Abstract
Extant arthropods are diverse and ubiquitous, forming a major constituent of most modern ecosystems. Evidence from early Palaeozoic Konservat Lagerstätten indicates that this has been the case since the Cambrian. Despite this, the details of arthropod origins remain obscure, although most hypotheses regard the first arthropods as benthic predators or scavengers such as the fuxianhuiids or megacheirans (‘great-appendage’ arthropods). Here, we describe a new arthropod from the Tulip Beds locality of the Burgess Shale Formation (Cambrian, series 3, stage 5) that possesses a weakly sclerotized thorax with filamentous appendages, encased in a bivalved carapace, and a strongly sclerotized, elongate abdomen and telson. A cladistic analysis resolved this taxon as the basal-most member of a paraphyletic grade of nekto-benthic forms with bivalved carapaces. This grade occurs at the base of Arthropoda (panarthropods with arthropodized trunk limbs) and suggests that arthrodization (sclerotization and jointing of the exoskeleton) evolved to facilitate swimming. Predatory and fully benthic habits evolved later in the euarthropod stem-lineage and are plesiomorphically retained in pycnogonids (sea spiders) and euchelicerates (horseshoe crabs and arachnids).
Keywords: Burgess shale, Cambrian, bivalved arthropod, phylogeny, arthrodization
1. Introduction
The origin of arthropods is a contentious issue [1–3]. Despite the ubiquity of fossil evidence from early Palaeozoic Konservat Lagerstätten [4,5], there is little consensus regarding the details of their origins. It is generally agreed that the anomalocaridids, a clade of large nektonic predators [6,7], represent the nearest non-arthropod outgroup [7–9], but the identity of the first arthropods remains obscure. A number of potential candidates have been identified, including fuxianhuiids [10–12] and ‘great-appendage’ arthropods [8,13]. These arthropods differed considerably in morphology and bore little resemblance to their supposed anomalocaridid ancestors. Recently, the anomalocaridid Hurdia from the middle Cambrian (stage 5) Burgess Shale Lagerstätten was redescribed [7]. This taxon possessed sclerotized lateral plates reminiscent of the carapace of bivalved arthropods, a common constituent of many early Palaeozoic fossil localities. Although usually represented by isolated valves, examples from sites such as the Burgess Shale offer considerable insight into their soft-part anatomy. Herein, we describe a new bivalved arthropod from the Tulip Beds of the Burgess Shale Formation (Cambrian, stage 5). This fossil documents a suite of primitive arthropod features, such as multi-podomerous limbs and a posterior tagma composed of three pairs of lateral flaps. This taxon and many other extinct early arthropods were coded into an extensive cladistic analysis of panarthropods to determine their affinities and explore relationships within the arthropod stem-lineage.
2. Material and methods
The new taxon, Nereocaris exilis gen. et sp. nov. (figure 1) was included in a dataset of 173 panarthropod taxa (76 extant; 97 fossil) and 580 characters. The dataset was based on a published matrix [14], from which five taxa were removed and to which 93 were added, most of the latter being early Palaeozoic fossils known from soft-part preservation. From the original list of 395 characters, four were removed, eight modified and 189 added (see electronic supplementary material S2). Cladistic analysis was performed using TNT v. 1.1 [15]. All characters were treated as non-additive (unordered) and weighted using both equal weights and implied weighting with a variety of concavity constants (k = 1, 3, 10) [16] (see electronic supplementary material S2). To find the most parsimonious trees, New Technology search options with 100 Random Addition Sequences using Ratchet [17], sectorial searches, tree drifting and tree fusing [18] were employed (figure 2).
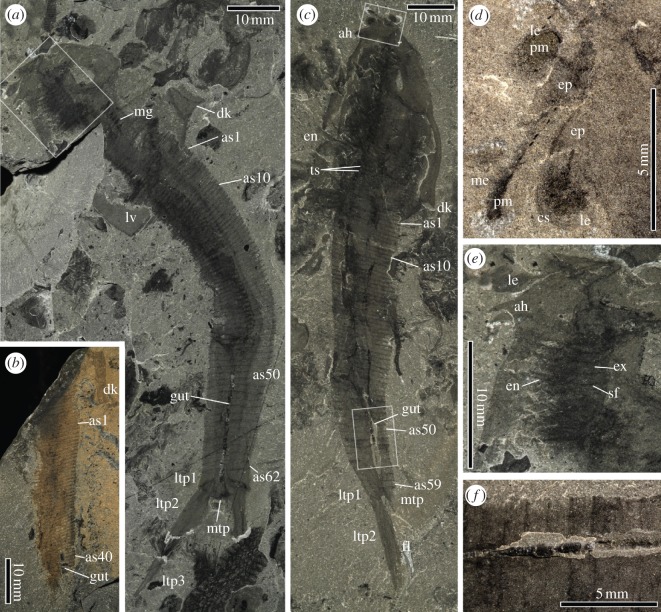
Figure 1.
