Abstract
The D2 polypeptide of the photosystem II (PSII) complex in the green alga Chlamydomonas reinhardtii is thought to be reversibly phosphorylated. By analogy to higher plants, the phosphorylation site is likely to be at residue threonine-2 (Thr-2). We have investigated the role of D2 phosphorylation by constructing two mutants in which residue Thr-2 has been replaced by either alanine or serine. Both mutants grew photoautotrophically at wild-type rates, and noninvasive biophysical measurements, including the decay of chlorophyll fluorescence, the peak temperature of thermoluminescence bands, and rates of oxygen evolution, indicate little perturbation to electron transfer through the PSII complex. The susceptibility of mutant PSII to photoinactivation as measured by the light-induced loss of PSII activity in whole cells in the presence of the protein-synthesis inhibitors chloramphenicol or lincomycin was similar to that of wild type. These results indicate that phosphorylation at Thr-2 is not required for PSII function or for protection from photoinactivation. In control experiments the phosphorylation of D2 in wild-type C. reinhardtii was examined by 32P labeling in vivo and in vitro. No evidence for the phosphorylation of D2 in the wild type could be obtained. [14C]Acetate-labeling experiments in the presence of an inhibitor of cytoplasmic protein synthesis also failed to identify phosphorylated (D2.1) and nonphosphorylated (D2.2) forms of D2 upon sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Our results suggest that the existence of D2 phosphorylation in C. reinhardtii is still in question.
The existence of phosphoproteins in thylakoid membranes from higher plants was first reported by Bennett (1977), who described a number of polypeptides from pea (Pisum sativum) that were reversibly phosphorylated in the presence of 32Pi. The most conspicuously phosphorylated bands belong to LHCII (Bennett, 1977). In addition to LHCII it is now known that four PSII proteins, the PsbH polypeptide, CP43, D1, and D2, are also reversibly phosphorylated in pea and spinach (Spinacia oleracea) (Ikeuchi et al., 1987a; Michel and Bennett, 1987; Telfer et al., 1987; Michel et al., 1988; Elich et al., 1992). For both the D1 and D2 proteins, the site of phosphorylation has been identified using tandem MS as the N-terminal Thr at position 2 (Michel et al., 1988).
Phosphorylation of the D1 and D2 subunits does not appear to be ubiquitous. In cyanobacteria neither D1 nor D2 are phosphorylated, and in the green alga Chlamydomonas reinhardtii only the D2 protein appears to be phosphorylated (Delepelaire, 1984). The analogous amino acid residue to D2 Thr-2 of higher plants is also a Thr residue (Erickson et al., 1986). Phosphorylation of D2 in C. reinhardtii was first suggested by Delepelaire (1984) after carrying out pulse-chase-labeling experiments with [14C]acetate in the presence of protein-synthesis inhibitors. After electrophoresis (using a urea-SDS gel system) and autoradiography, Delepelaire observed a doublet that he attributed to the phosphorylated and nonphosphorylated forms of D2 (D2.1 and D2.2, respectively). Based largely on the work of Delepelaire, various reports have appeared in the literature assigning a band with an apparent molecular mass of approximately 34 kD, labeled with 32P both in vivo and in vitro, and migrating between polypeptides 9 and 10 and 11 and 13 of the LHCII complex as the phosphorylated form of D2 (D2.1) (Delepelaire and Wollman, 1985; de Vitry et al., 1987, 1991; Ikeuchi et al., 1987b; de Vitry and Wollman, 1988). However, conclusive evidence indicating phosphorylation of D2 (e.g. by immunoprecipitation or amino acid sequencing) has not appeared in the literature to date.
It is now well established that LHCII phosphorylation is involved in the regulation of excitation energy distribution between PSI and PSII (Bennett et al., 1980; Allen et al., 1981; Bennett, 1991; Allen, 1992, 1995). In contrast to our knowledge concerning LHCII phosphorylation, very little is known about the role of phosphorylation of the PSII core proteins. Possible roles that have been suggested include the spatial separation of the two populations of PSII centers found in grana and stroma lamellae (PSII-α and PSII-β) (Mattoo et al., 1989), a role in biogenesis and assembly of PSII (Owens and Ohad, 1983; de Vitry et al., 1989; Summer et al., 1997), regulation of D1 and D2 degradation during photoinhibition (Elich et al., 1992; Aro et al., 1992, 1993; Giardi, 1993; Rintamaki et al., 1995, 1996), and stabilization of dimeric PSII complexes (Kruse et al., 1997).
