Abstract
Understanding the functional consequences of biodiversity loss is a major goal of ecology. Animal-mediated pollination is an essential ecosystem function and service provided to mankind. However, little is known how pollinator diversity could affect pollination services. Using a substitutive design, we experimentally manipulated functional group (FG) and species richness of pollinator communities to investigate their consequences on the reproductive success of an obligate out-crossing model plant species, Raphanus sativus. Both fruit and seed set increased with pollinator FG richness. Furthermore, seed set increased with species richness in pollinator communities composed of a single FG. However, in multiple-FG communities, highest species richness resulted in slightly reduced pollination services compared with intermediate species richness. Our analysis indicates that the presence of social bees, which showed roughly four times higher visitation rates than solitary bees or hoverflies, was an important factor contributing to the positive pollinator diversity–pollination service relationship, in particular, for fruit set. Visitation rate at different daytimes, and less so among flower heights, varied among social bees, solitary bees and hoverflies, indicating a niche complementarity among these pollinator groups. Our study demonstrates enhanced pollination services of diverse pollinator communities at the plant population level and suggests that both the niche complementarity and the presence of specific taxa in a pollinator community drive this positive relationship.
Keywords: biodiversity–ecosystem function relationship, complementary resource use, ecosystem services, plant–pollinator interactions, positive selection effect, social bees
1. Introduction
Understanding the consequences of biodiversity loss on ecosystem functioning and services has developed into a central theme in ecology [1–3]. Animal pollination is a critical ecosystem service as most angiosperms are pollen-limited [4] and rely on animals for sexual reproduction [5]. A large proportion of the human diet depends directly or indirectly on animal pollination [6]. However, there is evidence that pollinators are declining in many parts of the world as a consequence of environmental degradation [7–9]. Recent research has linked reductions in the pollination of crops and wild plants in intensively managed agro-ecosystems to declines in density and diversity of pollinators [10–18]. However, in these observational studies, aggregate abundance and diversity of pollinators are correlated. Thus, an experimental approach controlling for the confounding effect of aggregate abundance is needed in order to gain a better and more mechanistic understanding of the role of pollinator diversity in the provisioning of pollination services ([19–21], see also [22]).
Mainly three mechanisms have been proposed to explain positive pollinator diversity–pollination service relationships [20]: first, such relationships may result from a positive sampling [23,24] or selection effect [25], by which diverse communities are more likely to include functionally highly effective species or species groups. Pollination effectiveness among pollinator groups can vary in the quality of the pollen transfer as a consequence of morphological and behavioural differences, but primarily owing to variation in flower visitation rates [26]. Secondly, under functional facilitation some community members enhance the functional performance of others [27]. For example, honeybees switched more often between plant individuals of sunflower hybrids in the presence of foraging wild bees, thereby facilitating cross-pollination [28]. Third, functional complementarity through niche partitioning [29] in the flower-visitation patterns of pollinators may lead to enhanced pollination services provided by diverse pollinator communities [16,19,21]. Such niche partitioning may occur at multiple temporal and spatial scales [21]. At a large scale, inter-annual [18] or regional complementarity may enhance pollination services and their stability. Furthermore, diversity effects in pollination may be owing to a diverse plant community being pollinated (corresponding to resource heterogeneity) [19] or to differences among pollinators visiting a single-plant species [21,30]. The latter can occur, for example, when different species in a pollinator community partition their foraging activities during different daytimes [31] or among flowers at different positions within plant individuals [16]. However, experimental knowledge of the functional consequences of pollinator niche partitioning in diverse pollinator communities for the pollination success of single-plant species is currently lacking [but see 22].
In the present study, we experimentally manipulated functional group (FG) richness (one versus three FGs) and species richness (one, three and nine species) of caged pollinator communities in a substitutive design and analysed patterns of pollinator visitation to the single plant Raphanus sativus L. as a model species to address the following questions: (i) How does the presence and richness of pollinator species and FGs affect fruit and seed set of a self-incompatible, insect-pollinated plant species? (ii) Do single FGs and communities of pollinators differ in their temporal (diurnal) and spatial (flower visitation height) niches? While functional facilitation was not a focus of this study, we had the following expectations regarding positive sampling and complementarity effects: under a positive sampling effect, we would expect increased visitation rates and plant reproductive success in the presence of a given pollinator species or FG. If complementarity contributes to a positive pollinator diversity–pollination service relationship, we would expect complementary flower visitation patterns of different pollinator groups during the day or between different flower heights, and a significant contribution of these differential visitation patterns among pollinator groups to increased visitation rates at the pollinator community level in diverse communities.
2. Material and methods
(a). Study plant species
Radish, R. sativus ssp. oleiformes L. (Brassicaceae), is a hermaphroditic, annual herb native to Europe. It produces up to several hundred flowers per plant and is visited by a wide variety of flower visitors, including solitary and social bees, hoverflies and butterflies [13,26,32]. Raphanus sativus has a sporophytic self-incompatibility system and relies on animal pollination for reproduction [33]. Thus, R. sativus is an ideal model species to study the effects of diverse pollinator communities on life-time plant reproductive success [13,26].
(b). Experimental design
For the pollination treatments, 12 cubic cages (side length, 2 m, mesh width, 0.8 mm [19]) were set up in spring 2007 in the experimental garden of the University of Zurich (Switzerland). The cages prevented natural pollination of the experimental plant populations and a ground-covering plastic foil prevented natural plant establishment inside cages. During a pollination treatment a cage contained nine potted, abundantly flowering plants of R. sativus arranged in a grid with a width of 50 cm between plants. During spring 2007, R. sativus had been sown directly into these pots containing 5 l of standardized, nutrient-rich garden soil and grown in a pollinator-free glasshouse. To ensure that flowering plants of roughly the same age and size were available for the pollination treatments, they were sown at four different dates with a time-lag of roughly 1 week. Plants were randomly distributed among cages 3 to 4 days prior to a pollination treatment.
