovalent cation-induced decrease in chlorophyll a fluorescence and chloroplast structural changes. Arch. Biochem. Biophys. 164, 460–468 10.1016/0003-9861(74)90056-3 (doi:10.1016/0003-9861(74)90056-3) [DOI] [PubMed] [Google Scholar]
- 33.Duniec J. T., Sculley M. J., Thorne S. W. 1979. An analysis of the effect of mono- and di-valent cations on the forces between charged lipid membranes with special reference to the grana thylakoids of chloroplasts. J. Theor. Biol. 79, 473–484 10.1016/0022-5193(79)90238-8 (doi:10.1016/0022-5193(79)90238-8) [DOI] [PubMed] [Google Scholar]
- 34.Israelachvili J. N. 1973. van der Waals forces in biological systems. Q. Rev. Biophys. 6, 341–387 10.1017/S0033583500001566 (doi:10.1017/S0033583500001566) [DOI] [PubMed] [Google Scholar]
- 35.Sculley M. J., Duniec J. T., Thorne S. W., Chow W. S., Boardman N. K. 1980. The stacking of chloroplast thylakoids. Quantitative analysis of the balance of forces between thylakoid membranes of chloroplasts, and the role of divalent cations. Arch. Biochem. Biophys. 201, 339–346 10.1016/0003-9861(80)90519-6 (doi:10.1016/0003-9861(80)90519-6) [DOI] [PubMed] [Google Scholar]
- 36.Daum B., Nicastro D., Austin J., II, McIntosh J. R., Kühlbrandt W. 2010. Arrangement of photosystem II and ATP synthase in chloroplast membranes of spinach and pea. Plant Cell 22, 1299–1312 10.1105/tpc.109.071431 (doi:10.1105/tpc.109.071431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Standfuss J., Terwisscha van Scheltinga A. C., Lamborghini M., Kühlbrandt W. 2005. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution. EMBO J. 24, 919–928 10.1038/sj.emboj.7600585 (doi:10.1038/sj.emboj.7600585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson P. 2004. Biological physics. Energy, information and life, p. 272 New York, NY: W. H. Freeman & Co [Google Scholar]
- 39.Rubin B. T., Barber J. 1980. The role of membrane surface charge in the control of photosynthetic processes and the involvement of electrostatic screening. Biochim. Biophys. Acta 592, 87–102 10.1016/0005-2728(80)90116-4 (doi:10.1016/0005-2728(80)90116-4) [DOI] [PubMed] [Google Scholar]
- 40.Chow W. S. 1999. Grana formation: entropy-assisted local order in chloroplasts? Aust. J. Plant Physiol. 26, 641–647 10.1071/PP99024 (doi:10.1071/PP99024) [DOI] [Google Scholar]
- 41.Satini C., Tidu V., Tognon G., Magaldi A. G., Bassi R. 1994. Three-dimensional structure of the higher-plant photosystem II reaction centre and evidence for its dimeric organization in vivo. Eur. J. Biochem. 221, 307–315 10.1111/j.1432-1033.1994.tb18742.x (doi:10.1111/j.1432-1033.1994.tb18742.x) [DOI] [PubMed] [Google Scholar]
- 42.Boekema E. J., Hankamer B., Bald D., Kruip J., Nield J., Boonstra A. F., Barber J. 1995. Supramolecular structure of the photosystem II complex from green plants and cyanobacteria. Proc. Natl Acad. Sci. USA 92, 175–179 10.1073/pnas.92.1.175 (doi:10.1073/pnas.92.1.175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hugly S., Kunst L., Browse J., Somerville C. 1989. Enhanced thermal tolerance of photosynthesis and altered chloroplast structure in a mutant of Arabidopsis deficient in lipid desaturation. Plant Physiol. 90, 1134–1142 10.1104/pp.90.3.1134 (doi:10.1104/pp.90.3.1134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barber J., Chow W. S., Scoufflaire C., Lannoye R. 1980. The relationship between thylakoid stacking and salt induced chlorophyll fluorescence changes. Biochim. Biophys. Acta 591, 92–103 10.1016/0005-2728(80)90223-6 (doi:10.1016/0005-2728(80)90223-6) [DOI] [PubMed] [Google Scholar]
- 45.Kim E.-H., Chow W. S., Horton P., Anderson J. M. 2005. Entropy-assisted stacking of thylakoid membranes. Biochim. Biophys. Acta 1708, 187–195 10.1016/j.bbabio.2005.03.011 (doi:10.1016/j.bbabio.2005.03.011) [DOI] [PubMed] [Google Scholar]
- 46.Dewar R. C. 2010. Maximum entropy production and optimization theories. Phil. Trans. R. Soc. B 365, 1429–1435 10.1098/rstb.2009.0293 (doi:10.1098/rstb.2009.0293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dewar R. C., Juretic D., Županovic P. 2006. The functional design of the rotary enzyme ATP synthase is consistent with maximum entropy production. Chem. Phys. Lett. 430, 177–182 10.1016/j.cplett.2006.08.095 (doi:10.1016/j.cplett.2006.08.095) [DOI] [Google Scholar]
- 48.Chow W. S. 1984. The extent to which the spatial separation between photosystems I and II associated with granal formation limits non-cyclic electron flow in isolated lettuce chloroplasts. Arch. Biochem. Biophys. 232, 162–171 10.1016/0003-9861(84)90531-9 (doi:10.1016/0003-9861(84)90531-9) [DOI] [PubMed] [Google Scholar]