In this paper we describe the characterization of two mutants of C. reinhardtii that were constructed to investigate the role of D2 phosphorylation based on the reasonable assumption that the D2-phosphorylation site in C. reinhardtii, like that in higher plants, is at residue Thr-2 of D2. Mutant D2 Thr2Ala lacks the hydroxyl group required for attachment of the phosphate group and should provide information on the function of D2 phosphorylation. Mutant D2 Thr2Ser, on the other hand, contains a hydroxyl group and was constructed to investigate the substrate specificity of the kinase.
MATERIALS AND METHODS
Strains and Growth Conditions
Chlamydomonas reinhardtii strain CC125 (mating type +) was obtained from the Chlamydomonas Genetic Center (Department of Botany, Duke University, Durham, NC). This strain was used as the host for chloroplast-transformation experiments and as the reference wild-type strain. C. reinhardtii wild-type and mutant strains were grown in TAP medium or HSM and handled as described by Harris (1989). Solid media were supplemented with spectinomycin (Sigma) at 100 μg mL−1, ampicillin (Sigma) at 50 μg mL−1, and 10 μm DCMU (British Greyhound, Birkenhead, UK). The addition of DCMU at 10 μm should inhibit PSII activity and prevent competition from wild-type copies of the psbD gene and from PSII revertants. Liquid cultures were grown at 25 to 30°C in an orbital shaker/incubator, either at an incident light intensity of 30 to 50 μmol m−2 s−1 or in the dark.
Recombinant Plasmids and in Vitro Mutagenesis
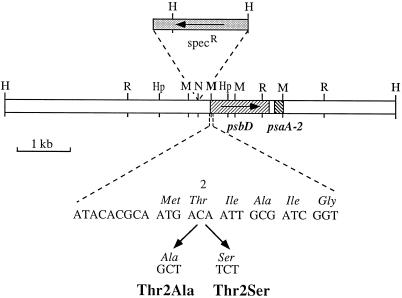
Plasmid pH3 (Erickson et al., 1986) was digested with HindIII to yield a 7.5-kb fragment containing the psbD gene, and was then partially digested with HpaI to give a 4.7-kb HpaI/HindIII fragment. The plasmid vector pTZ19U (Bio-Rad) was digested with HindIII and SmaI and ligated with the 4.7-kb HpaI/HindIII fragment to yield the plasmid pCA1. To select for transformed C. reinhardtii cells the aadA-selectable marker (Goldschmidt-Clermont, 1991), which confers resistance to spectinomycin and streptomycin, was inserted into pCA1 into a NsiI restriction site 261 bp upstream of the translation initiation codon of psbD. Plasmid pUC-atpX-AAD, which contains the aadA-selectable marker, was digested with EcoRV and SmaI to yield a 1.9-kb blunt-end fragment containing the spectinomycin-resistance cassette. pCA1 was digested with NsiI. Ends were blunt-ended with T4 DNA polymerase and then ligated with the 1.9-kb EcoRV/SmaI fragment, creating plasmid pNsi16.
Site-directed mutagenesis was carried out as described by Kunkel et al. (1987). Single-stranded DNA was prepared from pNsi16 and was annealed to the synthetic oligonucleotides 5′-ATACACGCAATGGCTATTGCGATCGGT-3′ and 5′-ATACACGCAATGTCTATTGCGATCCGT-3′ to create mutants Thr2Ala and Thr2Ser, respectively. Both mutagenic oligonucleotides were designed to delete a restriction site for the enzyme MunI along with the Thr-2 mutation (Fig. 1). Clones exhibiting a restriction pattern indicative of the presence of the desired mutation were subjected to sequence analysis using a double-stranded DNA-sequencing protocol (Sequenase 2.0, United States Biochemical). The whole psbD gene was sequenced in these clones to ensure that unwanted sequence alterations were not present in the gene and, subsequently, that the generated mutant plasmids were transformed into the chloroplast genome of C. reinhardtii.
Figure 1.
Restriction map of a 7.5-kb HindIII fragment from the chloroplast genome of C. reinhardtii showing the mutations made to the psbD gene. The positions and orientations of psbD, psaA-2, and the spectinomycin-resistance cassette (specR) are indicated. Restriction sites are HindIII (H), EcoRI (R), NsiI (N), HpaI (Hp), and MunI (M). The MunI site eliminated along with the creation of the Thr-2 mutations is shown in bold.