Pollinator species richness during the pollination treatments (one, three and nine species) and pollinator FG richness (one versus three groups) were manipulated in a substitutive design (figure 1) with a constant number of 18 pollinator individuals per community (including single-species communities). In preliminary experiments, we had identified this level of aggregate pollinator abundance to result in visitation rates very similar to those reported for radish under natural conditions ([32,34]; M. Albrecht 2004, unpublished data). The three-species communities were not overlapping and included either three species of a single FG or one species of each of the three FGs (figure 1). The three FGs used in the experiment were defined a priori as social bees (eusocial, large bees), solitary bees (solitary and primitively eusocial, smaller bees) and hoverflies. These three groups are generally considered the most important pollinator FGs in Europe, based on differences in foraging behaviour and morphology ([11,17,19] and references therein). They represent the main FGs pollinating radish [13,32,34], although butterflies can also be functionally important in some regions and ecosystems [26]. Each FG comprised three species, thus pollinator communities of up to nine species were used in the experiment: the social bee species Bombus terrestris L. (A1), Bombus pascuorum Scopoli (A2) and Apis mellifera L. (A3); the solitary bee species Halictus rubicundus Christ (B1), Andrena flavipes Panzer (B2), Lasioglossum sp. (B3); and the hoverfly species Eristalis tenax Latreille (C1), Episyrphus balteatus De Geer (C2) and Sphaerophoria sp. (C3) (figure 1). The Lasioglossum bees were most likely all L. morio Fabricius and the Sphaerophoria hoverflies most likely all S. scripta L., but we cannot totally rule out the possibility that also some individuals of morphologically very similar congeneric species were collected as identification of these species can be difficult. All species are highly generalized, polylectic flower visitors [35,36]. The nine-species community was replicated six times, whereas each unique one- and three-species community was replicated twice (except the single-species ‘community’ of E. tenax, which was replicated three times, while the single-species communities of H. rubicundus and B. pascuorum could not be replicated owing to the low number of individuals of these species available for the experiment).
Figure 1.
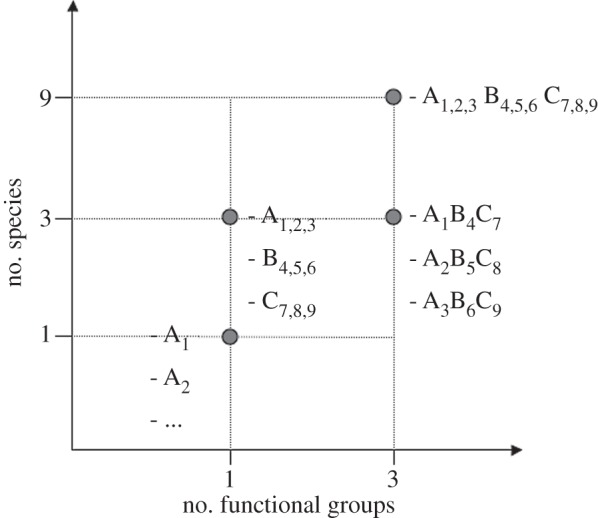
Experimental design. Increasing pollinator richness up to nine species and three functional groups (FGs) (social bees (A), solitary bees (B) and hoverflies (C)), including all single-species ‘communities’, were used in a substitutive design. Three-species communities contained one or three FGs. Communities with three species from three FGs did not overlap.
(c). Pollination rounds
The experiment was conducted during 4 days (four time blocks, hereafter ‘pollination rounds’) on 17 July, 25 July, 1 August and 9 August 2007 under sunny weather conditions. A 1-day period for each pollination round was used because flowers of R. sativus are open and receptive for roughly 1 day, with most ovules available for fertilization for a few hours [37]. Most pollinator species were captured at different locations in the northeast of Switzerland. Individuals of the hoverfly species E. balteatus were purchased as pupae from Katz Biotech AG, Germany. Two large colonies of B. terrestris were purchased from Leu & Gygax AG, Switzerland. For each community comprising B. terrestris half of the individuals required for a certain pollinator treatment were taken from colony 1 and the other half from colony 2. Pollinators (both purchased and captured individuals) were kept in boxes (acryl-glass and fine-meshed fabric; 50 × 50 × 150 cm) in a climate chamber (20°C, 60% humidity) and were fed with sugar- and honey-water and finely ground pollen (Leu & Gygax AG) until the day before using them for a pollination round. For each pollination round, pollinators were introduced into cages at 8 h and removed at 19 h on the same day. Immediately after a pollination round, stalks of open, not wilted flowers of each R. sativus plant were marked with a permanent marker. The next day, potted plants were brought back to the pollinator-free glasshouse until fruit collection. Seed set and fruit set was determined as the number of seeds and number of fruits set, respectively, per marked flower.
(d). Pollinator observations
To investigate possible mechanisms of pollinator-mediated consequences on plant reproduction, the number of visits—and the identity of the pollinator species performing the visit—was recorded for a randomly selected focal plant for each pollinator community treatment. Observations were made during each of the four pollination rounds. No observations were made before 9 h to ensure that pollinators had enough time to calm down after introduction into cages. Bees needed approximately 30 min to calm down before starting to visit flowers, while hoverflies usually started to visit flowers immediately (M. Albrecht 2007, personal observation; in agreement with observation by Fontaine et al. [19]). For each visit to a randomly selected focal plant the height of the flower visited by a pollinator was estimated and assigned to one of three flower height classes (basal, less than 40 cm; medium, between 40 and 80 cm; apical, more than 80 cm). Observations of pollinator communities were done during 30-min observation periods during each of four different daytime periods: 9.00–11.30, 11.30–14.00, 14.00–16.30 and 16.30–19.00. Despite extensive observations (3660 min of total observation time) and several people observing simultaneously during pollination rounds, it was not possible to observe all replicates of the different pollinator communities, and the three-species community A2B2C2 was not observed.
(e). Data analysis
Linear mixed-effects models were fitted using the lme-function of the nlme package supplied in the R-system of statistical computing [38]. A model selection procedure based on Akaike's information criterion (AIC) was used to select the most adequate model, using maximum likelihood for model comparisons and backward selection starting from the full model [39]. Most adequate models were fitted with restricted maximum likelihood and model fit was assessed by testing the residuals for normality and homoscedasticity and by plotting the residuals against the predicted values. Means ± 1 s.e. are reported. The relative importance of the predictor variables of the full model was calculated as the proportion of the total variance explained by each variable using increments of multiple R2 squared (i.e. percentage typ 1 sum of squares) of the fixed model versions of the fitted linear mixed models [40]. The calculated percentages can be used as measures of effect sizes [40].
