Abstract
Epithelial sodium channel (ENaC) is a Na+-selective, aldosterone-stimulated ion channel involved in sodium transport homeostasis. ENaC is rate-limiting for Na+ absorption in the epithelia of osmoregulatory organs of tetrapods. Although the ENaC/degenerin gene family is proposed to be present in metazoans, no orthologues or paralogues for ENaC have been found in the genome databases of teleosts. We studied full-length cDNA cloning and tissue distributions of ENaCα, β and γ subunits in the Australian lungfish, Neoceratodus forsteri, which is the closest living relative of tetrapods. Neoceratodus ENaC (nENaC) comprised three subunits: nENaCα, β and γ proteins. The nENaCα, β and γ subunits are closely related to amphibian ENaCα, β and γ subunits, respectively. Three ENaC subunit mRNAs were highly expressed in the gills, kidney and rectum. Amiloride-sensitive sodium current was recorded from Xenopus oocytes injected with the nENaCαβγ subunit complementary RNAs under a two-electrode voltage clamp. nENaCα immunoreactivity was observed in the apical cell membrane of the gills, kidney and rectum. Thus, nENaC may play a role in regulating sodium transport of the lungfish, which has a renin–angiotensin–aldosterone system. This is interesting because there may have been an ENaC sodium absorption system controlled by aldosterone before the conquest of land by vertebrates.
Keywords: evolution of ion channel, sodium transport, Xenopus oocyte, immunoreactivity, Dipnoi
1. Introduction
It is generally accepted now that the vertebrate transition from water to land was via a sarcopterygian fish ancestor. Sarcopterygians (lobe-finned fishes) separated from actinopterygians (ray-finned fishes) prior to the Devonian (great age of fishes), at least 416 Ma. Teleosts (the most numerous actinopterygian fish today) had not evolved at the time of this separation. Thus, they have been separated from tetrapods for more than 416 Myr. More recently, evidence has been accumulating to suggest that, of the living sarcopterygian fishes, lungfish (Dipnoi) are closer to the ancestral tetrapod than are the coelacanths [1]; and the Australian lungfish (Neoceratodus forsteri) is the most primitive lungfish [2]. So, it is to Neoceratodus that we must look for clues concerning the physiological pre-adaptations for terrestriality. One such pre-adaptation would be an efficient epithelial sodium channel (ENaC). The ENaC is a Na+-selective, non-voltage-gated, non-inactivating ion channel of the ENaC/degenerin (ENaC/Deg) gene family [3–5]. The ENaC/Deg gene family has been proposed as being present in metazoans [6]; ENaC/Deg proteins participate in several physiological processes, ranging from ion homeostasis in epithelia to mechanosensation and synaptic transmission in neurons. In the ENaC/Deg family, ENaC is essential for regulating Na+ transport, mediating electrogenic Na+ transport; it is rate-limiting for Na+ absorption in the epithelia of the kidney, colon, skin, airways, keratinocytes and taste cells in tetrapods [7]. ENaC comprises at least three homologous subunits: ENaCα, β and γ [3,7–9]. Each subunit (size: 85–95 kDa) shares approximately 30–40% sequence identity with the other two. It has two presumed membrane-spanning domains, a large extracellular loop and intracellular N- and C-termini. ENaC subunit stoichiometry has been the subject of several studies since the initial cloning of three subunits. Recent crystallographic data obtained for related acid-sensing ion channels suggest that ENaC most likely functions as an ENaCα, β and γ heterotrimer in the plasma membrane [10–12]. The ENaCα-subunit appears to be the core-conducting element, whereas the β and γ subunits are associated with trafficking and insertion of the channel in the cell membrane. A fourth ENaC subunit, δ, has been cloned and is a member of the ENaC/Deg family. The ENaCδ is distinct from the ENaCα, β and γ subunits, known for their role in sodium homeostasis, as ENaCδ is expressed in the brain and activated by external protons [13]. The ENaC subunits have been cloned in some non-mammalian vertebrates: chicken, lizard, salamander, clawed frog, toad, coelacanth and lamprey [14–17 and Ensemble Genome Browser (http://www.ensembl.org/index.html)]. However, no orthologues or paralogues have been identified in teleost fishes (e.g. zebrafish, fugu, stickleback, medaka). Because of the findings of the same three subunits for the coelacanth, it is assumed that ENaC was lost in teleosts, probably at the time of the whole genome duplication when a number of genes were lost or rearranged rather than duplicated [18].
Our recent cloning of ENaCα subunit from a primitive urodele species, expressed in the external gills and pronephric ducts of larvae and in the ventral skin and mesonephros of adults, leads us to conclude that ENaC plays an important role in the osmoregulation of aquatic and terrestrial vertebrates. Thus, ENaC is important for the evolution of terrestriality during the evolution of early tetrapods, in addition to the acquisition of aldosterone as the most potent sodium-retaining factor in the kidney and colon [19]. Aldosterone is present in tetrapods and in Neoceratodus [20], whereas it is absent in teleosts, elasmobranchs and agnathans [21]. Because Neoceratodus has been shown to have aldosterone, which is capable of increasing sodium retention, we thought to find out whether it may also possess ENaCs as precursors of osmoregulation for terrestriality. We sought to identify the three subunits described in all tetrapod species examined to date.
2. Material and methods
(a). Animals
Juvenile lungfish, N. forsteri, were obtained from the breeding colony at Macquarie University in Sydney, Australia (Macquarie University Animal Ethics Committee approval number: 2003/001). They were transported with CITES approval to the University of Toyama, Japan.
