Abstract
Sexual dichromatism, a form of sexual dimorphism in which males and females differ in colour, is widespread in animals but has been predominantly studied in birds, fishes and butterflies. Moreover, although there are several proposed evolutionary mechanisms for sexual dichromatism in vertebrates, few studies have examined this phenomenon outside the context of sexual selection. Here, we describe unexpectedly high diversity of sexual dichromatism in frogs and create a comparative framework to guide future analyses of the evolution of these sexual colour differences. We review what is known about evolution of colour dimorphism in frogs, highlight alternative mechanisms that may contribute to the evolution of sexual colour differences, and compare them to mechanisms active in other major groups of vertebrates. In frogs, sexual dichromatism can be dynamic (temporary colour change in males) or ontogenetic (permanent colour change in males or females). The degree and the duration of sexual colour differences vary greatly across lineages, and we do not detect phylogenetic signal in the distribution of this trait, therefore frogs provide an opportunity to investigate the roles of natural and sexual selection across multiple independent derivations of sexual dichromatism.
Keywords: sexual colour dimorphism, sexual niche partitioning, anuran
1. Introduction
Sexual dichromatism, a form of sexual dimorphism in which males and females differ in colour, is widespread in animals and is most commonly studied in birds [1,2], fishes [3] and butterflies [4]. In The descent of man [5], Darwin highlighted the strong association between sexually dimorphic traits and related courtship behaviours, thus setting the stage for sexual selection as a primary evolutionary mechanism for sexual dimorphism. In frogs and toads (anurans), the most common form of sexual dimorphism is body size (more than 90% of species), and these differences are attributed to fecundity (when females are larger [6]) or sexual selection (when males are larger [7]). Prior to this study, sexual dichromatism was only known from 25 species (or less than 0.5%) of frogs [8]. Although we have now documented sexual dichromatism in over 120 species (see the electronic supplementary material), both its function and evolution remain poorly understood. In this review we: (i) document the distribution and diversity of sexual dichromatism in frogs; (ii) test whether the phylogenetic distribution of sexual dichromatism reflects shared evolutionary history; (iii) identify circumstances in which sexual selection versus other selective mechanisms may be involved in maintaining sexual dichromatism; and (iv) outline areas of future research related to the evolution and function of sexual dichromatism in frogs.
2. The diversity of sexual dichromatism in frogs
Within frogs, we make a distinction between two broad classes of sexual dichromatism. In the first class, which we refer to as dynamic dichromatism, males undergo a temporary colour change during the breeding season (figure 1a,b). The duration of this dynamic colour change varies across species from only a few hours (e.g. Incilius luetkenii [9]) to several days or weeks during the breeding season (e.g. Rana temporaria [10]). In the second class of dichromatism, which we refer to as ontogenetic dichromatism, either males or females undergo a permanent colour and/or colour pattern change, generally at the onset of sexual maturation (figure 1c,d). The degree of colour differentiation between the sexes ranges from subtle differences in shade (e.g. Scaphiophryne gottlebei [11]) to dramatic differences in both colour and pattern (e.g. Hyperolius argus [12]).
Figure 1.

Examples of frog species showing (a,b) dynamic sexual dichromatism and (c,d) ontogenetic dichromatism. (a) Litoria leseueri (Hylidae): males turn yellow for several days during the breeding season (Photo credit: Stewart Macdonald); (b) Rana arvalis (Ranidae): males turn blue for several weeks during the breeding season (Photo credit: Lars Iversen); (c) Rhinella icterica (Bufonidae): at sexual maturity males are yellow and females are mottled brown and tan. Females retain the juvenile coloration (Photo credit: Célio F. B. Haddad). (d) Hyperolius ocellatus (Hyperoliidae): at sexual maturity males are green with white dorsolateral lines and females are rusty red to silver with small spots. Males retain the juvenile coloration (Photo credit: Rayna C. Bell).
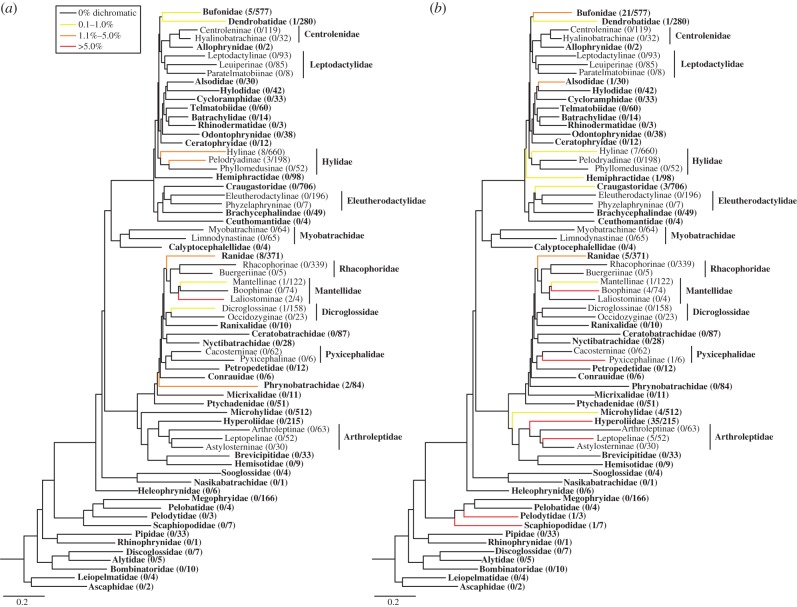
We distinguish between the two classes of dichromatism (dynamic and ontogenetic), and their respective phylogenetic distributions, because they may have important differences in terms of evolutionary lability and function. Dynamic sexual dichromatism is present in 31 species from nine families and subfamilies (see the electronic supplementary material) and is especially prevalent in the Ranidae, Bufonidae and Hylidae (figure 2a). Owing to its ephemeral nature, this class of dichromatism is probably under-documented in the literature and may be far more common among frogs. In particular, we anticipate future records of dynamic dichromatism within lineages where it has already been documented and is fairly common (e.g. Bufonidae). Ontogenetic dichromatism appears to be more taxonomically widespread and is present in 92 species from 18 families and subfamilies (see the electronic supplementary material), though the vast majority of these dichromatic species are in the Hyperoliidae, Bufonidae and Hylidae (figure 2b).