To test the effect of pollinator FG richness, species richness (and their interaction) and the presence of FGs on the response variables fruit and seed set, they were included as fixed effects in the full model. Species richness was log-transformed because this gave a better fit than linear species richness. However, we also calculated models in which species richness was fitted with a second degree polynomial (i.e. [linear species richness] + [linear species richness]2) to test for a hump-shaped relationship (results not shown). Cage (nested within pollination round) and community composition were included as random effects. Because this analysis indicated that species richness did not explain much variation when fitted after FG richness (see §3), we focused on FG identity and richness as explanatory variables in the subsequent analysis of niche complementarity. The full model, fitted to test whether spatio-temporal resource use (square-root transformed number of visited flowers) differed among pollinator FGs, contained the fixed factors pollinator FG identity, daytime (four daytime periods) and flower height (three height classes), and all their possible interactions, and pollinator species identity, cage and pollination round as random effects. To analyse visitation-rate patterns of whole pollinator communities, the same model but with the fixed effect FG richness instead of FG identity and the random effect pollinator community identity instead of pollinator species identity was fitted. Data have been deposited in the Dryad repository [41].
3. Results
(a). What are the functional consequences of pollinator richness?
Functional group richness of pollinators increased plant seed set (F1,27 = 9.60, p = 0.005; 1 FG: 2.91 ± 0.17, 3 FGs: 3.48 ± 0.28; figure 2a), explaining 55 per cent of the total variation owing to the fixed effects of the full model. However, log(species richness) only explained an additional non-significant amount of 9 per cent (fitted after FG richness in the full model; F1,27 = 1.45, p = 0.239; figure 2a). The presence or absence of social bees explained an additional marginally significant amount of 19 per cent (fitted after FG richness and log(species richness); F1,27 = 3.19, p = 0.085). Furthermore, there was no significant interaction between FG richness and log(species richness) (fitted after FG richness, log(species richness) and presence of social bees; F1,27 = 1.44, p = 0.241).
Figure 2.
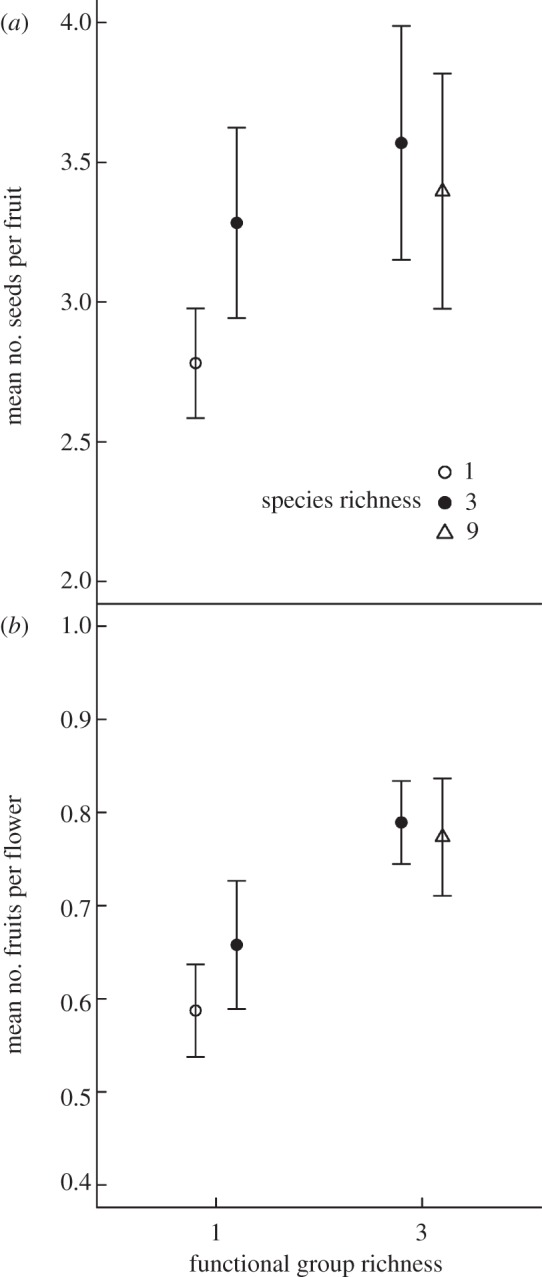
(a) Mean (±1 s.e.) number of seeds per fruit and (b) mean number of fruits per flower of R. sativus at different levels of pollinator FG (social bees, solitary bees, hoverflies) and species richness (see figure 1 for the experimental design).
Functional group richness of pollinators also increased plant fruit set (F1,26 = 10.86, p = 0.003; 1 FG: 0.61 ± 0.04, 3 FGs: 0.78 ± 0.04; figure 2b), explaining 37 per cent of the total variation owing to the fixed effects of the full model. Similar to seed set, the positive effect of log (species richness) on fruit set only explained an additional non-significant amount of 2 per cent (fitted after FG richness in the full model; F1,26 = 0.94, p = 0.342; figure 2b). Most of the total variation in fruit set owing to fixed effects, namely 60 per cent, was explained by the presence or absence of social bees (fitted after FG richness and log(species richness); F1,26 = 19.66, p < 0.001). The interaction between FG richness and log(species richness) was very small and not significant (fitted after FG richness, log(species richness) and presence of social bees; F1,27 = 1.44, p = 0.241). The most adequate model for fruit set contained social bee presence (present: 0.78 ± 0.04, absent: 0.52 ± 0.04) as the only fixed explanatory variable.
(b). Do pollinator functional groups differ in spatio-temporal flower visitation?