Nereocaris exilis gen. et sp. nov. from the Cambrian (Stage 3) of British Columbia. (a) Holotype, Royal Ontario Museum (ROM) 61831. (b) Paratype ROM 61832. (c) Paratype ROM 61833. (d) Details of ocular region located in top box of (c), immersed in water. (e) Details of the appendicular region located in box of (a). (f) Details of the posterior part of the gut located in the posterior box of (c), showing three-dimensional preservation. All specimens were photographed using low-angle cross-polarized light. Accompanying camera lucida drawings in electronic supplementary material S1, figure S1. ah, anterior hook-like processes; as1–62, abdominal somites 1–62; cs, corneal surface; dk, dorsal keel; en, endopod; ep, eye peduncle; ex, exopod; fl, fluke; gut, gut; le, lateral eyes; ltp1–3, lateral telson processes 1–3; lv, left valve; mg, midgut glands; mtp, medial telson process; pm, photoreceptive material; sf, setal fringe; ts, thoracic segments.
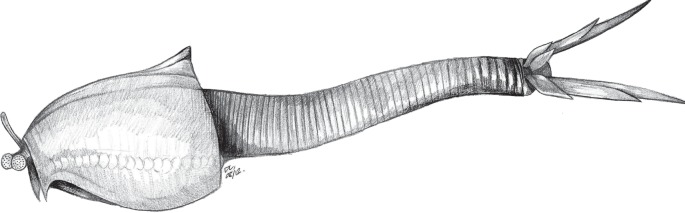
Figure 2.
Reconstruction of Nereocaris exilis. A detailed reconstruction of the appendages can be found in the electronic supplementary material S1, figure S2.
3. Systematic palaeontology
Phylum Arthropoda
Nereocaris exilis gen. et sp. nov.
(a). Etymology
After Nereus, the Greek titan often depicted in ancient artwork with a fish-like tail, caris (Latin, crab) and exilis (Latin, slender).
(b). Holotype
Royal Ontario Museum, ROM 61831, part (figure 1a) and counterpart of an almost complete specimen preserved in lateral aspect.
(c). Paratypes
Royal Ontario Museum, ROM 61832, part (figure 1b) and counterpart of a partial abdomen and postero-ventral section of a carapace; ROM 61833 (figure 1c), an almost complete specimen preserved in an oblique-lateral aspect.
(d). Locality and horizon
All material referred to this taxon was collected from the talus of slopes of the Tulip Beds locality (formerly S7 [19]), Mount Stephen, Yoho National Park, British Columbia, Canada. The lithology indicates that these specimens come from the Campsite Cliff Shale Member of the Burgess Shale Formation, Bathyuriscus–Elrathina biozone (Cambrian, series 3, stage 5) [20].
(e). Diagnosis for genus and species
Arthropod with stalked lateral eyes and a single rod-shaped median eye; a bivalved carapace with a postero-dorsal keel and hook-like antero-ventral spines; trunk composed of thorax of 30–40 somites bearing a homonomous series of biramous limbs and elongate abdomen of approximately 60 ring-like somites; telson composed of a small triangular medial process and elongate lateral processes comprising three segments.
(f). Description
Anatomical terminology is discussed in electronic supplementary material S2. The longest specimen, ROM 61832 (figure 1a), measures 142 mm from the anterior-most tip of the carapace to the distal tip of the telson processes. ROM 61833 has a preserved length of 127 mm (figure 1c), although the distal tips of the telson processes are absent.