Figure 2.
Phylogenetic distribution of (a) dynamic sexual dichromatism and (b) ontogenetic sexual dichromatism. Families are shown in bold and subfamilies in regular print. Branches are coloured according to the percentage of dichromatic species in each clade and the proportion of dichromatic species is shown in parentheses for each tip. The phylogeny is modified from [13].
Dynamic dichromatism is only present in the ‘neobatrachia’, or modern lineages, of frogs whereas ontogenetic dichromatism is present in several basal lineages as well as the three major modern lineages. These differences in phylogenetic distribution may provide insight into the underlying physiological mechanisms for each type of colour change, and whether similar pathways are employed in both types of dichromatism and across multiple independent evolutionary origins. The species-rich lineages in which sexual dichromatism are absent may be equally informative for understanding the evolution and genetic basis of this trait. For instance, dynamic sexual dichromatism is entirely absent from the primarily ground-dwelling Microhylidae, in which a heavy reliance on crypsis in leaf litter may render temporary male colour change too costly. Alternatively, dichromatism may be absent in these lineages owing to developmental constraint.
3. Characterizing the phylogenetic distribution of sexual dichromatism in frogs
To test for phylogenetic signal in each class of sexual dichromatism, we used the most comprehensive amphibian phylogeny to date [13], which includes representatives from more than 90 per cent of the currently recognized genera and approximately 2400 species (nearly 40% of total frog species diversity). We pruned the Pyron & Wiens tree [13] to the family or subfamily level as applicable, and created an ultrametric version of this tree using the function chronopl, with λ = 0 to approximate non-parametric rate smoothing [14]. Character states for dynamic and ontogenetic dichromatism were then assigned to the appropriate tips (families or subfamilies).
Phylogenetic signal is a measure of how well shared evolutionary history explains the distribution of trait values among terminal taxa and a particular phylogeny. We quantified the degree of phylogenetic signal in both classes of sexual dichromatism using Pagel's lambda (λ) [15], a test statistic that varies from zero to one, where a value of zero indicates that trait evolution is independent of phylogeny and a value of one indicates that shared character states among terminal taxa reflect shared ancestry. We optimized the value of lambda for both classes of sexual dichromatism using maximum likelihood in the fitDiscrete function of geiger with an equal rates character state transition model [16]. To determine whether our phylogenetic signal estimates were significantly greater than zero, we compared the negative log-likelihood values for our original phylogeny with those obtained after transforming the branches in the phylogeny by λ = 0 using the lambdaTree function of geiger [16], which results in a phylogeny without phylogenetic signal. All analyses were performed in R v. 2.13.1.
Although our current numbers of sexually dichromatic frogs are probably underestimated, this review significantly improves our current understanding of the phylogenetic distribution and diversity of this trait. Both ontogenetic dichromatism (λoriginal = 0.000045, log likelihood = −37.52307; λtransformed = 0, log likelihood = −37.52303) and dynamic dichromatism (λoriginal = 0.000045, log likelihood = −27.12687; λtransformed = 0, log likelihood = −27.12684) exhibit values of phylogenetic signal that are not significantly different from zero, indicating that trait evolution is independent of phylogeny. These values indicate that history alone cannot explain the phylogenetic distribution of either dynamic or ontogenetic dichromatism in frogs. Broad macroevolutionary patterns, however, point to specific lineages that merit further study and direct our attention to a diversity of evolutionary mechanisms that may result in sexual dichromatism.
4. Sexual dichromatism and sexual selection
In vertebrates, sexual dichromatism can exist in three general classes: (i) brightly coloured males and drab females, (ii) brightly coloured females and drab males, and (iii) both sexes equally conspicuous but with differences in colour and/or colour pattern. Regardless of the particular class of dichromatism, most studies of sexually dichromatic vertebrates find support for sexual selection as a driving force in the origin and maintenance of this trait. For instance, when males are the brighter sex, male–male competition and female choice are cited as evolutionary mechanisms in a number of vertebrate taxa, including birds [17], fishes [18], lizards [19,20], turtles [21], salamanders [22,23] and primates [24,25]. In cases where females are brighter than males, sexual colour differences may be explained by a sex-role reversal in the mating system in which females compete for males [26]. Finally, when both sexes are bright and differ in coloration, these differences are often attributed to mutual-mate choice, where males and females evaluate the quality of potential mates based on coloration [27].