(b). Molecular cloning of Australian lungfish N. forsteri epithelial sodium channel cDNA
Total RNA was extracted from the N. forsteri gill, using ISOGEN (Nippon Gene, Tokyo, Japan). First-strand gill cDNA was synthesized with a first-strand cDNA synthesis kit (Roche Diagnostics, Basel, Switzerland). Full-length nENaCα, ENaCβ and ENaCγ cDNAs were obtained by 5′- or 3′-rapid amplification of cDNA ends with adaptor primers (Takara Bio, Otsu, Japan) and ENaCα-, β- and γ-gene specific primers (see the electronic supplementary material, table S1).
(c). Tissue distribution of Neoceratodus ENaCα, ENaCβ and ENaCγ mRNA
Tissue expression of nENaCα, β and γ mRNA was examined by reverse transcriptase PCR (RT-PCR). Total RNA was isolated from various tissues (brain, eye, gill, heart, liver, lung, muscle, small intestine and rectum), using ISOGEN (Nippon Gene). To prepare first-strand cDNA, total RNA (1 μg) was reverse-transcribed with a first-strand cDNA synthesis kit (Roche Diagnostics). Specific PCR primers were synthesized based on nENaCα, β and γ. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal standard.
(d). Expression and function of Neoceratodus epithelial sodium channel subunits in Xenopus oocytes
Complementary RNAs (cRNAs) for nENaCα, β and γ subunits were transcribed in vitro with an mMESSAGE mMACHINE T7 ultra kit (Ambion, USA). For functional expression studies, isolated Xenopus oocytes were treated with collagenase and then injected with the cRNAs. Oocytes were analysed electrophysiologically 24 h after cRNA injection, using a two-electrode voltage clamp technique, as described previously [22]. Briefly, oocyte membrane potential was held at 0 mV and stepped to test potentials from −100 to +60 mV in increments of 10 mV. Current was recorded in a solution containing 96 mM NaCl, 2 mM KCl, 3 mM MgCl2 and 5 mM HEPES (pH 7.4). A macroscopic amiloride-sensitive nENaCαβγ current was obtained as the difference between the recorded currents from the same oocyte before and after adding 10 µM amiloride.
(e). Immunohistochemistry and Western blot for Neoceratodus epithelial sodium channel in Xenopus oocytes
For whole-mount immunostaining, oocytes were fixed overnight with 4 per cent paraformaldehyde at room temperature. Oocytes were incubated overnight in MAB (maleic buffer: 0.1 M maleic acid, 0.15 M NaCl, pH 7.5) solution containing 1 : 2000 rabbit anti-Bufo ENaCα polyclonal antiserum [23] at 4°C. The anti-ENaCα polyclonal antiserum was raised in Japanese white rabbits with a synthetic peptide, NH2–CNNTTIHGAIR–COOH, corresponding to amino acids 27–37 of the Bufo ENaCα-subunit. In the corresponding sites (aa 32–42) of the nENaCα-subunit, only one amino acid, alanine (Bufo ENaCα 35), is different from threonine (nENaCα 40). They were then incubated in blocking solution for 30 min, followed by incubation with a secondary antibody (1 : 500 Alexa-488 conjugated goat, anti-rabbit IgG; Invitrogen) for 2 h at room temperature. After rinses with PBT (1% bovine serum albumin, BSA/0.1% Triton X-100 in 1X phosphate-buffered saline, PBS), oocytes were observed with a dissecting microscope (Olympus SZX7) with a fluorescent module. For Western blot analysis of oocytes membrane preparations, whole membranes were isolated from oocytes. A total membrane sample was subjected to SDS–PAGE, using a 10 per cent resolving gel and a 5 per cent stacking gel. Proteins were transferred to nitrocellulose membranes, and Western blot analysis using an affinity-purified, anti-Bufo ENaC rabbit IgG fraction at 2 µg ml−1.
(f). Immunohistochemistry for Neoceratodus epithelial sodium channel in the kidney, gill and rectum
Tissue samples that had been immersion-fixed with 4 per cent paraformaldehyde/PBS were dehydrated and embedded in paraffin. Deparaffinized sections (6 μm thick) were incubated overnight at 4°C with rabbit anti-Bufo ENaCα antiserum [16] at 1 : 500–4000 dilutions in 1 per cent BSA–PBS. Rabbit anti-salmon Na+, K+-ATPase α-subunit antiserum was also used [23]. After incubation, the sections were treated with Alexa Fluor 488-labelled, anti-rabbit IgG (dilution 1 : 200; Molecular Probes, USA) for 2 h at room temperature. Stained sections were observed and photographed under a fluorescence microscope equipped with a digital imaging system (Axioplan/AxioCam; Zeiss, Germany).
(g). Statistical methods
Data are presented as the mean ± s.e. Statistical comparisons performed were by ANOVA followed by Bonferroni multiple comparisons post hoc test. A p-value of less than 0.05 was considered significant.
Further detailed methods and figures are provided in the electronic supplementary material, section ‘materials and methods’ and figure legends.
3. Results
(a). Molecular cloning of Neoceratodus epithelial sodium channel subunits
Neoceratodus ENaC (nENaC) comprises three subunits: nENaCα, β and γ. A full-length cDNA sequence for nENaCα was 2558 bp long with a 1965 bp open reading frame translating to a putative sequence of 655 amino acids (DDBJ accession no. AB675922; figure 1(α)). A full-length cDNA sequence for nENaCβ was 2512 bp long with a 1953 bp open reading frame translating to a putative sequence of 651 amino acids (DDBJ accession no. AB675923; figure 1(β)). A full-length cDNA sequence for nENaCγ was 2351 bp long with a 1959 bp open reading frame translating to a putative sequence of 653 amino acids (DDBJ accession no. AB675944; figure 1(γ)).