The anterior region of all specimens is preserved in lateral aspect (figure 1a–c). In ROM 61831 the telson is preserved in dorsoventral aspect and the abdomen has a distinct torsion (figure 1a). This indicates that the stable orientation of the carapace upon death was lateral, and hence that it was laterally compressed in life (in contrast with other bivalved arthropods with a laterally expansive carapace (e.g. Odaraia) which typically preserve in dorsoventral aspect) [21]. The carapace is subovoid with a restricted anterior gape and expands strongly towards the posterior, reaching its tallest near the postero-dorsal margin where it expands into a subtriangular fin-like keel. The posterior margin is only slightly curved, meeting the ventral margin at an approximate right angle at the posterior-most point of the carapace. Short recurved hook-like processes occur on the antero-ventral margin of the carapace (figure 1a,c,e).
The head region is poorly delimited as no evidence for limb specialization is preserved. The lateral eyes protrude from the anterior margin of the carapace (figure 1c,d) and consist of two parts: a proximal peduncle and a distal corneal surface. The attachment site of the peduncle is unclear, but appears to converge on a single point, presumably an anterior sclerite. The lateral eyes of ROM 61833 are 2.3 mm in diameter. The central region of each lateral eye is preserved as a highly reflective material, and is surrounded by a narrow margin of unreflective material (figure 1d). A small rod of reflective material extends from the reflective area of the eye into the peduncle. In other bivalved arthropods (e.g. Odaraia) this reflective material has been interpreted as fossilized photoreceptive tissue [21]. A single elongate medial process, 3.9 mm long, originates between the lateral eyes (figure 1d). This projection appears unsegmented, bears reflective material in the form of a medial filament extending from the base of the tip, and is distally bulbous; it is hence tentatively interpreted not as an appendage but a medial eye, as proposed for a similar structure in Jugatacaris [22].
The limbs are best preserved in ROM 61831 (figure 1e). They represent a homonomous series of biramous arthropodized appendages, comprising a long (8.6 mm), thin endopod of more than10 podomeres, and a small subovoid exopod fringed with fine setae (see electronic supplementary material S1, figure S2). Appendages are restricted to the thorax, and decrease in size towards the posterior carapace margin. The thorax (anterior of as1 in figure 1a,c) is poorly sclerotized, but annulated, and consists of 30–40 segments; a one-to-one correspondence between appendages and these segments is likely, but cannot be demonstrated. In contrast to the thorax, the abdomen is well sclerotized and extremely long, accounting for over half (69% in ROM 61831 and 67% in ROM 61833) of the total body length. The abdomen consists of approximately 60 somites (62 in ROM 61831, figure 1a; 59 in ROM 61833, figure 1c). Separate tergites and sternites are not evident; each somite instead consists of a complete ring. The anterior somites are more closely spaced (12 per 10 mm) than the posterior ones (6.5 per 10 mm). An elongate and dark medial structure within the abdomen is interpreted as a gut trace and is present in all specimens (figure 1a–c). It is preserved in a range of styles, varying between and within individual specimens from faint staining to highly reflective areas with noticeable relief, the latter preferentially occurring posteriorly (figure 1f). The gut terminates within the telson (figure 1c). Darkly stained villi preserved adjacent to the gut in the thorax may represent midgut glands (figure 1a).
The telson bears three sets of spinose processes: one medial and two sets of lateral processes. The medial process is short (6 mm in ROM 61833) and subtriangular (figure 1c). Each lateral process set consists of three elements. The most proximal processes are subrectangular and possess short spines on their postero-lateral margin (figure 1a). The remaining processes are long (33 mm in ROM 61831 and 23 mm in ROM 61833), and appear fused and spinose. A fluke-like expansion of the telson processes is present in ROM 61833 (figure 1c).
4. Discussion
The Cambrian seas contained a diverse and polyphyletic fauna of bivalved arthropods, including mandibulate-like taxa (phosphatocopines and bradoriids) and a variety of more enigmatic taxa (Jugatacaris, Pectocaris, Odaraia, Branchiocaris, Canadaspis, Perspicaris and others). Many of the latter have been traditionally allied with the crustaceans [21–26], but unequivocal crustacean synapomorphies (e.g. second antennae) are lacking (although they were tentatively identified in Pectocaris [23], they appear absent), and while mandibles have been identified in some of these taxa in previous studies, we do not consider any of these interpretations to be valid (see electronic supplementary material S1, figure S3). These taxa either exhibit no limb-specialization (Nereocaris, Jugatacaris, Pectocaris) or have a raptorial second head appendage (Branchiocaris, Canadaspis, Odaraia, Perspicaris; see electronic supplementary material S1, figure S3). For these reasons, others have considered them part of the euarthropod stem-lineage [8,11]. These hypotheses were tested by coding a variety of bivalved arthropods (including Nereocaris) into a cladistic analysis.