Chloroplast Transformation
Plasmid DNA precipitation onto M10 tungsten particles (Bio-Rad) and chloroplast transformation were performed essentially as described by Boynton and Gillham (1993) and Sanford et al. (1993). The particle-delivery instrument used was the gunpowder-driven model Mk2 from Shearline (Cambridge, UK).
Genetic Analysis of Site-Directed Mutants
Total cellular DNA was isolated using a slight modification of the procedure described by Newman et al. (1990). Instead of precipitating the nucleic acids after phenol/chloroform extraction, the top aqueous phase was mixed with 1 mL of resin (Wizard, Promega), and the DNA was recovered using a miniprep DNA-purification system (Wizard, Promega). ctDNA was prepared according to the method of Roffey et al. (1991).
Southern-blot hybridizations were performed following standard protocols. Sequencing of algal DNA was performed after amplification of miniprep DNA with primers C10 (5′-ctcggatccAAATACACAATGATTAAAAT-3′) and C70 (5′-tggaagcttAAAAATATATTATAGAGCGT-3′), which incorporate into the PCR product restriction sites for the enzymes BamHI and HindIII, respectively. The amplified DNA was digested with BamHI and HindIII and then cloned into the corresponding restriction sites of a pBluescript KS vector (Stratagene). The altered nucleotides in the Thr2Ala and Thr2Ser mutants were identified using primer C8 (5′-CAAGGAATAGTAATAAACC-3′).
Phenotypic Analysis of Site-Directed Mutants
The growth pattern of transformants was studied by growing liquid cultures photoautotrophically in 100 mL of HSM at 25°C and at an incident light intensity of 50 to 70 μmol m−2 s−1. The cultures were bubbled with air and stirred continuously. The optical density at 750 nm was monitored in a spectrophotometer (model MPS-2000, Shimadzu, Kyoto, Japan) at regular intervals until the cultures had reached the stationary phase.
Oxygen evolution of whole cells was measured with an oxygen electrode (model DW, Hansatech, Kings Lynn, UK). The measurements were performed in HSM at 25°C and at saturating light intensities (4000–6000 μmol m−2 s−1).
Fluorescence and Thermoluminescence Measurements
For the measurements of the thermoluminescence characteristics in whole cells of wild-type and transformant strains, cells were grown in TAP medium at 25°C and at an incident light intensity of 10 μmol m−2 s−1. Samples were dark adapted for 3 min at 20°C in the absence or presence of 10 μm DCMU, and were then excited by a single saturating flash at −10°C, followed by fast cooling to −40°C. The warming-ramp speed for these measurements was 10°C/min. Fluorescence induction was measured with a pulse-amplitude-modulated fluorometer (PAM 101, Walz, Effeltrich, Germany) for up to 3 s in the absence or presence of 10 μm DCMU. Measurements of the decay of Fv were performed according to the method of Whitelegge et al. (1995).
Photoinhibition Measurements
C. reinhardtii cells were grown with air bubbling and stirring in HSM until their middle- or late-exponential phase. The cultures were subjected to heat-filtered high-light illumination of 1000 μmol m−2 s−1 (provided by an apparatus equipped with a 1-kW halogen lamp). CAP at 200 μg/mL or lincomycin at 100 μg/mL (both from Sigma) was added to some of the samples to inhibit chloroplast-protein synthesis. One- to 2-mL samples were taken at set intervals during a time course of 4 to 5 h, and light-saturated oxygen evolution was measured.
32P Labeling of Whole Cells and Thylakoid Membranes
32P labeling of C. reinhardtii whole cells was performed according to the method of de Vitry et al. (1991). Cells grown in TAP medium until the late-exponential phase were incubated with 1 mCi of 32Pi for 16 h at room temperature under low light. Thylakoid membranes were isolated according to the method of Diner and Wollman (1980), except that all buffers contained 20 mm NaF to inhibit phosphatase. PSII-RC particles were prepared as described by Alizadeh et al. (1995). All buffers until the stage of the column wash contained 20 mm NaF.