Pollinator FGs tended to differ in the number of flowers visited (F2,6 = 4.86, p = 0.055), which was primarily a result of the roughly four times higher visitation rate of social bees compared with solitary bees or hoverflies (figure 3). The three FGs differed in their flower-visitation patterns during the day, irrespective of whether only communities consisting of a single FG were analysed (FG × daytime interaction: F6,87 = 4.81, p < 0.001) or also three-FG communities were included in the analysis (F6,229 = 3.21, p = 0.005; figure 3). However, when only the communities consisting of three FGs were analysed, the three FGs did not significantly differ in flower visitation among the four different daytime periods (F6,108 = 0.92, p = 0.484), but social bees still tended to differ from solitary bees and hoverflies in visitation rate early (morning and noon) compared with later during the day (afternoon and evening; F1,115 = 3.82, p = 0.053). Social bees visited most flowers between 14.00 and 16.30 h, in contrast to solitary bees, visiting most flowers in the morning hours between 9.00 and 11.30 h, while hover flies visited most flowers in the morning and noon, with similar numbers recorded between 9.00 and 11.30 and between 11.30 and 14.00 h, respectively (figure 3). The three FGs did not differ in the relative number of visits to flowers at lower parts of plants compared with more upper parts when single and three FG communities were analysed separately, although there was a trend for social bees to visit a relatively higher number of basal flowers when all communities were analysed (interaction FG × flower height contrast (basal versus medium and apical): F2,69 = 2.56, p = 0.085). While including the FG × daytime interaction (indicating diurnal visitation differences among pollinator groups) substantially enhanced the model fit (ΔAIC 7.18; all communities included), including the FG × flower height interaction only slightly increased model performance (ΔAIC < 2), confirming that diurnal differences in visitation rates among FGs played a more important role than spatial differences (visits to flowers at different heights).
Figure 3.
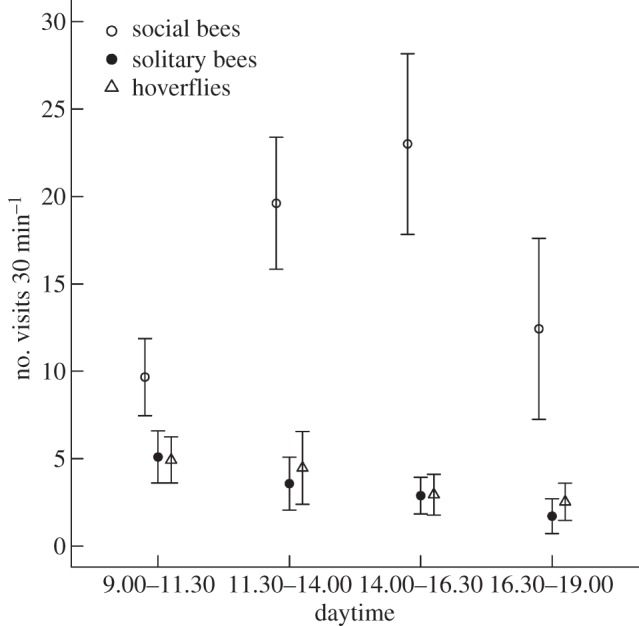
Mean (±1 s.e.) number of flower visits during 30 min of the three FGs social bees, solitary bees and hoverflies at four different daytime periods: 9.00–11.30, 11.30–14.00, 14.00–16.30, 16.30–19.00.
(c). Do pollinator communities differ in spatio-temporal flower visitation?
Analysing visitation rates of whole communities, the average number of visited flowers increased with FG richness from 14.4 (±3.4) to 22.7 (±6.9) visits per plant during 30 min, but this increase was statistically not significant (F1,13 = 1.17, p = 0.299). However, visitation rate was significantly increased in communities including the FG social bees (present: 25.4 ± 3.1, absent: 7.9 ± 2.8; F1,13 = 13.63, p = 0.003). Moreover, the number of flowers a pollinator community visited varied among different daytimes: visitation rate was highest during noon and afternoon (11.30–14.00: 19.3 ± 3.3; 14.00–16.30: 17.1 ± 3.2), somewhat lower in the morning (9.00–11.30: 14.2 ± 1.8) and lowest in the evening (16.30–18.00: 10.4 ± 2.4). The number of visited flowers tended also to be influenced by the position of the flowers within the plant: 22.3 (±2.8) visits per 30 min to flowers at a height of 40–80 cm, 17.2 (±2.5) visits to flowers at a height of more than 80 cm and 7.1 (±1.2) visits to flowers at a height of less than 40 cm. Furthermore, daytime visitation was contingent on whether it included social bees or not (social bee presence in community × daytime interaction: F3,111 = 8.24, p < 0.001): visitation rate of communities containing social bees was roughly twice as high during noon and afternoon (31.3 ± 5.3 and 34.1 ± 5, respectively) than during morning (9.00–11.30: 17.9 ± 3.3) and evening (16.30–18.00: 17 ± 4.7), while visitation rate in communities without social bees was highest in the morning (9.00–11.30: 11.9 ± 2; 11.30–14.00: 8.7 ± 3.1; 14.00–16.30: 6.5 ± 1.8; 16.30–18.00: 5 ± 1.6). The most adequate model explaining variation in flower visitation by the entire pollinator community contained the fixed effect social bee presence in the community, daytime and flower height and the interactions of the latter two with social bee presence.
4. Discussion
(a). What are the functional consequences of pollinator diversity?
Our study demonstrates enhanced population-level reproductive success of an insect-pollinated model species, R. sativus, as a consequence of increased pollination services provided by a higher functional richness of its pollinators. As shown in figure 2, the increase in the fruit and seed set of R. sativus was most pronounced between single- and three-FG communities. The increase in fruit and seed set from single- to three-species assemblages consisting of a single FG was clearly less pronounced. The nine-species communities, however, did not perform significantly better than the communities consisting of three species from three FGs, or their performance was even slightly lower in the case of seed set. This suggests a saturating or, alternatively, a hump-shaped relationship between species richness and pollination service [42,43]. However, our analysis indicates that the latter did not adequately describe the observed patterns. A saturating relationship between biodiversity and ecosystem functioning is predicted by niche theory assuming complementary resource use but increasing niche overlap with increasing richness, and systems characterized by rather generalized interactions [29,44–46]. A hump-shaped relationship between pollinator richness and pollination service is predicted, if increasing species richness leads to a lower proportion of visits a plant receives by the more effective pollinator species [30,42].