While sexual selection may be the prevailing evolutionary mechanism underlying sexual dichromatism in vertebrates, alternative mechanisms need to be considered. Sexual niche partitioning, in which males and females use different resources or experience different predation pressures, is implicated in a number of sexually dimorphic taxa [28–30]. Relative to sexual selection, this theory remains largely unexplored in the scientific literature, particularly in the context of sexual dichromatism (but see [31]). The historical bias towards sexual selection may be inherent to the groups that have traditionally been studied, such as birds and fishes that typically have polygynous or promiscuous mating systems with highly visual courtship displays for mate selection. Though dynamic dichromatism in diurnal frogs may be consistent with sexual selection [9], ontogenetic dichromatism in nocturnal species where females and males are equally conspicuous indicates that ecological selection may also be an important selective force. Therefore, sexual dichromatism in frogs, and in particular ontogenetic dichromatism, provides the opportunity to investigate the relative roles of natural and sexual selection across multiple independent derivations of this trait.
5. Evolutionary mechanisms for dynamic sexual dichromatism
Dynamic sexual dichromatism in frogs is probably driven by sexual selection because these temporary colour changes only occur in males and coincide with the mating season [9,32]. Within the realm of sexual selection, this class of dichromatism may serve a variety of functions that are well characterized in other taxa. These potential functions include male–male competition, which is well documented in birds [26], visual signalling between the sexes, which is recognized in at least one frog species [33], and as an honest indicator of mate quality, which has been proposed in birds [34], lizards [35] and some frogs [36]. Though these functions are well characterized in other vertebrate groups, the specific functions of dynamic dichromatism may vary greatly across frog lineages depending on certain aspects of mating system biology, such as reproductive mode and degree of parental care. For instance, in birds, male coloration is a common signal of male quality, including paternal investment in offspring and male genotypic quality [37], whereas in frogs, females typically assess male quality based on body size and advertisement call [38]. Nonetheless, females may use carotenoid-based colour as an honest indicator of male quality in breeding aggregations where acoustic signals are more difficult to assess [36]. Likewise, the duration of temporary colour change across dichromatic species probably varies with mating aggregation size and duration of the breeding season. Migratory birds are 23 times more likely to be dichromatic than non-migratory species, which is hypothesized to be owing to a shorter mate-sampling period for migratory species [2,39]. Therefore, if male coloration in frogs is in fact used to evaluate mate quality, we expect that dynamic dichromatism will be more common in ‘explosive breeders’, or species with shorter breeding seasons [40]; however, the data to test this hypothesis is not yet available.
The underlying physiology of temporary changes in skin coloration and the range of anuran visual acuity may limit the diversity of temporary coloration observed in male frogs. One of the most dramatic temporary colour changes in frogs occurs in Rana arvalis where males are bright blue for several weeks. This colour change may result from destruction of yellow pigments in xanthophores such that blue wavelengths reflected by the iridophores are unfiltered (box 1). In most dynamically dichromatic species, however, temporary coloration in males is either yellower or slightly darker or lighter than the non-breeding coloration. These temporary colour changes are probably accomplished by modulating pigment distribution in xanthophores or melanophores [42]. Although there may be physiological limits as to which temporary colour changes are possible, the high prevalence of yellow or ‘brighter’ colour changes (23 of the 31 dynamic species; figure 3) may provide some insight into anuran vision, female sensory bias or developmental constraint in the types of temporary colour changes that are possible in frogs.
Box 1. Colour variation from three pigment cell types in frog skin.
Interactions between three pigment cell types in the dermis underlie both permanent and temporary coloration in frogs. The layer of pigment and light-reflecting cells (the dermal chromatophore unit) in frog skin includes melanophores, which contain melanin, non-reflecting chromatophores called xanthophores or erythrophores, and reflecting chromatophores called iridophores. The upper layer of this dermal chromatophore unit is composed of non-reflecting chromatophores that are called xanthophores when they bestow yellow coloration and erythrophores when they bestow red coloration. The pigments found in these cells include pteridines, which can be synthesized by the chromatophores, or carotenoids, which are metabolized from the diet. The second cell type, the iridophore, is located below the non-reflecting chromatophores and reflects light with platelets of purine ‘pigments’. This layer creates iridescence by diffracting light within the platelets and interacts with the overlying non-reflecting chromatophores to produce bright colours, such as the bright green coloration present in many frogs [41]. In the absence of non-reflecting chromatophores, iridophores may bestow a structural blue colour [42]. Likewise, when iridophores are reduced, non-reflecting chromatophores may impart bright red and yellow coloration [43]. The third cell type, the melanophore, is the basal-most chromatophore and contains eumelanin that appears black or dark brown. These three layers interact to produce general skin lightening and darkening in response to physiological change [44]. Short-duration colour changes result from hormonal stimulation (primarily melanocyte stimulating hormone and steroid hormones) that causes dispersion or aggregation of pigment-containing organelles [45]. By contrast, permanent or semi-permanent colour changes may involve the synthesis or destruction of pigments [46].
Figure 3.