In vitro 32P labeling of thylakoid membrane proteins was carried out in 100 mm Suc, 50 mm Hepes-KOH, pH 8.0, 10 mm MgCl2, 0.2 mm ATP, and 10 mm NaF at a chlorophyll concentration of 0.2 mg mL−1. [γ-32P]ATP (specific activity, approximately 3000 Ci mmol−1; Amersham) at a concentration of 10 μCi for every 100 μg of chlorophyll was added and the suspension was incubated for 15 min at 50 to 70 μmol m−2 s−1 of heat-filtered light at 25°C. The reaction was stopped by centrifugation for 10 min in a microcentrifuge.
Pulse-Chase Labeling of Whole Cells Using [14C]Acetate
The following protocol was based on the procedure of Kuras and Wollman (1994). Cells grown in TAP medium to an optical density (750 nm) of 0.2 to 0.5 were harvested, washed twice in HSM, and resuspended in HSM to a chlorophyll concentration of 25 μg mL−1. Cells were depleted of acetate by incubating them at 25°C in HSM for 1 h at an incident light intensity of 100 μmol m−2 s−1. Ten minutes before the addition of the label, the cytoplasmic protein-synthesis inhibitor cycloheximide was added at a concentration of 10 μg mL−1. [1-14C]Sodium acetate (specific activity, 56 mCi/mmol; Amersham) was then added at 5 μCi mL−1, and the incubation was continued for 5 min. For the chase, the labeled cells were washed twice in HSM containing 25 mm cold sodium acetate to deplete them of the radioactive label, and then resuspended in the same medium plus 10 μg mL−1 cycloheximide. The cell suspension was then incubated under the same conditions (100 μmol m−2 s−1 at 25°C) for an additional 90 min. Ten-milliliter aliquots were withdrawn at 0, 45, and 90 min, and thylakoid membranes were isolated for SDS-PAGE and autoradiography.
SDS-PAGE and Immunoblotting
SDS-PAGE was carried out according to the method of Hankamer et al. (1997). Before loading, protein samples were solubilized in the dark by the addition of an equal volume of solubilization buffer (187 mm Tris, pH 6.8, 30% glycerol, 9% SDS, 15% β-mercaptoethanol). Proteins from whole cells were solubilized by boiling the cell suspension (at a chlorophyll concentration of 0.5 μg/μL) for 1 min in an equal volume of solubilization buffer. Pigmented samples were loaded on the gel on an equal-chlorophyll basis. Protein samples resolved by SDS-PAGE were transferred to a nitrocellulose membrane according to the method of Dunn (1986). Immunodecoration of protein was achieved by incubating membranes with one of the following antisera: a D2 antibody raised against a synthetic peptide corresponding to the last 12 amino acids of PsbD (Nixon et al., 1990), a spinach LHCII antibody (Hilditch, 1986), or a monospecific antibody to phospho-Thr (Sigma). The specificity of the latter was tested by adding phospho-Thr or phospho-Tyr (Sigma) during incubation with the primary antibody. Cross-reacting proteins were detected using an anti-rabbit IgG-alkaline phosphatase conjugate (Sigma) and 5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt and nitroblue tetrazolium chloride (Sigma).
RESULTS
Construction of D2 Thr2Ala and D2 Thr2Ser Mutants
The mutants D2 Thr2Ala and D2 Thr2Ser were constructed in C. reinhardtii using the biolistic technique pioneered by Boynton and co-workers (1988). To select for transformants, a spectinomycin-resistance cassette was inserted upstream of the psbD gene (Fig. 1). After bombardment of wild-type cells with a plasmid carrying the mutant psbD gene, the site-directed mutation was incorporated into the psbD gene on the chloroplast genome through homologous recombination. Figure 2 shows a DNA gel blot of ctDNA isolated from mutants Thr2Ser and Thr2Ala, the wild-type control strain Nsi16, which contains the spectinomycin-resistance cassette upstream of a wild-type copy of psbD, and the wild type. Figure 2 confirms that the transformants were homoplasmic.
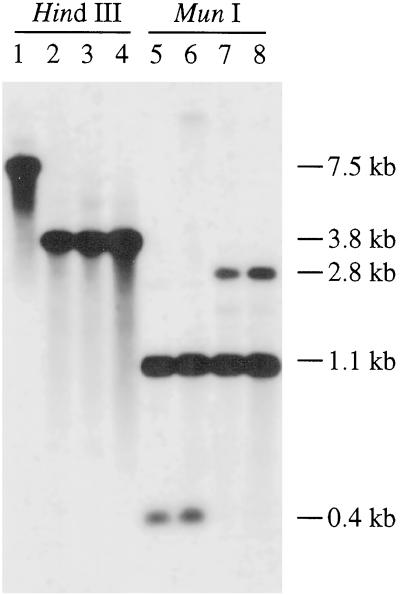
Figure 2.