In a previous study using caged pollinator communities, Fontaine et al. [19] was able to show that pollinator communities containing the two FGs hoverflies and social bees can increase aggregated plant reproductive success of plant communities consisting of species with open versus tubular flowers, compared with single functional-group assemblages, owing to morphological constraints in short-tongued hoverflies to pollinate plants with tubular flowers, while more efficiently pollinating open flowers. In this case, similar to experiments with plant or bacterial communities, the functional consequences of diversity were stronger in more heterogeneous resource environments [47,48]. In contrast, our study is among the first to experimentally demonstrate positive effects of pollinator richness in a more homogeneous pollinator resource environment, namely that of a single-plant species. From the plant's point of view this indicates that even at the population level a diverse pollinator community may increase plant reproductive success.
Apart from the study of Fontaine et al. [19] and the present study, existing evidence for positive effects of pollinator species diversity on pollination services comes mainly from correlational studies of animal-pollinated crop [10,12,16] and wild-plant species [13,42]. Some of these correlations between pollinator diversity and pollination service have been attributed to temporal complementarity among years [10] or combined spatio-temporal complementarity [16]. However, collinearity among aggregate abundance and diversity of pollinators in these studies makes it difficult to assess the importance of different components of pollinator diversity and the mechanisms driving the observed patterns [20,21]. Indeed, a recent simulation study suggested that similar functional patterns may arise from the relationship between relative abundance and the effectiveness of the pollinator species present in diverse communities [30]. Our results demonstrate positive pollinator diversity effects on pollination services and plant reproductive success that are independent of aggregate pollinator abundance.
Despite the clear need for controlled experiments to address some of the important aspects of the functional consequences of pollinator diversity [19–21], they come at the cost of simplifying some of the real-world complexity. Cages represent an artificial environment to pollinators, hindering them in the performance of some types of natural behaviour, such as the provisioning of nests in the case of bees. However, in agreement with observations of Fontaine et al. [19], visitation rates and duration of flower visits of foraging pollinators were in the range of those observed under natural conditions for the model plant species (M. Albrecht 2004, unpublished data). Pollinator communities of a generalized plant species such as R. sativus are likely to be more species-rich, at least those of plant populations in relatively un-degraded habitats [13,42]. From our study using a relatively low maximum pollinator number, we cannot rule out the possibility that at considerably higher levels of pollinator richness the richness–pollination service relationship becomes humped-shaped [42] if negative selection effects owing to many highly inefficient pollinators or nectar-robbing flower visitors [49] play a more important role. Furthermore, it is conceivable that at high richness antagonistic pollinator interactions in the simple one-resource plant environment, e.g. through disturbance or even competitive exclusion of functionally superior pollination service providers by inferior ones from some pollination niches, could lead to negative complementarity effects. Such increases in antagonistic interactions in simple resource environments leading to negative biodiversity effects have recently been found in bacterial biodiversity–ecosystem functioning experiments [46,50]. More complex resource environments including larger temporal and spatial scales are expected to considerably broaden the scope for complementarity effects and associated increased ecosystem functioning [18,51–53].
(b). What are the drivers of the positive pollinator richness effects?
Our results indicate that the presence of social bees in a community was an important factor explaining positive pollinator richness effects on seed set, and in particular fruit set, of R. sativus, suggesting that a positive selection effect [25] played an important role in the observed diversity effects. Indeed, social bees visited roughly four times more flowers than solitary bees or hoverflies, and the three social bee species showed the highest pollination service, measured as fruit and seed set, in the single-species treatments. The pollinator species providing the highest pollination service was the bumblebee B. pascuorum.
However, we found strong evidence that—in addition to the higher overall visitation rates of social bees—niche complementarity was a key mechanism driving the positive pollinator richness–pollination service relationship: the three FGs differed in their relative foraging activity at different times of the day—social bees showing particularly distinct diurnal visitation patterns compared with solitary bees and hoverflies—and they tended to differ in the relative number of flowers visited at different heights within plants.
By exploiting different spatio-temporal niches, pollinators can maximize their resource–use efficiency, while simultaneously increasing pollination efficiency at the community level [20,21]. Diurnal foraging activity is determined by intrinsic factors, such as physiological attributes and environmental tolerances, and behavioural responses in relation to the daily course of extrinsic factors [54] that may result in pollinator species-specific or pollinator group-specific ‘daily activity windows’ [31,51,55,56]. In our study, solitary bees foraged most in the morning, hoverflies in the morning and noon and social bees in the afternoon. Similarly, peak visitation rates of bumblebees and honeybees in the afternoon have been observed for other wild plant and crop species in temperate climates, which have mostly been attributed to positive temperature–foraging activity relationships [57]. Our findings are also in agreement with the scarce existing data on diurnal foraging activity of hoverflies, suggesting that pollen feeding of most species is highest in the morning hours [58]. Previous observations of foraging activity patterns of solitary bees indicate that they can be highly variable, with some solitary bee species visiting more flowers in the morning than in the afternoon [59], while others show bimodal patterns with peaks in the morning and afternoon [31]. In our study, social bumblebees and honeybees, in contrast to the other pollinator groups, also continued to forage after 18 h (M. Albrecht 2007, personal observation). In many plant species, including R. sativus, stigma receptivity is rather short (usually a few hours) and can show some variation among plant individuals during the day [37], which could have contributed to the importance of diurnal complementarity in pollinator foraging activity.
Spatio-temporal niche partitioning in pollinator communities is likely to be greatest in heterogeneous or plant species-rich landscapes that offer a broad array of niches to be partitioned [20,53]. This is in accordance with the general finding that biodiversity effects increase with biotope space [48]. Indeed, our results indicate that even within a simple environment with a single flowering plant as a resource, such spatio-temporal niche partitioning was effective, with the temporal component of complementarity in diurnal visitation times being clearly more important than the spatial component of complementarity in flower visitation heights.