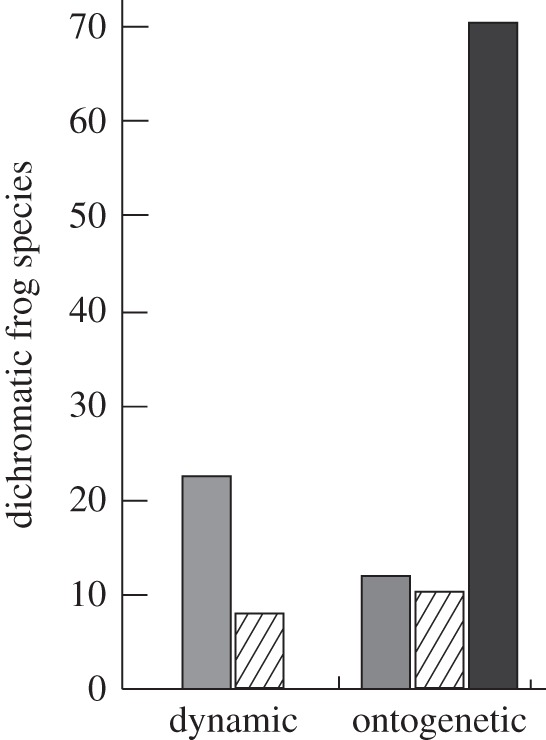
Of the frogs that exhibit dynamic sexual dichromatism, males undergo a temporary colour change to become yellower or brighter than females in 75 per cent of species (grey bar), while in the remaining 25 per cent of species, males become bluish or darker than females (hashed bar). Of the frogs that exhibit ontogenetic dichromatism, males are more conspicuously coloured than females in 13 per cent of species (grey bar), females are more conspicuously coloured than males in 11 per cent of species (hashed bar) and males and females are different coloured but equally conspicuous in 76 per cent of species (black bar).
6. Evolutionary mechanisms for ontogenetic sexual dichromatism
Ontogenetic dichromatism, where one sex undergoes a colour change that is generally coincident with sexual maturation, may potentially result from a combination of both sexual and natural selection [31]. The first sub-class of ontogenetic dichromatism, where males are more brightly coloured than females, is documented in more than 10 frog species (figure 3), the majority of which are found in the Bufonidae and Hylinae. This sub-class of ontogenetic dichromatism is probably subject to similar types of sexual selection as dynamic dichromatism with the exception that sexual colour differences are maintained beyond the mating season. Therefore, the relative contribution of sexual selection versus natural selection in these species will presumably depend on the strength of selection for bright and conspicuous coloration during the breeding season and the strength of selection for (or against) that same coloration during non-breeding periods. For chemically defended frogs, bright coloration in males serves a dual purpose to attract females and as aposematic signals to potential predators (e.g. Bufonidae and Dedrobatidae); therefore, both sexual and natural selection may act in concert in these species to produce brighter coloration in males [47].
The second sub-class of ontogenetic sexual dichromatism, in which females are equally as conspicuous or more conspicuously coloured than males, is especially common in the African hyperoliid treefrogs (35 of the 80 species in this sub-class; figure 3) among which dichromatic species repeatedly evolve from monochromatic species [48]. In several species in this family, sex steroids at the onset of maturation trigger a change in dorsal coloration [49] that results in either bright sexual monochromatism (both sexes become bright at maturity) or sexual dichromatism (females undergo a colour change and males retain the juvenile coloration). There are few hypotheses as to the function of colour differences in frogs with female-biased ontogenetic dichromatism [8]. Bright coloration in females may be sexually selected, providing a benefit in mutual mate choice [27] and female–female competition for limited resources or territoriality [50]. Alternatively, males and females may use different habitats, and differences in coloration may simply provide better camouflage in their respective habitats (e.g. sexual niche partitioning [30,31].
Though sexual niche partitioning has never been formally tested as a mechanism for sexual dichromatism in frogs, sexual differences in habitat use have been examined in other dichromatic vertebrates. For instance, in the Eclectus parrot, where females are bright red/purple and males are emerald green, both intra-sexual competition and inter-sexual differences in exposure to visual predators contribute to sexual dichromatism [31]. Likewise, in many Old World vipers bright striped coloration in males, which confuses visual predators [51,52], is thought to result from increased male exposure to predators when they actively seek females during the breeding season [53,54]. Quantifying sexual ecological differences in habitat use and diet (if colour differences are carotenoid-based) across multiple lineages of sexually dichromatic taxa may indicate that sexual niche partitioning is a more pervasive mechanism than currently appreciated.
7. Future directions and conclusions
Broad ecological factors, such as latitude and range size, correlate with the global distribution of sexual dichromatism in other vertebrates [2,39] and these macroecological patterns point to specific mechanisms driving the distribution of sexual dichromatism; some of these mechanisms may also be relevant in frogs. For example, birds exhibit higher prevalence of dichromatism in temperate regions, and this pattern may result from increased predation pressure at high latitudes [55] resulting in reduced coloration in females [2]. Conversely, sexual dichromatism in frogs appears to be more common among tropical than temperate species (108 and 15 species, respectively, in the electronic supplementary material). Frogs are ancestrally temperate, and the extensive species diversity in the tropics is driven by diversification in a few, more derived lineages [56]; therefore, accounting for the historical effects of latitude on diversification will be necessary to identify whether differences in predation pressure between temperate and tropical environments affect the global distribution of sexual dichromatism in frogs.