Southern-blot analysis of the wild type (lanes 1 and 5), Nsi16 (lanes 2 and 6), and mutants Thr2Ser (lanes 3 and 7) and Thr2Ala (lanes 4 and 8). ctDNA was digested with HindIII (lanes 1–4) and MunI (lanes 5–8), and hybridized to a 0.6-kb psbD probe.
The 7.5-kb HindIII fragment in the wild type, which contains psbD, is replaced by a 3.8-kb fragment in the transformants (Fig. 2, lanes 2–4). The presence of the aadA cassette in the transformants was confirmed in DNA gel blots by probing with an aadA-specific probe (data not shown). In addition, mutants Thr2Ser and Thr2Ala have lost a MunI site because of their mutations, so a 0.4-kb MunI fragment found in the wild type and in Nsi16 is replaced by a 2.8-kb fragment in the mutants (Fig. 2, lanes 7 and 8). The psbD gene in the transformants was recloned and sequenced to confirm the existence of the desired mutations at Thr-2. DNA sequencing also confirmed that no other spontaneous mutations were incorporated into the psbD gene during construction of the mutants (data not shown).
Biophysical Characterization of the Thr-2 Mutants
The photoautotrophic growth rates of the wild type, Nsi16, and the Thr-2 mutants in HSM were indistinguishable, with a doubling time of approximately 10 h at a light intensity of 50 to 70 μmol m−2 s−1 (data not shown). Table I confirms that PSII activity, assayed by the rate of light-saturated oxygen evolution in the presence of the artificial electron acceptors ferricyanide and DCBQ, was largely unaffected in the mutants, although a slight reduction was observed in Thr2Ala. A number of noninvasive techniques were therefore used to determine if substitution of the Thr-2 residue had subtle effects on the electron-transfer reactions within PSII. The fluorescence-induction characteristics of the mutants were similar to those of the wild type, suggesting little perturbation to photosynthetic electron flow (data not shown). The Fv/Fm value in the presence of DCMU was approximately 0.69 for both the wild type and the mutants. The Fv/Fm ratio is a measure of the efficiency of light capture by PSII (Krause and Weis, 1991).
Table I.
Phenotypic characteristics of the D2-Thr2 mutants
| Strain | Photosynthetic Growth | Oxygen Evolutiona | Thermoluminescence
Characteristics
|
|
|---|---|---|---|---|
| Q band | B band | |||
| Wild type | + | 100 | 14–16°C | 30–32°C |
| Nsi16 | + | 102 ± 5 | 14–16°C | 30–32°C |
| Thr2Ser | + | 119 ± 38 | 14–16°C | 30–32°C |
| Thr2Ala | + | 75 ± 15 | 14–16°C | 30–32°C |
Measurements were carried out in HSM in the presence of 1 mm potassium ferricyanide and 1 mm DCBQ. Data presented are means of four independent experiments.
The light-saturated steady-state rates of oxygen evolution in whole cells of wild type were 266 ± 21 μmol O2 mg−1 chlorophyll h−1.
Thermoluminescence measurements confirmed that the peak positions of the Q and B bands were also unaffected in the mutants (Table I). The Q and B bands are associated with charge recombination between S2QA− and S2QB−, respectively, with the peak position indicative of the degree of charge stabilization (Vass et al., 1981).