Figure 1.

The deduced amino acid sequence of nENaCα, β and γ proteins isolated from the gills of Neoceratodus forsteri. Plus symbols represent corresponding region of amino acids used as the anitigen to generate ENaC antibody. The grey shadows represent a consensus site for furin cleavage (RXXR). The dark shadows represent PY motif (PPxY) that is highly conserved in the C-terminus of ENaC subunits.
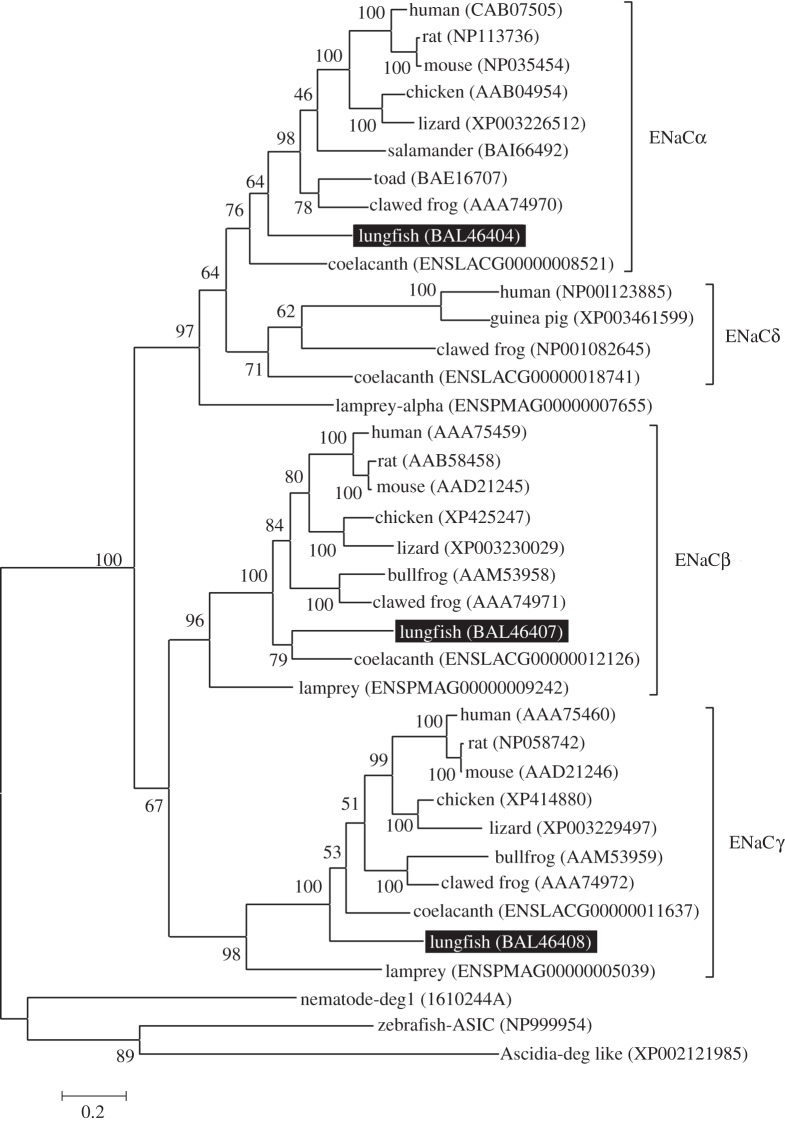
Hydropathy analysis using the Kyte–Doolittle algorithm for ENaCs predicted two putative transmembrane regions with N- and C-termini located in the cytoplasm; the large hydrophilic region was extracellular (see the electronic supplementary material, figure S1). ENaC subunits comprised two hydrophobic segments, the first (TM1) and second (TM2) transmembrane domains, which probably form transmembrane α helices. Predicted TM2 segment sequences show a high degree of homology among all members of the ENaC gene family identified in epithelial tissues of tetrapods. According to an ENaC pore model of mouse ENaC subunits by Kashlan & Kleyman [12], a Gly/Ser–X–Ser tract and selective sites for degenerin, amiloride blockade, voltage sensitivity and Na+/K+ selectivity are present in the TM2 domain of nENaC subunits. A phylogenetic tree using the Ascidia-deg like (XP002121985) as an outgroup indicated that there were three main branches supported by a bootstrap value of 100 per cent: nENaCα, nENaCβ and nENaCγ.
The nENaCα protein was related to rodent ENaCα (52% identity) and salamander and Bufo (both 53% identity), coelacanth (49% identity) and lamprey (49% identify), based on the NCBI protein database research program (BlastP). The nENaCβ protein was related to ENaCβ of rodent (52% identity), chicken (54% identity), clawed frog (54% identity), coelacanth (50% identity) and lamprey (42%). The nENaCγ protein was 52 per cent, 54 per cent, 54 per cent, 57 per cent and 39 per cent identical to the corresponding regions of rat ENaCγ, chicken, clawed frog, coelacanth and lamprey, respectively. Molecular phylogenetic relationships were analysed by the maximum-likelihood method, using the molecular evolutionary genetics analysis software (v. 4.0). The nENaC subunits were most closely related to amphibian and coelacanth ENaC subunits (figure 2).
Figure 2.