Species range size and species richness of a particular breeding community may also be important predictors of sexual dichromatism in vertebrates. Sexually dichromatic birds tend to have broader distributions than monochromatic species [57,58] and sexual dichromatism is often lost on islands [59]. One potential explanation is that selection for dichromatism is correlated with increasing importance of species recognition [21,60]. If sexual dichromatism in frogs enhances species recognition, we might expect that sexual dichromatism is more common in frog communities that form diverse breeding assemblages where other mating signals, such as call, may be insufficient for correctly identifying conspecifics [61].
Finally, ontogenetic dichromatism may also be non-adaptive, particularly in species with distinct juvenile and adult colour phases, such as in hyperoliid treefrogs. The ontogenetic colour change in these species can result in sexual monochromatism if both sexes undergo an identical colour change at sexual maturity, or sexual dichromatism if the ontogenetic pathway is disrupted in one sex such that it retains the juvenile coloration. Because steroid hormones have a similar effect on chromatophores as melanocyte stimulating hormone (box 1), a change in chromatophore sensitivity to either male or female sex hormones could result in the loss of ontogenetic colour change in only one sex. Characterizing the underlying genetics of ontogenetic colour change pathways will be essential for assessing whether non-adaptive evolution can explain the multiple losses of ontogenetic monochromatism, and therefore sexual dichromatism, in this group that accounts for 29 per cent of sexually dichromatic frogs.
Developmental and hormonal skin colour regulation is well characterized in several frog species [43,62,63], providing an excellent framework for studies of the underlying physiology of dynamic and ontogenetic sexual dichromatism (box 1). Likewise, the capacity to discern colour differences is well documented for several diurnal frog species [64–66], therefore applying appropriate vision models to studies of sexual selection in diurnal frog species should be feasible. The extent of anuran spectral sensitivity in low light conditions, however, is largely unknown (but see [67]) and will be a necessary component of dichromatism research in nocturnal species.
Our review highlights that we are rapidly gathering data on the distribution of sexual dichromatism among frog species, but that we still know very little about the function of sexual dichromatism in this group of vertebrates. Our review also underscores the potential benefits of using frogs for investigating the relative roles of natural selection and sexual selection in the evolution of sexual dichromatism, and the opportunity for interpreting those patterns in a comparative framework. In particular, studies that focus on lineages in which dynamic or ontogenetic dichromatism evolve repeatedly hold the most promise for addressing hypotheses about the origin and maintenance of this phenotype in frogs as well as other groups of dichromatic organisms.
Acknowledgements
We thank K. Adler, S. D. Biju, S. Donnellan, D. Edwards, H. W. Greene, C. F. B. Haddad, R. Inger, A. Lima, A. V. Longo, C. P. A. Prado, C. Raxworthy, S. Richards, J. Rowley, B. Stuart and G. Velo-Antón for contributing to our database of dichromatic species. We are grateful to A. Pyron for providing the phylogenetic tree and R. E. Glor, L. J. Harmon, A. V. Longo, C. E. Wagner, M. G. Weber and the PhyloPhunGroup at Cornell for discussion of comparative methods. H. W. Greene, E. Hoffman, C. Moritz, J. L. Parra and J. M. Robertson provided comments that greatly improved the manuscript.
References
- 1.Kimball R. T., Ligon J. D. 1999. Evolution of avian plumage dichromatism from a proximate perspective. Am. Nat. 154, 182–193 10.1086/303228 (doi:10.1086/303228) [DOI] [Google Scholar]
- 2.Badyaev A. V., Hill G. E. 2003. Avian sexual dichromatism in relation to phylogeny and ecology. Annu. Rev. Ecol. Evol. Syst. 34, 27–49 10.1146/annurev.ecolsys.34.011802.132441 (doi:10.1146/annurev.ecolsys.34.011802.132441) [DOI] [Google Scholar]
- 3.Kodric-Brown A. 1998. Sexual dichromatism and temporary color changes in the reproduction of fishes. Am. Zool. 31, 70–81 10.1093/icb/38.1.70 (doi:10.1093/icb/38.1.70) [DOI] [Google Scholar]
- 4.Allen C. E., Zwaan B. J., Brakefield P. M. 2011. Evolution of sexual dimorphism in the Lepidoptera. Annu. Rev. Entomol. 56, 445–464 10.1146/annurev-ento-120709-144828 (doi:10.1146/annurev-ento-120709-144828) [DOI] [PubMed] [Google Scholar]