5. Conclusions
Our study demonstrates enhanced pollination-mediated reproductive success in a single-plant species owing to higher pollinator functional-group richness—and if only one FG is present also at higher species richness—independent of aggregate pollinator abundance. Such fine-scale functional effects of pollinator richness are likely to be important for the population dynamics of local populations of natural plant species, and have economic implications for the many animal-pollinated plant crops worldwide, which are typically grown as monocultures of single-plant species [6]. In agreement with predictions of biodiversity–ecosystem functioning relationships for simple resource environments, highest species richness resulted in slightly reduced pollination services compared with intermediate levels of species richness. Our results suggest that both complementarity effects, primarily resulting from different realized daytime niches among pollinator FGs, and the presence of particular taxa in a pollinator community, in our case social bees, contributed to the positive pollinator richness–pollination service relationship. These findings provide an important step towards a more mechanistic understanding of the effect of pollinator diversity on pollination services. Our results emphasize the importance of the conservation and restoration of diverse pollinator communities for the provisioning of pollination services to animal-pollinated plants.
Acknowledgements
We thank Jochen Fründ and Colin Fontaine for valuable discussions and advice, and two anonymous reviewers for excellent suggestions that considerably improved an earlier version of the manuscript. We further thank Dominique Bühler, Lauren Cottle, Anna Kopps, Angelica Lopez, Kevin Richards, Daniel Trujillo and Tobias Züst for assistance at various stages of the experiment and the analysis of fruit and seed set. Thanks for funding the project go to the Swiss National Science Foundation (grant to CBM; 631-065950).
References
- 1.Hooper D. U., et al. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35 10.1890/04-0922 (doi:10.1890/04-0922) [DOI] [Google Scholar]
- 2.Balvanera P., Pfisterer A. B., Buchmann N., He J.-S., Nakashizuka T., Raffaelli D., Schmid B. 2006. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 9, 1146–1156 10.1111/j.1461-0248.2006.00963.x (doi:10.1111/j.1461-0248.2006.00963.x) [DOI] [PubMed] [Google Scholar]
- 3.Cardinale B. J., Srivastava D. S., Duffy J. E., Wright J. P., Downing A. L., Sankaran M., Jouseau C. 2006. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443, 989–992 10.1038/nature05202 (doi:10.1038/nature05202) [DOI] [PubMed] [Google Scholar]
- 4.Ashman T.-L., et al. 2004. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85, 2408–2421 10.1890/03-8024 (doi:10.1890/03-8024) [DOI] [Google Scholar]
- 5.Kearns C. A., Inouye D. W., Waser N. M. 1998. Endangered mutualisms: the conservation of plant–pollinator interactions. Annu. Rev. Ecol. Syst. 29, 83–112 10.1146/annurev.ecolsys.29.1.83 (doi:10.1146/annurev.ecolsys.29.1.83) [DOI] [Google Scholar]
- 6.Klein A. M., Vaissiere B. E., Cane J. H., Steffan-Dewenter I., Cunningham S. A., Kremen C., Tscharntke T. 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 274, 303–313 10.1098/rspb.2006.3721 (doi:10.1098/rspb.2006.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biesmeijer J. C., et al. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354 10.1126/science.1127863 (doi:10.1126/science.1127863) [DOI] [PubMed] [Google Scholar]
- 8.Winfree R., Aguilar R., Vázquez D. P., LeBuhn G., Aizen M. A. 2009. A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 90, 2068–2076 10.1890/08-1245.1 (doi:10.1890/08-1245.1) [DOI] [PubMed] [Google Scholar]
- 9.Potts S. G., Biesmeijer J. C., Kremen C., Neumann P., Schweiger O., Kunin W. E. 2010. Global pollinator declines: trends, impacts and drivers. TREE 25, 345–353 10.1016/j.tree.2010.01.007 (doi:10.1016/j.tree.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 10.Kremen C., Williams N. M., Thorp R. W. 2002. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl Acad. Sci. USA. 99, 16 812–16 816 10.1073/pnas.262413599 (doi:10.1073/pnas.262413599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steffan-Dewenter I., Münzenberg U., Bürger C., Thies C., Tscharntke T. 2002. Scale-dependent effects of landscape context on three pollinator guilds. Ecology 83, 1421–1432 10.1890/0012-9658(2002)083[1421:SDEOLC]2.0.CO;2 (doi:10.1890/0012-9658(2002)083[1421:SDEOLC]2.0.CO;2) [DOI] [Google Scholar]
- 12.Klein A. M., Steffan-Dewenter I., Tscharntke T. 2003. Fruit set of highland coffee increases with the diversity of pollinating bees. Proc. R. Soc. Lond. B 270, 955–961 10.1098/rspb.2002.2306 (doi:10.1098/rspb.2002.2306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albrecht M., Duelli P., Muller C., Kleijn D., Schmid B. 2007. The Swiss agri-environment scheme enhances pollinator diversity and plant reproductive success in nearby intensively managed farmland. J. Appl. Ecol. 44, 813–822 10.1111/j.1365-2664.2007.01306.x (doi:10.1111/j.1365-2664.2007.01306.x) [DOI] [Google Scholar]
- 14.Kremen C., et al. 2007. Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol. Lett. 10, 299–314 10.1111/j.1461-0248.2007.01018.x (doi:10.1111/j.1461-0248.2007.01018.x) [DOI] [PubMed] [Google Scholar]
- 15.Winfree R., Williams N. M., Dushoff J., Kremen C. 2007. Native bees provide insurance against ongoing honey bee losses. Ecol. Lett. 10, 1105–1113 10.1111/j.1461-0248.2007.01110.x (doi:10.1111/j.1461-0248.2007.01110.x) [DOI] [PubMed] [Google Scholar]