Phylogenetic tree showing the relative homologies of nENaCα, β and γ subunit orthologues. Ascidia-deg like (XP002121985) was set as an outgroup. This analysis used the maximum-likelihood method with 100 bootstrap replicates. Numbers indicate the bootstrap values. Numbers in parentheses are the GenBank and Ensemble accession numbers of the sequences. ENaC, epithelial sodium channel; ASIC, acid-sensing ionic channel; deg, degenerin.
(b). Neoceratodus epithelial sodium channel mRNA expressions and tissue distributions
mRNA expressions and tissue distributions of nENaCs were examined by RT-PCR, using total RNA isolated from various tissues. As shown in figure 3, nENaCα mRNA expression was strong in the gill, kidney and rectum, and moderate or weak in the muscle, brain, heart, liver and intestine. No mRNA signal was detected in the eye and lung. nENaCβ mRNA expression was strong in the gill, kidney and rectum, and moderate in the brain, eye, liver and muscle. No mRNA signal was detected in the heart, lung and intestine. nENaCγ mRNA expression was strong in the gill, liver, kidney and rectum, and moderate or weak in the heart, muscle and intestine. No mRNA signal was detected in the brain, eye and lung.
Figure 3.
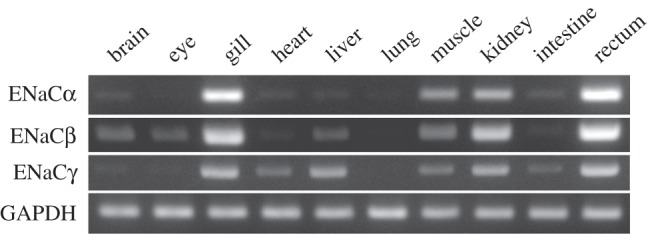
Tissue distributions of nENaCα, β and γ subunit mRNAs by RT-PCR. Semi-quantitative RT-PCR used specific primers for the ENaC subunits and nGAPDH (Neoceratodus glyceraldehyde-3-phosphate dehydrogenase). mRNAs of ENaCα, β and γ subunits were strongly expressed in the gill, kidney and rectum, whereas no mRNA expression was observed in the lung or intestine.
(c). Electrophysiological recordings
We recorded clear membrane currents under a two-electrode voltage clamp from Xenopus oocytes injected with nENaCα, β and γ subunit cRNAs. Such a clear current was not obtained from non-injected or nENaCα cRNA-injected oocytes under the same condition (figure 4a). The current–voltage relation for the nENaCαβγ current showed a weak inward rectification (figure 4b). The resting membrane potential of oocytes expressing nENaCαβγ was 23 ± 5 mV (n = 6). The less-depolarized value in comparison with expected reversal potential of Na+ channel current (approx. +60 mV) was judged to be owing to a contamination of a slight leakage current. The reversal potentials of oocytes expressing nENaCα only and non-injected oocytes were −13.8 ± 1.0 mV (n = 5) and −33.4 ± 5.2 mV (n = 5), respectively. nENaC-mediated Na+ currents excluding the leakage current were obtained as the difference between the currents recorded from the same oocyte before and 2 min after application of 10 µM amiloride in the bath solution. The subtracted current as well as its current–voltage relationship is shown in figure 4c. It showed an inward rectification, and the reversal potential was +59.0 ± 2.5 mV (n = 4; figure 4c). These results indicate that amiloride-sensitive Na+ current was expressed in Xenopus oocytes injected with nENaCαβγ cRNA.
Figure 4.
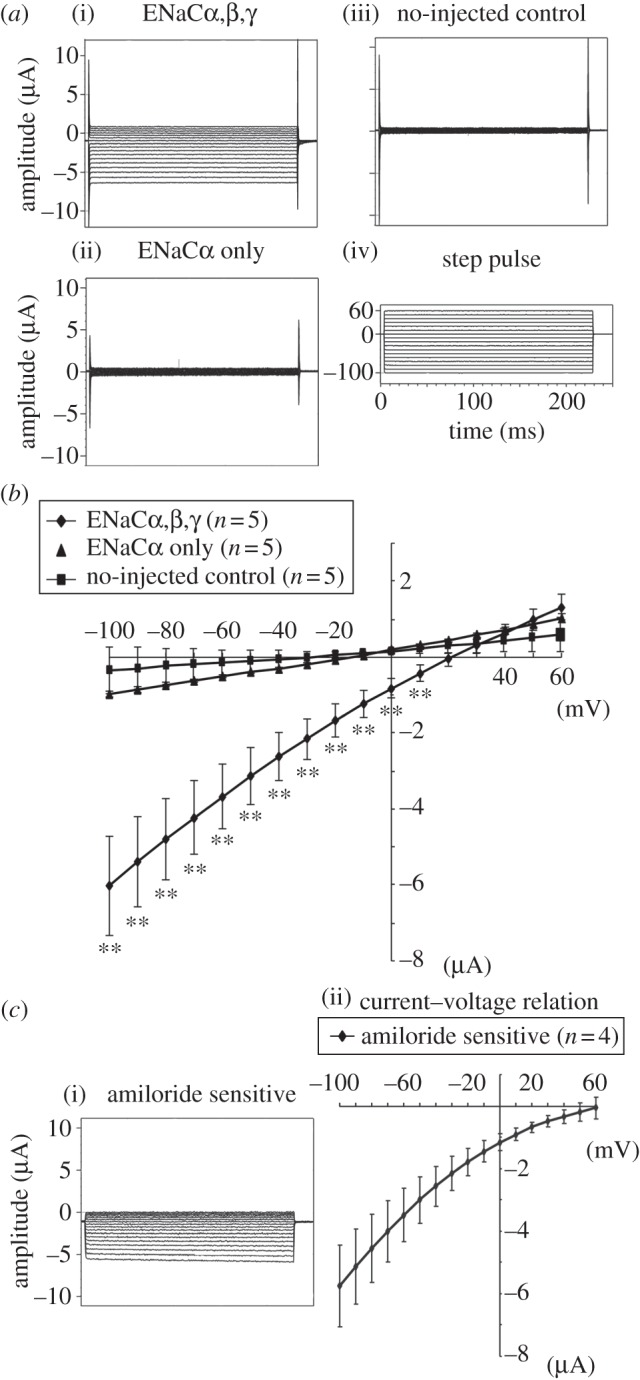