- 5.Darwin C. 1874. The descent of man, and selection in relation to sex. London, UK: John Murray [Google Scholar]
- 6.Salthe S. N., Duellman W. 1973. Quantitative constrains associated with reproductive mode in anurans. In Evolutionary biology of the anurans (ed. Vial J. L.), pp. 229–249 Columbia, SC: University of Missouri Press [Google Scholar]
- 7.Shine R. 1979. Sexual selection and sexual dimorphism in the amphibia. Copeia 2, 297–306 10.2307/1443418 (doi:10.2307/1443418) [DOI] [Google Scholar]
- 8.Hoffman E. A., Blouin M. S. 2000. A review of colour and pattern polymorphism in anurans. Biol. J. Linnean Soc. 70, 633–665 10.1111/j.1095-8312.2000.tb00221.x (doi:10.1111/j.1095-8312.2000.tb00221.x) [DOI] [Google Scholar]
- 9.Doucet S. M., Mennill D. J. 2010. Dynamic sexual dichromatism in an explosively breeding neotropical toad. Biol. Lett. 6, 63–66 10.1098/rsbl.2009.0604 (doi:10.1098/rsbl.2009.0604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedengren I. 1987. Selection on body size, arm length and colour in male and female moor frogs (Rana arvalis). MSc thesis, Section of Ethology, Department of Zoology, University of Stockholm, Stockholm, Sweden [Google Scholar]
- 11.Glaw F., Vences M. 1994. A field guide to the amphibians and reptiles of Madagascar. Leverkusen & Koln, Germany: Moos Druck/FARBO [Google Scholar]
- 12.Stewart M. M. 1967. Amphibians of Malawi. Albany, NY: State University of New York Press [Google Scholar]
- 13.Pyron R. A., Wiens J. J. 2011. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders and caecilians. Mol. Phylogenet. Evol. 61, 543–583 10.1016/j.ympev.2011.06.012 (doi:10.1016/j.ympev.2011.06.012) [DOI] [PubMed] [Google Scholar]
- 14.Sanderson M. J. 2002. Estimating absolute rates of molecular evolution and divergence times: a penalized likelihood approach. Mol. Biol. Evol. 19, 101–109 10.1093/oxfordjournals.molbev.a003974 (doi:10.1093/oxfordjournals.molbev.a003974) [DOI] [PubMed] [Google Scholar]
- 15.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884 10.1038/44766 (doi:10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 16.Harmon L., Weir J., Brock C., Glor R., Wendell C., Hunt G. 2009. geiger: analysis of evolutionary diversification. R package v. 1.3–1. See http://CRAN.R-project.org/package=geiger
- 17.Andersson M. 1982. Sexual selection, natural selection and quality advertisement. Biol. J. Linnean Soc. 17, 375–393 10.1111/j.1095-8312.1982.tb02028.x (doi:10.1111/j.1095-8312.1982.tb02028.x) [DOI] [Google Scholar]
- 18.Kodric-Brown A., Brown J. H. 1984. Truth in advertising: the kinds of traits favored by sexual selection. Am. Nat. 124, 309–323 10.1086/284275 (doi:10.1086/284275) [DOI] [Google Scholar]
- 19.Olsson M. 1992. Sexual selection and reproductive strategies in the sand lizard (Lacerta agilis). PhD dissertation, University of Göteborg, Göteborg, Sweden [Google Scholar]
- 20.Wiens J. J., Reeder T. W., De Oca A. N. M. 1999. Molecular phylognetics and evolution of sexual dichroamtism among populations of the Yarrow's spiny lizard (Sceloporus jarrovii). Evolution 53, 1884–1897 10.2307/2640448 (doi:10.2307/2640448) [DOI] [PubMed] [Google Scholar]
- 21.Moll E. O., Matson K. E., Krehbiel E. B. 1981. Sexual and seasonal dichromatism in the Asian river turtle Callagur borneoensis. Herpetologica 37, 181–194 [Google Scholar]
- 22.Salthe S. N. 1967. Courtship patterns and the phylogeny of urodeles. Copeia 1967, 100–117 10.2307/1442181 (doi:10.2307/1442181) [DOI] [Google Scholar]
- 23.Todd B. D., Davis A. K. 2007. Sexual dichromatism in the marbled salamander, Ambystoma opacum. Can. J. Zool. 85, 1008–1013 10.1139/Z07-082 (doi:10.1139/Z07-082) [DOI] [Google Scholar]
- 24.Cooper V. J., Hosey G. R. 2003. Sexual dichromatism and female preference in Eulemur fulvus subspecies. Int. J. Primatol. 24, 1177–1188 10.1023/B:IJOP.0000005986.21477.ad (doi:10.1023/B:IJOP.0000005986.21477.ad) [DOI] [Google Scholar]
- 25.Caro T. 2009. Contrasting coloration in terrestrial mammals. Phil. Trans. R. Soc. B 364, 537–548 10.1098/rstb.2008.0221 (doi:10.1098/rstb.2008.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 27.Hanssen S. A., Folstad I., Erikstad K. E. 2006. White plumage reflects individual quality in female eiders. Anim. Behav. 71, 337–343 10.1016/j.anbehav.2005.04.021 (doi:10.1016/j.anbehav.2005.04.021) [DOI] [Google Scholar]
- 28.Feduccia A., Slaughter B. H. 1973. Sexual dimorphism in skates (Rajidae) and its possible role in differential niche utilization. Evolution 28, 164–168 10.2307/2407249 (doi:10.2307/2407249) [DOI] [PubMed] [Google Scholar]