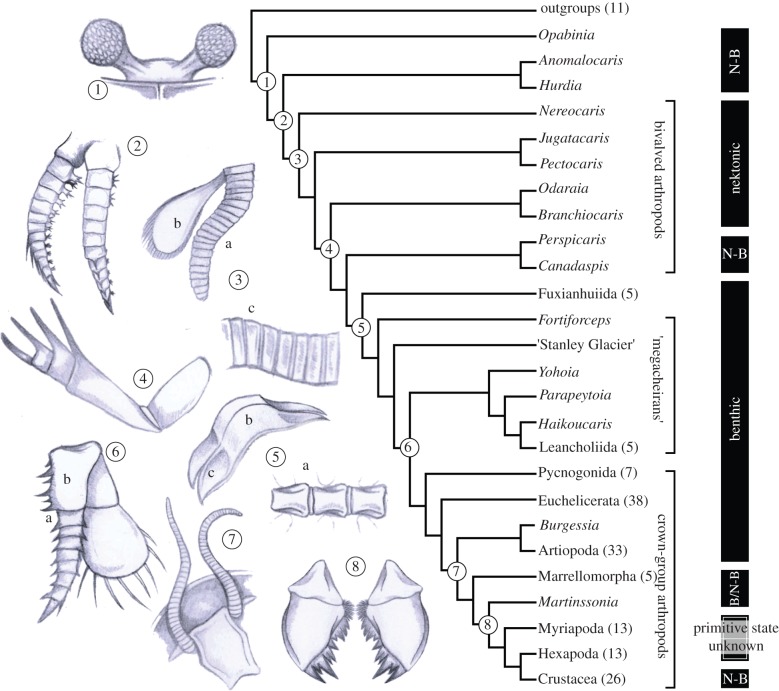
A paraphyletic grade of bivalved arthropods is resolved at the base of Arthropoda sensu Bergström et al. [10]—that is, panarthropods with jointed trunk appendages (=arthropodization) and an exoskeleton composed of stiff, segmental sclerites separated by softer membranous areas (=arthrodization) (figure 3). Nereocaris resolved as the basal-most member of this bivalved-arthropod grade, and hence the most basal arthropod. This position is stable in the face of changes to character weights (see electronic supplementary material S2) and is not dependent on either the interpretation of the median eye or assumptions of homology between tripartite lateral telson processes of Nereocaris and the posterior tagma of dinocaridids; coding either or both of these characters as uncertain does not change the topology. Primitive features of Nereocaris include a poorly sclerotized thorax and a posterior tagma composed of three lanceolate lateral elements, both also present in dinocaridids (e.g. Anomalocaris [6] and Opabinia). This placement would be compatible with the lateral valves of the anomalocaridid Hurdia [7] and the carapace valves of bivalved stem-group arthropods being homologous, although they were not coded as such in the current study.
Figure 3.
The phylogenetic position of Nereocaris and the origin of key innovations in Arthropoda. Summary of three most parsimonious trees (MPTs) of 91.98 steps (implied character weighting, k = 3; CI = 0.565; RI = 0.863; all nodes in the arthropod stem group are identical under equal weights). Complete phylogeny in electronic supplementary material S1, figure S4. Numbers in parentheses are the number of terminal taxa within supra-generic clades. Column at right is locomotory habit (B, benthic; N-B, nekto-benthic). Key innovations include: 1 compound eyes (as figured for Odaraia), although this character has disappeared and ‘re-evolved’ a number of times with Arthropoda; 2 arthropodized limbs (Anomalocaris)— it is unclear if the arthropodized cephalic limbs of radiodontans (Anomalocaris and Hurdia) are homologous to the arthropodized trunk limbs of arthropods; 3 (a) arthropodization and (b) biramy of the trunk appendages (Fuxianhuia), and (c) arthrodization of the trunk exoskeleton (Nereocaris); 4 specialization of the cephalic appendages into raptorial ‘great-appendages’ (Yohoia); 5 (a) division of trunk somites into distinct ventral sternites (Misszhouia) and (b) dorsal tergites with (c) paratergal folds (Shankouia); 6 (a) reduction in the number of trunk limb podomeres associated with (b) the acquisition of a rigid gnathobasic protopodite (Alalcomenaeus); 7 modification of the anterior (deutocerebral) appendages into antennae (Olenoides); 8 the origin of mandibular mastication (Locusta).