Current recordings under a two-electrode voltage clamp for Xenopus oocytes expressing nENaCα and nENaCαβγ. (a) Representative traces were obtained from an nENaCαβγ-expressing oocyte (i: ENaCαβγ), and an oocyte-expressing nENaCα (ii: nENaCα only) and a non-injected oocyte (iii: non-injected control). (iv) Step pulse. Oocytes were voltage-clamped at 0 mV and step pulses were given from −100 to +60 mV in increments of 10 mV. (b) Current–voltage relationships for oocytes expressing nENaCα only and nENaCαβγ, and no-injected oocytes were shown. Results from five oocytes in each group were summarised. There were no significant differences in current values for ENaCα-expressing oocytes compared with the values for non-injected controls. There were significant inwardly rectifying currents in nENaCαβγ-expressing oocytes compared with the non-injected control oocytes. (c) Subtracted current before and after application of 10 µM amiloride from the same oocyte-expressing nENaCαβγ. Average of current–voltage relationship is shown in the right-hand side of the figure. Plotted data are shown as mean ± s.e. **p < 0.01 different from control.
(d). Immunochemical and immunohistochemical expressions of Neoceratodus ENaCα in the Xenopus oocytes
Western blot analysis of total protein extracted from oocytes used an affinity-purified Bufo ENaCα polyclonal antibody. On the basis of its amino acid sequence, the predicted molecular weight of nENaCα was 73.36 kDa, using the PeptideMass tool on the ExPASy server (http://www.expasy.org/tools/peptide-mass.html). The immunopositive bands on an SDS–PAGE gel were proteins of about 73 and 79 kDa (see the electronic supplementary material, figure S2). Immunofluorescence experiments using the same polyclonal antibody and low-power magnification revealed a polarized distribution of nENaC in Xenopus oocytes (see the electronic supplementary material, figure S3), with a bright signal in the plasma membrane region at the vegetal pole. In non-injected or water-injected oocytes, no immunofluorescent signals were observed in the plasma membrane.
(e). Immunohistochemical tissue localization of Neoceratodus ENaCα
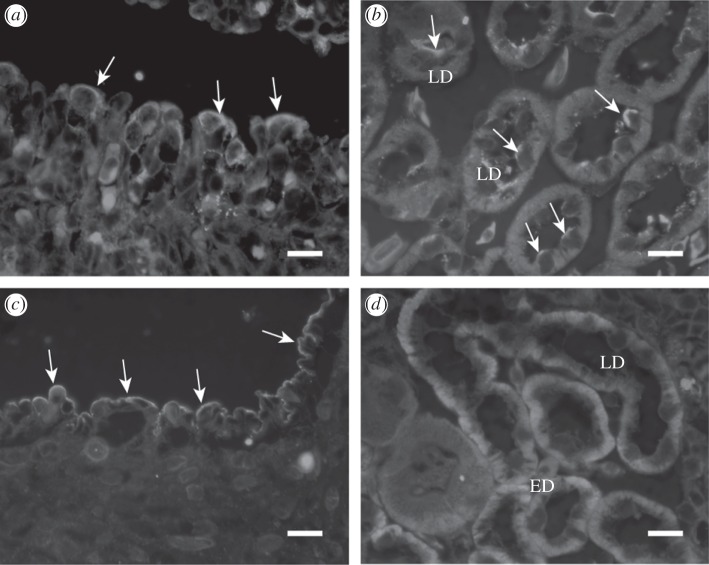
Immunohistochemistry using the polyclonal antibody was performed to determine the cellular expression and localization of nENaCα in the kidney, rectum and gills. Neoceratodus forsteri gills consist of lobe-like filaments of varying sizes. nENaCα immunoreactivity was observed in the cell membrane of oval-shaped cells in lobe-like filaments (figure 5a). nENaCα was also located in the apical membranes of cells in the late distal tubule of the kidney. No nENaCα-immunoreactive cells were found in the early distal nephron although Na+, K+-ATPase positive interdigitation in the basolateral membrane was prominent (figure 5b,d). In the rectum, the surface epithelial cells in the distal end of the spiral valve showed dense immunostaining (figure 5c). By contrast, immunostaining with the anti-ENaCα antibody was absent in the ventral skin of N. forsteri, where ENaCα-immunopositive cells are prominently present in amphibians.
Figure 5.
Immunohistochemical localization of nENaCα and Na+, K+-ATPase in the gills, kidney and rectum, using a polyclonal Bufo ENaCα antibody or a polyclonal Na+, K+-ATPase antibody. (a) Immunoreactivity (green fluorescence) for nENaCα was observed in the apical cell membrane (arrows) in the epithelial cells of gill lamella. (b) Apical cell membranes (arrows) of some cells in the late distal tubules of the nephron were also immunoreactive. (c) Immunoreactivity (green fluorescence) was observed in the apical membrane of rectum epithelium. (d) Na+, K+-ATPase immunoreactivity was observed in the basolateral cell membrane of the cells along the early distal (ED) to the late distal tubules (LD) in the kidney. Scale bars, 20 µm.