- 29.Partridge L., Green P. 1985. Intraspecific feeding specializations and population dynamics. In Behavioral ecology: ecological consequences of adaptive behavior (eds Sibly R. M., Smith R. H.), pp. 207–226 Oxford, UK: Blackwell Scientific Publications [Google Scholar]
- 30.Shine R. 1989. Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Q. Rev. Biol. 64, 419–461 10.1086/416458 (doi:10.1086/416458) [DOI] [PubMed] [Google Scholar]
- 31.Heinsohn R., Legge S., Endler J. A. 2005. Extreme reversed sexual dichromatism in a bird without sex role reversal. Science 309, 617–619 10.1126/science.1112774 (doi:10.1126/science.1112774) [DOI] [PubMed] [Google Scholar]
- 32.Hettyey A., Herczeg G., Hoi H. 2009. Testing the phenotype-linked fertility hypothesis in male moor frogs (Rana arvalis) exhibiting a conspicuous nuptial colouration. Amphibia-Reptilia 30, 581–586 10.1163/156853809789647086 (doi:10.1163/156853809789647086) [DOI] [Google Scholar]
- 33.Taylor R. C., Buchana B. W., Doherty J. L. 2007. Sexual selection in the squirrel treefrog Hyla squirella: the role of multi-modal cue assessment in female choice. Anim. Behav. 74, 1753–1763 10.1016/j.anbehav.2007.03.010 (doi:10.1016/j.anbehav.2007.03.010) [DOI] [Google Scholar]
- 34.Hamilton W. D., Zuk M. 1982. Heritable true fitness and bright birds: a role for parasites? Science 218, 384–387 10.1126/science.7123238 (doi:10.1126/science.7123238) [DOI] [PubMed] [Google Scholar]
- 35.Martín J., López P. 2010. Multimodal sexual signals in male ocellated lizards Lacerta lepida: vitamin E in scent and green coloration may signal male quality in different sensory channels. Naturwissenschaften 97, 545–553 10.1007/s00114-010-0669-8 (doi:10.1007/s00114-010-0669-8) [DOI] [PubMed] [Google Scholar]
- 36.Vásquez T., Pfennig K. S. 2007. Looking on the bright side: females prefer coloration indicative of male size and condition in the sexually dichromatic spadefoot toad, Scaphiopus couchii. Behav. Ecol. Sociobiol. 62, 127–135 10.1007/s00265-007-0446-7 (doi:10.1007/s00265-007-0446-7) [DOI] [Google Scholar]
- 37.Hill G. E. 1991. Plumage coloration is a sexually selected indicator of male quality. Nature 350, 337–339 10.1038/350337a0 (doi:10.1038/350337a0) [DOI] [Google Scholar]
- 38.Pfennig K. S., Tinsley R. C. 2002. Different mate preferences by parasitized and unparasitized females potentially reduces sexual selection. J. Evol. Biol. 15, 399–406 10.1046/j.1420-9101.2002.00406.x (doi:10.1046/j.1420-9101.2002.00406.x) [DOI] [Google Scholar]
- 39.Friedman N. R., Hofmann C. M., Kondo B., Omland K. E. 2009. Correlated evolution of migration and sexual dichromatism in the new world orioles (Icterus). Evolution 63, 3269–3274 10.1111/j.1558-5646.2009.00792.x (doi:10.1111/j.1558-5646.2009.00792.x) [DOI] [PubMed] [Google Scholar]
- 40.Wells H. D. 1977. The social behaviour of anuran amphibians. Anim. Behav. 25, 666–693 10.1016/0003-3472(77)90118-X (doi:10.1016/0003-3472(77)90118-X) [DOI] [Google Scholar]
- 41.Lyerla T. A., Jameson D. L. 1968. Development of color in chimeras of pacific tree frogs. Copeia 1968, 113–128 10.2307/1441558 (doi:10.2307/1441558) [DOI] [Google Scholar]
- 42.Bagnara J. T. 1998. Comparative anatomy and physiology of pigment cells in non-mammalian tissues. In The pigmentary system: physiology and pathophysiology (eds Nordlund J. J., Boissy R. E., Hearing V. J., King R. A., Ortonne J.-P.), pp. 9–40 New York, NY: Oxford University Press [Google Scholar]
- 43.Frost S. K., Robinson S. J. 1984. Pigment cell differentiation in the fire-bellied toad, Bombina orientalis. I. Structural, chemical, and physical aspects of the adult pigment pattern. J. Morphol. 179, 229–242 10.1002/jmor.1051790303 (doi:10.1002/jmor.1051790303) [DOI] [PubMed] [Google Scholar]
- 44.Frost-Mason S., Morrison R., Masok K. 1994. Pigmentation. In Amphibian biology (ed. Heatwole H.). New South Wales, Australia: Surrey Beatty & Sons [Google Scholar]
- 45.Bagnara J. T. 1976. Color change. In Physiology of the amphibia, vol. 3 (ed. Lofts B.), pp. 1–44 New York, NY: Academic Press [Google Scholar]
- 46.Baker A. S. 1951. A study of the expression of the burnsi gene in adult Rana pipiens. J. Exp. Zool. 116, 191–229 10.1002/jez.1401160202 (doi:10.1002/jez.1401160202) [DOI] [PubMed] [Google Scholar]