Nereocaris lacks specialized sensory and gnathal head appendages. We do not consider a taphonomic explanation for this absence to be particularly likely, as labile structures anterior and posterior to the expected region (e.g. the eyes and thoracic limbs) are preserved; we also note that other bivalved taxa resolving proximally demonstrably lack antennae (Jugatacaris, Odaraia) or modified gnathal appendages. However, the lack of head appendages renders interpretations of feeding mode difficult. Predation would require specialized appendages for the capture and mastication of prey, and can hence be excluded; a scavenging mode is conceivable, but also problematic. A filter-feeding autecology has been proposed for related taxa by some previous authors (see electronic supplementary material S1, table S1), although this has been contested [27]. If Nereocaris employed filter-feeding, it would have been relatively inefficient, judging from the underdeveloped endites and lack of obvious enditic setal armature on its thoracic appendages.
The limbs of Nereocaris were completely enclosed by the carapace, and their filamentous nature would have rendered them unsuitable for walking; we hence interpret the taxon as a member of the nekto-benthos. The appendages are small and delicate, and could have provided little propulsive force for swimming. Instead propulsion must have been generated by the elongate abdomen and fluke-like telson; sclerotization of the abdomen would have accommodated the attachment of powerful musculature. Nereocaris was probably a highly agile swimmer, the flattened carapace and expanded postero-dorsal keel providing stability during high-speed turning. This combination of features and enlarged lateral eyes would have aided in predator detection and evasion. Similar morphologies are also present in other bivalved Cambrian stem-group arthropods, and their modes of life were probably comparable. Both Jugatacaris [22] and Pectocaris [23] have an elongate abdomen with ring-like somites and fluke-like, segmented telson. Jugatacaris also possesses a flattened carapace, dorsal keel and a rod-like, unpaired median eye [22].
The topology of our cladogram implies that a nekto-benthic habit is primitive for arthropods (figure 3). This contrasts with other hypotheses, which have suggested that either fuxianhuiids [10–12] or megacheirans [8,13] represent the primitive arthropod condition. Both these taxa are generally considered to have been benthic predators or scavengers, the latter based on evidence from gut contents [28,29] (see electronic supplementary material S1, table S1). A transition to a benthic habit is associated with an inferred reduction of the bivalved carapace into either a head capsule (in fuxianhuiids) or a cephalic shield (in megacheirans and euarthropods). This transition is also associated with the acquisition of separate sternites and tergites, and accompanying paratergal folds; the latter would have afforded protection for the respiratory exites in the absence of a bivalved carapace.
The acquisition of an arthrodized exoskeleton was one of the most important innovations in arthropod evolution; by providing a tough, durable barrier it allowed its bearers to adapt to a variety of environmental stresses. Among the few studies that have considered its origin is a recent proposal that sclerotization and arthrodization evolved to increase extrinsic muscular control of the appendages [30]. On the basis of the phylogenetic placement and ecology of Nereocaris and other bivalved arthropods, which we also interpret as nekto-benthic (see electronic supplementary material S1, table S1), we can refine this hypothesis. These taxa indicate that arthrodization originated in a flexible natatory trunk, and its original function was the strengthening of the exoskeleton to accommodate swimming musculature, rather than a musculature for walking, as proposed elsewhere [30].
The number and diversity of taxa in the euarthropod stem-group demonstrates that the origin of the arthropods was one of gradual transitions, with key innovations such as compound eyes, arthropodized limbs, an arthrodized exoskeleton and gnathobasic limbs evolving at different times in arthropod evolution, often associated with shifts in ecology (figure 3). The current topology indicates that a benthic predatory or scavenging habit was primitive for Euarthropoda (crown-group arthropods), as it is present in the nearest outgroups (megacheirans, fuxianhuiids), and is retained in pycnogonids (sea spiders) and euchelicerates (horseshoe crabs and arachnids).
Acknowledgements
We thank Peter Fenton for help and advice at the Royal Ontario Museum (ROM); Mark Florence and Doug Erwin for helpful discussion and access to collections at the NMNH; J. Ortega-Hernández for comments on early versions of the manuscript; A. Daley for helpful discussion; the referees for constructive advice; and the Willi Hennig Society for TNT. D.A.L. is funded by a Janet Watson Scholarship (Imperial College London). Specimens were collected under Parks Canada Research Permits delivered to D. Collins in 1996 (ROM 61831 and ROM 61832) and to J.-B. Caron in 2008 (ROM 61833). The 2008 ROM expedition was made possible thanks to the support of Natural Sciences and Engineering Research Council (NSERC) Discovery grant 341944, and the ROM Reproductions Acquisitions and Research Fund. This is Royal Ontario Museum Burgess Shale research project no. 36.