4. Discussion
This is, to our knowledge, the first report on the cDNA and genomic sequences of ENaC (α, β and γ) subunits and their expression pattern in the tissues of Australian lungfish N. forsteri. Taken together with the presence of a functional aldosterone system for ion uptake and salt secretion in N. forsteri [20], this further confirms the position of N. forsteri as the closest living ancestor to the tetrapods (land vertebrates).
(a). Molecular identification and characterization of Neoceratodus epithelial sodium channel subunits
The nENaCα, β and γ subunit sequences were most closely related to amphibian ENaC subunits (54%, 56% and 54% identities, respectively). Homology among nENaC subunits was low; each nENaC subunit shared only about 30 per cent sequence identity with the other subunits, suggesting that gene duplication occurred early in this subfamily. The nENaCα, β and γ subunits (ca 650 amino acids) had two presumed membrane-spanning domains (TM1 and TM2) connected by a large extracellular loop (>60% of the total protein) with short intracellular N- and C-termini projecting into the cytosol. Although little is known about the overall channel architecture and secondary structures of ENaC, the second membrane-spanning domain (TM2) is thought to contribute to the channel pore.
All three nENaC subunits had conserved proline-rich regions conforming to the consensus PPXY motifs, where P is proline, Y is tyrosine and X is any amino acid, in their C-terminal end. The PPXY motif is important for interactions with the ubiquitin–protein ligases Nedd4 and Nedd4-2, which promote the ubiquitination, endocytosis and proteasomal degradation of ENaC [24]. ENaC activity is regulated by numerous intracellular and extracellular factors through alterations of either channel density, open probability or both. Channel activity is regulated by several serine proteases, including prostasin, furin and elastase. Hughey et al. [25] reported that furin cleaved specific sites within the extracellular loops of the ENaCα- and γ-subunits. Consensus sequences for furin-dependent proteolysis at two sites within ENaCα- and at single sites within γ-subunits are R–X–X–R, where R is arginine and X is any residue [25,26]. These consensus sequences of the ENaCα- and γ-subunits interosculate among the ENaC subunits of tetrapods. In this study, there was no furin cleavage consensus site within the nENaCα-subunit. Conversely, there were two sites of consensus sequences for furin-dependent proteolysis within the nENaCγ subunits (figure 1). Thus, a furin cleavage site domain is not conserved in nENaCα-subunits. This may be related to our results that insignificant amiloride-sensitive sodium current was observed in Xenopus oocytes that expressed nENaCα cRNA only.
(b). Expression of Neoceratodus ENaCα, β and γ subunit mRNA and ENaCα protein
In amphibians, ENaC mRNA and protein are expressed in the urinary bladder and ventral skin in addition to the kidney [16,19]. We recently studied the ontogeny of ENaC expression in the external gills and the kidney in a primitive urodele, Hynobius nigrescens [19]. Hynobius ENaC mRNA and protein were expressed in the external gills and pronephric ducts of aquatic larvae, and in the urinary bladder, ventral and dorsal skin and kidney in amphibious adults. It would seem that ENaCs change their tissue expression sites to play an important role in hydromineral regulation throughout life. For lungfishes, a few studies have outlined the structures of gills, kidneys and the alimentary canal, although the functions of these organs were not described in detail [27–29]. In this study, mRNA for ENaCα, β and γ subunits were highly expressed in common in the gills, kidney and rectum. ENaCα protein was also highly expressed immunohistochemically in the gills, kidney and rectum. Fish gills are major sites for multiple functions [30]. The expression pattern of ENaCα immunoreactive cells differed from Hynobius ENaCα expression in the external gills of salamander larvae. High Hynobius ENaCα immunoreactivity was observed in the apical cell membrane of a population of pavement cells and in mitochondria-rich (MR) cells in the primary filaments and secondary lamellae of the external gills [23]. Lungfish have MR cells in the gills and skin epithelia [27]. In this study, nENaCα was localized on the apical membrane of several cells, possibly MR cells. In the kidneys of mammals and amphibians, ENaC are expressed on the apical membranes of the principal cells in the distal nephron [3,16]. The major stimulus for Na+ reabsorption by principal cells is the hormone aldosterone, which stimulates both ENaC and Na+, K+-ATPase activities [5]. It was recently proposed that the renin–angiotensin–aldosterone system and angiotensin itself directly control ENaC activity and expression in the distal nephron of mammalian kidney [31]. Because angiotensin regulates aldosterone secretion in the Australian lungfish [20], as it does in mammals, a renin–angiotensin–aldosterone system may control ENaC expression and its function in the lungfish. In freshwater, ingested food may substantially contribute to ion uptake.
In this study, nENaCα was expressed specifically on the apical membrane of epithelial cells of the rectum, and the distal end of the spiral valve but not in the midgut region [29]. The present observations may indicate that dietary sodium uptake contributes significantly to ion regulation in the lungfish. In mammals, ENaC has been found to regulate airway mucosal hydration and mucus clearance in the lung. It was not observed in N. forsteri lung, however, probably because this lungfish preferentially uses its gills for respiration and cannot survive long out of water, unlike the other two genera of living lungfish, which are renowned for their ability to aestivate out of water for long periods of drought [32].