- 47.Mann M. E., Cummings M. E. 2009. Sexual dimorphism and directional sexual selection on aposematic signals in a poison frog. Proc. Natl Acad. Sci. USA 106, 19 072–19 077 10.1073/pnas.0903327106 (doi:10.1073/pnas.0903327106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veith M., Kosuch J., Rödel M. O., Hillers A., Schmitz A., Burger M., Lötters S. 2009. Multiple evolution of sexual dichromatism in African reed frogs. Mol. Phylogenet. Evol. 51, 388–393 10.1016/j.ympev.2008.12.022 (doi:10.1016/j.ympev.2008.12.022) [DOI] [PubMed] [Google Scholar]
- 49.Hayes T. B. 1997. Hormonal mechanisms as potential constraints on evolution: examples from the Anura. Am. Zool. 37, 482–490 10.1093/icb/37.6.482 (doi:10.1093/icb/37.6.482) [DOI] [Google Scholar]
- 50.Murphy T. G., Hernández-Muciño D., Osorio-Beristain M., Montgomerie R., Omland K. 2009. Carotenoid-based status signaling by females in the tropical streak-backed oriole. Behav. Ecol. 2, 1000–1006 10.1093/beheco/arp089 (doi:10.1093/beheco/arp089) [DOI] [Google Scholar]
- 51.Jackson J. F., Ingram W., Campbell H. W. 1976. The dorsal pigmentation pattern of snakes as an antipredator strategy: a multivariate approach. Am. Nat. 110, 1029–1053 10.1086/283125 (doi:10.1086/283125) [DOI] [Google Scholar]
- 52.Pough F. H. 1976. Multiple cryptic effects of crossbanded and ringed patterns of snakes. Copeia 1976, 834–836 10.2307/1443481 (doi:10.2307/1443481) [DOI] [Google Scholar]
- 53.Shine R., Madsen T. 1994. Sexual dichromatism in snakes of the genus Vipera: a review and a new evolutionary hypothesis. J. Herpetol. 28, 114–117 10.2307/1564692 (doi:10.2307/1564692) [DOI] [Google Scholar]
- 54.Lindell L. E., Forsman A. 1996. Sexual dichromatism in snakes: support for the flicker-fusion hypothesis. Can. J. Zool. 74, 2254–2256 10.1139/z96-256 (doi:10.1139/z96-256) [DOI] [Google Scholar]
- 55.Martin T. E. 1996. Life history evolution in tropical and south temperate birds: what do we really know? J. Avian Biol. 27, 263–272 10.2307/3677257 (doi:10.2307/3677257) [DOI] [Google Scholar]
- 56.Wiens J. J. 2007. Global patterns of diversification and species richness in amphibians. Am. Nat. 170, S86–S106 10.1086/519396 (doi:10.1086/519396) [DOI] [PubMed] [Google Scholar]
- 57.Badyaev A. V., Ghalambor C. K. 1998. Does a trade-off exist between sexual ornamentation and ecological plasticity? Sexual dichromatism and occupied elevational range in finches. Oikos 82, 319–24 10.2307/3546972 (doi:10.2307/3546972) [DOI] [Google Scholar]
- 58.Price T. 1998. Sexual selection and natural selection in bird speciation. Phil. Trans. R. Soc. Lond. B 353, 251–260 10.1098/rstb.1998.0207 (doi:10.1098/rstb.1998.0207) [DOI] [Google Scholar]
- 59.Peterson A. T. 1996. Geographic variation in sexual dichromatism in birds. Bull. Br. Ornithol. Club 116, 156–172 [Google Scholar]
- 60.Figuerola J., Green A. J. 2000. The evolution of sexual dimorphism in relation to mating patterns, cavity nesting, insularity, and sympatry in the Anseriformes. Funct. Ecol. 14, 701–710 10.1046/j.1365-2435.2000.00474.x (doi:10.1046/j.1365-2435.2000.00474.x) [DOI] [Google Scholar]
- 61.Hebets E. A., Papaj D. R. 2005. Complex signal function: developing a framework of testable hypotheses. Behav. Ecol. Sociobiol. 57, 197–214 10.1007/s00265-004-0865-7 (doi:10.1007/s00265-004-0865-7) [DOI] [Google Scholar]
- 62.Bagnara J. T. 1960. Pineal regulation of the body lightening reaction in amphibian larvae. Science 132, 1481–1483 10.1126/science.132.3438.1481-a (doi:10.1126/science.132.3438.1481-a) [DOI] [PubMed] [Google Scholar]
- 63.Bagnara J. T., Frost S. K., Matsumoto J. 1978. On the development of pigment patterns in amphibians. Am. Zool. 18, 301–312 10.1093/icb/18.2.301 (doi:10.1093/icb/18.2.301) [DOI] [Google Scholar]
- 64.Hailman J. P., Jaeger R. G. 1974. Phototactic responses to spectrally dominant stimuli and use of colour vision by adult anuran amphibians: a comparative study. Anim. Behav. 22, 757–795 10.1016/0003-3472(74)90002-5 (doi:10.1016/0003-3472(74)90002-5) [DOI] [PubMed] [Google Scholar]
- 65.Kondrashev S. L., Gnyubkin V. F., Dimentman A. M. 1976. Role of visual stimuli in the breeding behavior of males of the common frog Rana temporaria the common toad Bufo bufo and the green toad Bufo viridis. Zoologicheskii Zhurnal 55, 1027–1037 [Google Scholar]
- 66.Siddiqi A., Cronin T. W., Loew E. R., Vorobyev M., Summers K. 2004. Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J. Exp. Biol. 207, 2471–2485 10.1242/jeb.01047 (doi:10.1242/jeb.01047) [DOI] [PubMed] [Google Scholar]
- 67.Gomez D., Richardson C., Lengagne T., Derex M., Plenet S., Joly P., Léna J. P., Théry M. 2010. Support for a role of colour vision in mate choice in the nocturnal European treefrog (Hyla arborea). Behaviour 147, 1753–1768 10.1163/000579510X534227 (doi:10.1163/000579510X534227) [DOI] [Google Scholar]