References
- 1.Edgecombe G. D. 2010. Arthropod phylogeny: an overview from the perspectives of morphology, molecular data and the fossil record. Arthropod Struct. Dev. 39, 74–87 10.1016/j.asd.2009.10.002 (doi:10.1016/j.asd.2009.10.002) [DOI] [PubMed] [Google Scholar]
- 2.Budd G. E., Telford M. J. 2009. The origin and evolution of arthropods. Nature 457, 812–817 10.1038/nature07890 (doi:10.1038/nature07890) [DOI] [PubMed] [Google Scholar]
- 3.Legg D. A., Ma X., Wolfe J. M., Ortega-Hernández J., Edgecombe G. D., Sutton M. D. 2011. Lobopodian phylogeny reanalysed. Nature 476, E2–E3 10.1038/nature10267 (doi:10.1038/nature10267) [DOI] [PubMed] [Google Scholar]
- 4.Briggs D. E. G., Erwin D. H., Collier F. J. 1994. The fossils of the Burgess Shale. Washington, DC: Smithsonian Institution [Google Scholar]
- 5.Hou X. G., Aldridge R. J., Bergström J., Siveter D. J., Siveter D. J., Feng X. H. 2004. The Cambrian fossils of Chengjiang, China: the flowering of early animal life. Oxford, UK: Blackwell [Google Scholar]
- 6.Collins D. 1996. The ‘evolution’ of Anomalocaris and its classification in the arthropod class Dinocarida (nov.) and order Radiodonta (nov.). J. Paleontol. 70, 280–293 [Google Scholar]
- 7.Daley A. C., Budd G. E., Caron J.-B., Edgecombe G. D., Collins D. 2009. The Burgess Shale anomalocaridid Hurdia and its significance for early euarthropod evolution. Science 323, 1597–1600 10.1126/science.1169514 (doi:10.1126/science.1169514) [DOI] [PubMed] [Google Scholar]
- 8.Budd G. E. 2002. A palaeontological solution to the arthropod head problem. Nature 417, 271–275 10.1038/417271a (doi:10.1038/417271a) [DOI] [PubMed] [Google Scholar]
- 9.Kühl G., Briggs D. E. G., Rust J. 2009. A great-appendage arthropod with a radial mouth from the Lower Devonian Hunsrück Slate, Germany. Science 323, 771–773 10.1126/science.1166586 (doi:10.1126/science.1166586) [DOI] [PubMed] [Google Scholar]
- 10.Bergström J., Hou X.-G., Zhang X., Clausen S. 2008. A new view of the Cambrian arthropod Fuxianhuia. GFF 130, 189–201 [Google Scholar]
- 11.Hou X.-G., Bergström J. 1997. Arthropods of the Lower Cambrian Chengjiang fauna, southwest China. Fossils and Strata 45, 1–116 [Google Scholar]
- 12.Waloszek D., Chen J.-Y., Maas A., Wang X.-Q. 2005. Early Cambrian arthropods—new insights into arthropod head and structural evolution. Arthropod Struct. Dev. 34, 189–205 10.1016/j.asd.2005.01.005 (doi:10.1016/j.asd.2005.01.005) [DOI] [Google Scholar]
- 13.Bousfield E. L. 1995. A contribution to the natural classification of Lower and Middle Cambrian arthropods: food-gathering and feeding mechanisms. Amphipacifica 2, 3–34 [Google Scholar]
- 14.Rota-Stabelli O., et al. 2011. A congruent solution to arthropod phylogeny: phylogenomics, microRNAs and morphology support Mandibulata. Proc. R. Soc. B 278, 298–306 10.1098/rspb.2010.0590 (doi:10.1098/rspb.2010.0590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goloboff P. A., Farris J. S., Nixon K. C. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786 10.1111/j.1096-0031.2008.00217.x (doi:10.1111/j.1096-0031.2008.00217.x) [DOI] [Google Scholar]
- 16.Goloboff P. A. 1993. Estimating character weights during tree search. Cladistics 9, 83–91 10.1111/j.1096-0031.tb00209.x (doi:10.1111/j.1096-0031.tb00209.x) [DOI] [PubMed] [Google Scholar]