According to recent studies, terrestrialization of vertebrates occurred in two steps: (i) the first tetrapods diverged from sarcopterygians during the Late Devonian period (ca 380 Ma); (ii) this was followed by adaptation to terrestrial life during the earliest Carboniferous period (ca 350 Ma). During the first step, the lungfishes represent an important step in the direct transition from water breathing to air-breathing vertebrates in that they possess both gills and lungs [32]. Neoceratodus forsteri has a single dorso-lateraly placed lung and preferentially uses its four pairs of holobranch gills for respiration and only uses its lungs when oxygen levels in water are very low. Thus, ENaC may play an important role in regulating sodium transport in gills, kidney and rectum under normal circumstances, relying on its rennin–angiotensin–aldosterone system along with an active ENaC system. The other two genera of lungfish are more recently derived and appear to have moved towards a different path of semi-terrestriality, involving aestivation when water-deprived. The presence of ENaC subunits and their expressions during aestivation for Protopterus spp. and Lepidosiren would need to be studied to confirm or not, that all living lungfishes possess ENaC similar to those of tetrapod species.
Acknowledgements
All experiments were performed according to the regulations of the Ethics Committee of the University of Toyama.
We thank Akihiro Isumi, Natsumi Watanabe and Tomomi Yamamoto for technical assistance. This work was supported, in part, by a grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan to M.U.
References
- 1.Brinkmann H., Venkatesh B., Brenner S., Meyer A. 2004. Nuclear protein-coding genes support lungfish and not the coelacanth as the closest living relatives of land vertebrates. Proc. Natl Acad. Sci. USA 101, 4900–4905 10.1073/pnas.0400609101 (doi:10.1073/pnas.0400609101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavin L., Suteethorn V., Buffetaut E., Tong H. 2007. A new Thai Mesozoic (Sarcopterygii, Dipnoi) with an insight into post-Palaeozoic dipnoan evolution. Zool. J. Linn. Soc. 149, 141–177 10.1111/j.1096-3642.2007.00238.x (doi:10.1111/j.1096-3642.2007.00238.x) [DOI] [Google Scholar]
- 3.Garty H., Palmer L. G. 1997. Epithelial sodium channels: function, structure, and regulation. Physiol. Rev. 77, 359–396 [DOI] [PubMed] [Google Scholar]
- 4.Alvarez de la Rosa D., Canessa C. M., Fyfe G. K., Zhang P. 2000. Structure and regulation of amiloride-sensitive sodium channels. Annu. Rev. Physiol. 62, 573–594 10.1146/annurev.physiol.62.1.573 (doi:10.1146/annurev.physiol.62.1.573) [DOI] [PubMed] [Google Scholar]
- 5.Kellenberger S., Schild L. 2002. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. 82, 735–767 10.1152/physrev.00007.2002 (doi:10.1152/physrev.00007.2002) [DOI] [PubMed] [Google Scholar]
- 6.Studer R. A., Person E., Robinson-Rechavi M., Rossier B. C. 2011. Evolution of the epithelial sodium channel and the sodium pump as limiting factors of aldosterone action on sodium transport. Physiol. Genomics 43, 844–854 10.1152/physiolgenomics.00002.2011 (doi:10.1152/physiolgenomics.00002.2011) [DOI] [PubMed] [Google Scholar]
- 7.Canessa C. M., Schild L., Buell G., Thorens B., Gautschi I., Horisberger J.-D., Rossier B. C. 1994. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367, 463–467 10.1038/367463a0 (doi:10.1038/367463a0) [DOI] [PubMed] [Google Scholar]
- 8.Benos D. J., Stanton B. A. 1999. Functional domains within the degenerin/epithelial sodium channel (Deg/ENaC) superfamily of ion channels. J. Physiol. 520, 631–644 10.1111/j.1469-7793.1999.00631.x (doi:10.1111/j.1469-7793.1999.00631.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fyfe G. K., Canessa C. M. 1998. Subunit composition determines the single channel kinetics of the epithelial sodium channel. J. Gen. Physiol. 112, 423–432 10.1085/jgp.112.4.423 (doi:10.1085/jgp.112.4.423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jasti J., Furukawa H., Gonzales E. B., Gouaux E. 2007. Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature 449, 316–323 10.1038/nature06163 (doi:10.1038/nature06163) [DOI] [PubMed] [Google Scholar]
- 11.Stewart A. P., Haerteis S., Diakov A., Korbmacher C., Edwardson J. M. 2011. Atomic force microscopy reveals the architecture of the epithelial sodium channel (ENaC). J. Biol. Chem. 286, 31 944–31 952 10.1074/jbc.M111.275289 (doi:10.1074/jbc.M111.275289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashlan O. B., Kleyman T. R. 2011. ENaC structure and function in the wake of a resolved structure of a family member. Am. J. Physiol. Renal Physiol. 301, F684–F696 10.1152/ajprenal.00259.2011 (doi:10.1152/ajprenal.00259.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waldmann R., Champigny G., Bassilana F., Voilley N., Lazdunski M. 1995. Molecular cloning and functional expression of a novel amiloride-sensitive Na+ channel. J. Biol. Chem. 270, 27 411–27 414 10.1074/jbc.270.46.27411 (doi:10.1074/jbc.270.46.27411) [DOI] [PubMed] [Google Scholar]