- 16.Hoehn P., Tscharntke T., Tylianakis J. M., Steffan-Dewenter I. 2008. Functional group diversity of bee pollinators increases crop yield. Proc. R. Soc. B 275, 2283–2291 10.1098/rspb.2008.0405 (doi:10.1098/rspb.2008.0405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricketts T. H., et al. 2008. Landscape effects on crop pollination services: Are there general patterns? Ecol. Lett. 11, 499–515 10.1111/j.1461-0248.2008.01157.x (doi:10.1111/j.1461-0248.2008.01157.x) [DOI] [PubMed] [Google Scholar]
- 18.Winfree R., Kremen C. 2009. Are ecosystem services stabilized by differences among species? A test using crop pollination. Proc. R. Soc. B 276, 229–237 10.1098/rspb.2008.0709 (doi:10.1098/rspb.2008.0709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontaine C., Dajoz I., Meriguet J., Loreau M. 2006. Functional diversity of plant–pollinator interaction webs enhances the persistence of plant communities. PloS Biol. 4, 129–135 10.1371/journal.pbio.0040001 (doi:10.1371/journal.pbio.0040001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein A. M., Müller C. B., Hoehn P., Kremen C. 2009. Understanding the role of species richness for crop pollination services. In Biodiversity, ecosystem functioning, and human wellbeing (eds Naeem S., Bunker D. E., Hector A., Loreau M., Perrings C.), pp. 195–208 New York, NY: Oxford University Press [Google Scholar]
- 21.Blüthgen N., Klein A. M. 2011. Functional complementarity and specialisation: the role of biodiversity in plant–pollinator interactions. Basic Appl. Ecol. 12, 282–291 10.1016/j.baae.2010.1011.1001 (doi:10.1016/j.baae.2010.1011.1001) [DOI] [Google Scholar]
- 22.Paschke M., Abs C., Schmid B. 2002. Effects of population size and pollen diversity on reproductive success and offspring size in the narrow endemic Cochlearia bavarica (Brassicaceae). Am. J. Bot. 89, 1250–1259 10.3732/ajb.89.8.1250 (doi:10.3732/ajb.89.8.1250) [DOI] [PubMed] [Google Scholar]
- 23.Huston M. A. 1997. Hidden treatments in ecological experiments: re-evaluating the ecosystem function of biodiversity. Oecologia 110, 449–460 10.1007/s004420050180 (doi:10.1007/s004420050180) [DOI] [PubMed] [Google Scholar]
- 24.Tilman D., Lehman C. L., Thomson K. T. 1997. Plant diversity and ecosystem productivity: theoretical considerations. Proc. Natl Acad. Sci. USA 94, 1857–1861 10.1073/pnas.94.5.1857 (doi:10.1073/pnas.94.5.1857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loreau M., Hector A. 2001. Partitioning selection and complementarity in biodiversity experiments. Nature 412, 72–76 10.1038/35083573 (doi:10.1038/35083573) [DOI] [PubMed] [Google Scholar]
- 26.Sahli H. F., Conner J. K. 2007. Visitation, effectiveness, and efficiency of 15 genera of visitiors to wild radish, Raphanus raphanistrum (Brassicaceae). Am. J. Bot. 94, 203–209 10.3732/ajb.94.2.203 (doi:10.3732/ajb.94.2.203) [DOI] [PubMed] [Google Scholar]
- 27.Cardinale B. J., Palmer M. A., Collins S. L. 2002. Species diversity increases ecosystem functioning through interspecific facilitation. Nature 415, 426–429 10.1038/415426a (doi:10.1038/415426a) [DOI] [PubMed] [Google Scholar]
- 28.Greenleaf S. S., Kremen C. 2006. Wild bees enhance honey bees’ pollination of hybrid sunflower. Proc. Natl Acad. Sci. USA 103, 13 890–13 895 10.1073/pnas.0600929103 (doi:10.1073/pnas.0600929103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loreau M. 1998. Biodiversity and ecosystem functioning: a mechanistic model. Proc. Natl Acad. Sci. USA 95, 5632–5636 10.1073/pnas.95.10.5632 (doi:10.1073/pnas.95.10.5632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perfectti F., Gómez J. M., Bosch J. 2009. The functional consequences of diversity in plant–pollinator interactions. Oikos 118, 1430–1440 (doi:10.1111/j.1600–0706.2009.17491.x) [DOI] [Google Scholar]
- 31.Stone G. N., Gilbert F., Willmer P., Potts S., Semida F., Zalat S. 1999. Windows of opportunity and the temporal structuring of foraging activity in a desert solitary bee. Ecol. Entomol. 24, 208–221 10.1046/j.1365-2311.1999.00181.x (doi:10.1046/j.1365-2311.1999.00181.x) [DOI] [Google Scholar]
- 32.Stanton M., Young H. J., Ellstrand N. C., Clegg J. M. 1991. Consequences of floral variation for male and female reproduction in experimental populations of wild radish (Raphanus sativus L.). Evolution 45, 268–280 10.2307/2409662 (doi:10.2307/2409662) [DOI] [PubMed] [Google Scholar]
- 33.Karron J. D., Marshall D. L., Oliveras D. M. 1990. Numbers of sporophytic self-incompatibility alleles in populations of wild radish. Theor. Appl. Genet. 79, 457–460 10.1007/BF00226152 (doi:10.1007/BF00226152) [DOI] [PubMed] [Google Scholar]
- 34.Conner J. K., Rush S. 1996. Effects of flower size and number on pollinator visitation to wild radish, Raphanus raphanistrum. Oecologia 105, 509–516 10.1007/BF00330014 (doi:10.1007/BF00330014) [DOI] [PubMed] [Google Scholar]
- 35.Westrich P. 1990. Die Wildbienen Baden-Württembergs: Volume 2. Stuttgart, Germany: Ulmer Verlag [Google Scholar]
- 36.Kormann K. 1988. Schwebfliegen Mitteleuropas. München, Germany: Landsberg [Google Scholar]
- 37.Ashman T.-L., Galloway L. F., Stanton Ms. L. 1993. Apparent versus effective mating in an experimental population of Raphanus sativus. Oecologia 96, 102–107 10.1007/BF00318036 (doi:10.1007/BF00318036) [DOI] [PubMed] [Google Scholar]
- 38.R Development Core Team 2009. R: A language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