- 17.Nixon K. C. 1999. The Parsimony Ratchet, a new method for rapid parsimony analysis. Cladistics 15, 407–414 10.1111/j.1096-0031.1999.tb00277.x (doi:10.1111/j.1096-0031.1999.tb00277.x) [DOI] [PubMed] [Google Scholar]
- 18.Goloboff P. A. 1999. Analyzing large data sets in reasonable times: solutions for composite optima. Cladistics 15, 415–428 10.1111/j.1096-0031.1999.tb00278.x (doi:10.1111/j.1096-0031.1999.tb00278.x) [DOI] [PubMed] [Google Scholar]
- 19.Fletcher T. P., Collins D. 1998. The Middle Cambrian Burgess Shale and its relationship to the Stephen Formation in the southern Canadian Rocky Mountains. Can. J. Earth Sci. 35, 413–436 10.1139/e97-120 (doi:10.1139/e97-120) [DOI] [Google Scholar]
- 20.O'Brien L. J., Caron J.-B. 2012. A new stalked filter-feeder from the Middle Cambrian Burgess Shale, British Columbia, Canada. PLoS ONE 7, 1–21 10.1371/journal.pone.0029233 (doi:10.1371/journal.pone.0029233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briggs D. E. G. 1981. The arthropod Odaraia alata Walcott, Middle Cambrian, Burgess Shale, British Columbia. Phil. Trans. R. Soc. Lond. B 291, 541–582 10.1098/rstb.1981.0007 (doi:10.1098/rstb.1981.0007) [DOI] [Google Scholar]
- 22.Fu D., Zhang X. 2011. A new arthropod Jugatacaris agilis n. gen. n. sp. from the Early Cambrian Chengjiang Biota, South China. J. Paleontol. 85, 567–586 10.1666/09-173.1 (doi:10.1666/09-173.1) [DOI] [Google Scholar]
- 23.Hou X.-G., Bergström J., Xu G.-H. 2004. The Lower Cambrian crustacean Pectocaris from the Chengjiang Biota, Yunnan, China. J. Paleontol. 78, 700–708 (doi:10.1666/0022-3360(2004)078<0700:TLCCPF>2.0.CO;2) [DOI] [Google Scholar]
- 24.Briggs D. E. G. 1977. Bivalved arthropods from the Cambrian Burgess Shale of British Columbia. Palaeontology 20, 595–621 [Google Scholar]
- 25.Briggs D. E. G. 1978. The morphology, mode of life, and affinities of Canadaspis perfecta (Crustacea: Phyllocarida), Middle Cambrian, Burgess Shale, British Columbia. Phil. Trans. R. Soc. Lond. B 281, 439–487 10.1098/rstb.1978.0005 (doi:10.1098/rstb.1978.0005) [DOI] [Google Scholar]
- 26.Wills M. A., Briggs D. E. G., Fortey R. A., Wilkinson M., Sneath P. H. A. 1998. An arthropod phylogeny based on fossil and Recent taxa. In Arthropod fossils and phylogeny (ed. Edgecombe G. D.), pp. 33–105 New York, NY: Columbia University Press [Google Scholar]
- 27.Budd G. E. 2001. Ecology of nontrilobite arthropods and lobopods in the Cambrian. In The ecology of the Cambrian radiation (eds Zhuravlev A. Yu., Riding R.), pp. 404–427 New York, NY: Columbia University Press [Google Scholar]
- 28.Butterfield N. J. 2002. Leanchoilia guts and the interpretation of three-dimensional structures in Burgess Shale-type fossils. Paleobiology 28, 155–171 (doi:10.1666/0094-8373(2002)028<0155:LGATIO>2.0.CO;2) [DOI] [Google Scholar]
- 29.Zhu M.-Y., Vannier J., Van Iten H., Zhao Y.-L. 2004. Direct evidence for predation on trilobites in the Cambrian. Proc. R. Soc. Lond. B 271, S277–S280 10.1098/rsbl.2004.0194 (doi:10.1098/rsbl.2004.0194). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Budd G. E. 2001. Why are arthropods segmented? Evol. Dev. 3, 332–342 10.1046/j.1525-142X.2001.01041.x (doi:10.1046/j.1525-142X.2001.01041.x) [DOI] [PubMed] [Google Scholar]