- 14.Goldstein O., Asher C., Garty H. 1997. Cloning and induction by low NaCl intake of avian intestine Na+ channel subunits. Am. J. Physiol. Cell Physiol. 272, C270–C277 [DOI] [PubMed] [Google Scholar]
- 15.Puoti A., May A., Canessa C. M., Horisberger J. D., Schild L., Rossier B. C. 1995. The highly selective low-conductance epithelial Na channel of Xenopus laevis A6 kidney cells. Am. J. Physiol. Cell Physiol. 269, C188–C197 [DOI] [PubMed] [Google Scholar]
- 16.Konno N., Hyodo S., Yamada T., Matsuda K., Uchiyama M. 2007. Immunolocalization and mRNA expression of the epithelial Na+ channel α-subunit in the kidney and urinary bladder of the marine toad, Bufo marinus, under hyperosmotic condition. Cell Tissue Res. 328, 583–594 10.1007/s00441-007-0383-9 (doi:10.1007/s00441-007-0383-9) [DOI] [PubMed] [Google Scholar]
- 17.Giraldez T., Rojas P., Jou J., Flores C., Alvarez de la Rosa D. 2012. Review: the epithelial sodium channel δ-subunit: new notes for an old song. Am. J. Physiol. Renal Physiol. 303, F328–F338 10.1152/ajprenal.00116.2012 (doi:10.1152/ajprenal.00116.2012) [DOI] [PubMed] [Google Scholar]
- 18.Meyer A., Van de Peer Y. 2005. From 2R to 3R: evidence for a fish-specific genome duplication (FSGD). BioEssays 27, 937–945 10.1002/bies.20293 (doi:10.1002/bies.20293) [DOI] [PubMed] [Google Scholar]
- 19.Uchiyama M., Kumano T., Konno N., Yoshizawa H., Matsuda K. 2011. Ontogeny of ENaC expression in the gills and the kidneys of the Japanese black salamander (Hynobius nigrescens Stejneger). J. Exp. Zool. B 316B, 135–145 10.1002/jez.b.21384 (doi:10.1002/jez.b.21384) [DOI] [PubMed] [Google Scholar]
- 20.Joss J. M. P., Arnold-Reed D. E., Balment R. J. 1994. The steroidogenic response to angiotensin II in the Australian lungfish, Neoceratodus forsteri. J. Comp. Physiol. B 164, 378–382 10.1007/BF00302553 (doi:10.1007/BF00302553) [DOI] [Google Scholar]
- 21.Bentley P. J. 2002. Endocrines and osmoregulation: a comparative account in vertebrates, 2nd edn Berlin, Germany: Springer [Google Scholar]
- 22.Fujiwara Y., Keceli B., Nakajo K., Kubo Y. 2009. Voltage-and [ATP]-dependent gating of the P2X2 ATP receptor channel. J. Gen. Physiol. 133, 93–109 10.1085/jgp.200810002 (doi:10.1085/jgp.200810002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchiyama M., Kumano T., Komiyama M., Yoshizawa H., Matsuda K. 2011. Immunohistological classification of ionocytes in the external gills of larval Japanese black salamander, Hynobius nigrescens Stejneger. J. Morphol. 272, 1017–1024 10.1002/jmor.10977 (doi:10.1002/jmor.10977) [DOI] [PubMed] [Google Scholar]
- 24.Synder P. M., Steines J. C., Olson D. R. 2004. Relative contribution of Nedd4 and Nedd4-2 to ENaC regulation in epithelia determined by RNA interference. J. Biol. Chem. 279, 5042–5046 10.1074/jbc.M312477200 (doi:10.1074/jbc.M312477200) [DOI] [PubMed] [Google Scholar]
- 25.Hughey R. P., Bruns J. B., Kinlough C. L., Harkleroad K. L., Tong Q., Carattino M. D., Johnson J. P., Stockand J. D., Kleyman T. R. 2004. Epithelial sodium channels are activated by furin-dependent proteolysis. J. Biol. Chem. 279, 18 111–18 114 10.1074/jbc.C400080200 (doi:10.1074/jbc.C400080200) [DOI] [PubMed] [Google Scholar]
- 26.Bruns J. B., Carattino M. D., Sheng S., Maarouf A. B., Weisz O. A., Pilewski J. M., Hughey R. P., Kleyman T. R. 2007. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the γ-subunit. J. Biol. Chem. 282, 6153–6160 10.1074/jbc.M610636200 (doi:10.1074/jbc.M610636200) [DOI] [PubMed] [Google Scholar]
- 27.Sturla M., Masini M. A., Prato P., Grattarola C., Uva B. 2001. Mitochondria-rich cells in gills and skin of an African lungfish, Protopterus annectens. Cell Tissue Res. 303, 351–358 10.1007/s004410000341 (doi:10.1007/s004410000341) [DOI] [PubMed] [Google Scholar]
- 28.Konno N., Hyodo S., Yamaguchi Y., Matsuda K., Uchiyama M. 2010. Vasotocin/V2-type receptor/aquaporin axis exists in African lungfish kidney but is functional only in terrestrial condition. Endocrinology 151, 1089–1096 10.1210/en.2009-1070 (doi:10.1210/en.2009-1070) [DOI] [PubMed] [Google Scholar]
- 29.Hassanpour M., Joss J. 2009. Anatomy and histology of the spiral valve intestine in juvenile Australian lungfish, Neoceratodus forsteri. Open Zool. J. 2, 62–85 10.2174/1874336600902000062 (doi:10.2174/1874336600902000062) [DOI] [Google Scholar]
- 30.Evans D. H., Caliborne J. B. 2009. Osmotic and ionic regulation in fishes. In Osmotic and ionic regulation: cell and animals (ed. Evans D. H.), pp. 295–366 Boca Raton, FL: CRC Press [Google Scholar]
- 31.Mamenko M., Zaika O., Ilatovskaya D., Staruschenko A., Pochynyuk O. 2012. Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J. Biol. Chem. 287, 660–671 10.1074/jbc.M111.298919 (doi:10.1074/jbc.M111.298919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jørgensen J. M., Joss J. 2010. The biology of lungfishes. Enfield, NH: Science Publishers [Google Scholar]