- 39.Burnham K. P., Anderson D. R. 2002. Model selection and multimodel inference, 2nd edn New York, NY: Springer [Google Scholar]
- 40.Grömping U. 2006. Relative importance for linear regression in R: the package relaimpo. J. Stat. Softw. 17, 1–27 [Google Scholar]
- 41.Albrecht M., Schmid B., Hautier Y., Müller C. B. Data from: Diverse pollinator communities enhance plant reproductive success. Dryad Digital Repository. See http://dx.doi.org/10.5061/dryad.8gj1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gómez J. M., Bosch J., Perfectti F., Fernández J., Abdelaziz M. 2007. Pollinator diversity affects plant reproduction and recruitment: the tradeoffs of generalization. Oecologia 153, 597–605 10.1007/s00442-007-0758-3 (doi:10.1007/s00442-007-0758-3) [DOI] [PubMed] [Google Scholar]
- 43.Finke D. L., Snyder W. E. 2008. Niche partitioning increases resource exploitation by diverse communities. Science 321, 1488–1490 10.1126/science.1160854 (doi:10.1126/science.1160854) [DOI] [PubMed] [Google Scholar]
- 44.Schmid B., Joshi J., Schläpfer F. 2002. Empirical evidence for biodiversity–ecosystem functioning relationships. In Functional consequences of biodiversity: experimental progress and theoretical extensions (eds Kinzig A., Tilman D., Pacala P.), pp. 120–150 Princeton, NJ: Princeton University Press [Google Scholar]
- 45.Srinivasan U. T., Dunne J. A., Harte J., Martinez N. D. 2007. Response of complex food webs to realistic extinction sequences. Ecology 88, 671–682 10.1890/06-0971 (doi:10.1890/06-0971) [DOI] [PubMed] [Google Scholar]
- 46.Schmid B., Balvaneara P., Cardinale B. J., Godbold J., Pfisterer A. B., Raffaelli D., Solan M., Srivastava D. S. 2009. Consequences of species loss for ecosystem functioning: meta-analyses of data from biodiversity experiments. In Biodiversity, ecosystem functioning and human wellbeing: an ecological and economic perspective (eds Naeem S., Bunker D. E., Hector A., Loreau M., Perrings C.), pp. 14–29 New York, NY: Oxford University Press [Google Scholar]
- 47.Jousset A., Schmid B., Scheu S., Eisenhauer N. 2011. Genotypic richness and dissimilarity opposingly affect ecosystem functioning. Ecol. Lett. 14, 537–545 (doi:10.1111/j.1461–0248.2011.01613.x) [DOI] [PubMed] [Google Scholar]
- 48.Dimitrakopoulos P. G., Schmid B. 2004. Positive biodiversity effects increase linearly with biotope space. Ecol. Lett. 7, 574–583 10.1111/j.1461-0248.2004.00607.x (doi:10.1111/j.1461-0248.2004.00607.x) [DOI] [Google Scholar]
- 49.Irwin R. E., Bronstein J. L., Manson J. S., Richardson L. 2010. Nectar robbing: ecological and evolutionary perspectives. Annu. Rev. Ecol. Evol. Syst. 41, 271–292 10.1146/annurev.ecolsys.110308.120330 (doi:10.1146/annurev.ecolsys.110308.120330) [DOI] [Google Scholar]
- 50.Becker J., Eisenhauer N., Scheu S., Jousset A. 2012. Increasing antagonistic interactions cause bacterial communities to collapse at high diversity. Eocl. Lett. 15, 468–474 10.1111/j.1461-0248.2012.01759.x (doi:10.1111/j.1461-0248.2012.01759.x) [DOI] [PubMed] [Google Scholar]
- 51.Herrera C. M. 1990. Daily patterns of pollinator activity, differential pollinating effectiveness, and floral resource availability, in a summer-flowering Mediterranean shrub. Oikos 58, 277–288 10.2307/3545218 (doi:10.2307/3545218) [DOI] [Google Scholar]
- 52.Cardinale B. J., Ives A. R., Inchausti P. 2004. Effects of species diversity on the primary productivity of ecosystems: extending our spatial and temporal scales of inference. Oikos 104, 437–450 10.1111/j.0030-1299.2004.13254.x (doi:10.1111/j.0030-1299.2004.13254.x) [DOI] [Google Scholar]
- 53.Tylianakis J. M., Rand T. A., Ansgar K., Klein A. M., Buchmann N., Perner J., Tscharntke T. 2008. Resource heterogeneity moderates the biodiversity–function relationship in real world ecosystems. PLoS Biol. 6, e122. 10.1371/journal.pbio.0060122 (doi:10.1371/journal.pbio.0060122) [DOI] [Google Scholar]
- 54.Stone G. N. 1994. Activity patterns of females of the solitary bee Anthophora plumipes in relation to temperature, nectar supplies and body-size. Ecol. Entomol. 19, 177–189 10.1111/j.1365-2311.1994.tb00408.x (doi:10.1111/j.1365-2311.1994.tb00408.x) [DOI] [Google Scholar]
- 55.Willmer P. G., Corbet S. A. 1981. Temporal and microclimatic partitioning of the floral resources of Justicia aurea amongst a concourse of pollen vectors and nectar robbers. Oecologia 51, 67–78 10.1007/BF00344655 (doi:10.1007/BF00344655) [DOI] [PubMed] [Google Scholar]
- 56.Vicens V., Bosch J. 2000. Weather-dependent pollinator activity in an apple orchard, with special reference to Osmia cornuta and Apis mellifera (Hymenoptera, Megachilidae and Apidae). Environ. Entomol. 29, 413–420 10.1603/0046-222X-29.3.413 (doi:10.1603/0046-222X-29.3.413) [DOI] [Google Scholar]
- 57.Free J. B. 1968. Foraging behaviour of honeybees (Apis mellifera), and bumblebees (Bombus spp) on blackcurrant (Ribes nigrum), raspberry (Rubus idaeus) and strawberry (Fragaria x Ananassa) flowers. J. Appl. Ecol. 5, 157–168 10.2307/2401280 (doi:10.2307/2401280) [DOI] [Google Scholar]
- 58.Gilbert F. 1985. Diurnal activity patterns in hoverflies (Diptera, Syrphidae). Ecol. Entomol. 10, 385–392 10.1111/j.1365-2311.1985.tb00736.x (doi:10.1111/j.1365-2311.1985.tb00736.x) [DOI] [Google Scholar]
- 59.Motten A. F., Campbell D. R., Alexander D. E., Miller H. L. 1981. Pollination effectiveness of specialist and generalist visitors of a North-Carolina population of Claytonia virginica. Ecology 62, 1278–1287 10.2307/1937292 (doi:10.2307/1937292) [DOI] [Google Scholar]