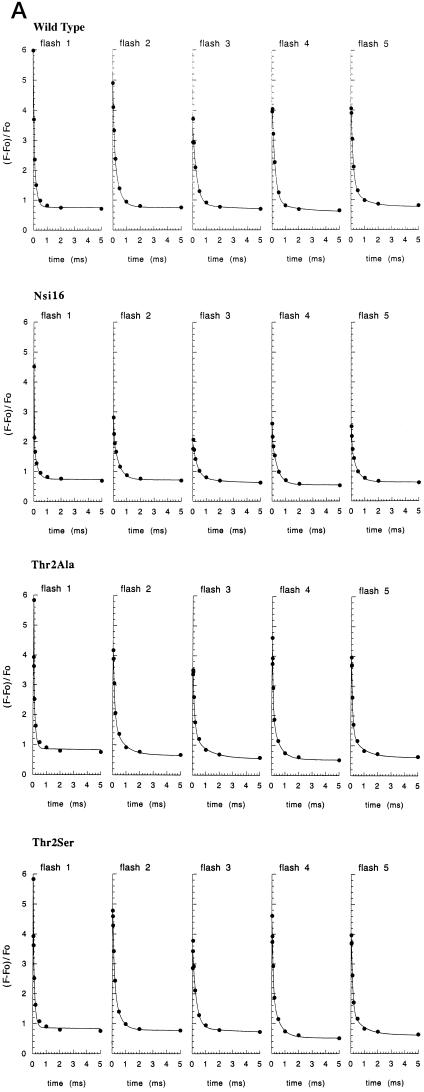
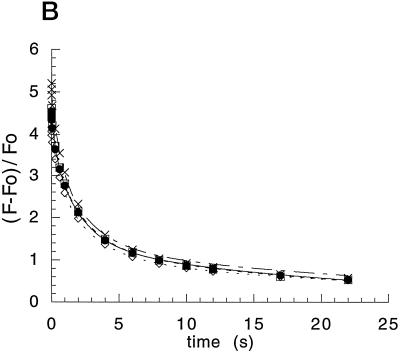
The rate of decay of chlorophyll fluorescence from whole cells after a saturating flash of light is indicative of the rate of electron transfer between QA and QB (Whitelegge et al., 1995). From the fluorescence-decay measurements shown in Figure 3A, recorded after each of five saturating flashes spaced 0.6 s apart, the rate of electron transfer between QA and QB appears similar in the wild type and the mutants. Figure 3A also shows that the QA-to-QB electron transfer is faster on the odd flashes than on the even. Such an oscillation of period two is consistent with the normal operation of electron transfer on the acceptor side of PSII (Robinson and Crofts, 1983). Figure 3B shows the decay of chlorophyll fluorescence in intact cells in the presence of DCMU, an herbicide that blocks electron transfer between QA and QB. After a single saturating flash, the decay in fluorescence is now a measure of the rate of charge recombination between the S2 state of the water-oxidizing complex and QA−. No differences were observed between the different strains. These analyses therefore suggested that electron transfer within PSII and the redox properties of the various cofactors are largely unaffected in the Thr-2 mutants.
Figure 3.
Relaxation of Fv in the Thr-2 mutants. A, Relaxation of the Fv after each of a series (1.67 Hz) of five saturating 2-μs flashes in whole cells of the C. reinhardtii mutants Thr2Ala and Thr2Ser. Shown is the 5-ms range after each flash starting at 50 μs. The 50-μs values of Fv are normalized to Fo [(F − Fo)/Fo]. B, Relaxation of Fv resulting from charge recombination between QA− and the PSII donor side after a single, saturating 2-μs flash excitation of whole cells of C. reinhardtii. Wild type (•) is compared with transformant Nsi16 (×) and with the mutants Thr2Ala (⋄) and Thr2Ser (□).
Photoinhibition of the Thr-2 Mutants
To determine whether the Thr-2 residue played a role in protecting PSII from photoinactivation, cells of the wild type and the Thr-2 mutants were exposed to a high-light irradiance (1000 μmol m−2 s−1), and PSII activity was monitored as a function of time. PSII activity in both the wild type and Thr2Ala, measured as the rate of light-saturated oxygen evolution in the presence of the artificial electron acceptors DCBQ (1 mm) and ferricyanide (1 mm), was stable over the time period of the experiment (data not shown). When chloroplast-protein synthesis was blocked by the addition of CAP to the medium, the loss of PSII activity could be used to monitor the rate of photoinactivation of PSII, since repair of PSII by de novo protein synthesis was unable to proceed. Both the wild type and Thr2Ala showed similar rates of photoinactivation (t1/2 approximately 80 min; Fig. 4), indicating that the wild-type and mutant PSII complexes were similarly susceptible to photodamage. Photoinactivation of PSII activity in the Thr2Ser mutant was slightly faster (t1/2 approximately 50–60 min; Fig. 4). Similar results were obtained using lincomycin instead of CAP to inhibit chloroplast-protein synthesis (data not shown). Immunoblotting experiments confirmed that the steady-state levels of D2 in photoinhibited cells were similar between the wild type and Thr2Ala (data not shown).
Figure 4.
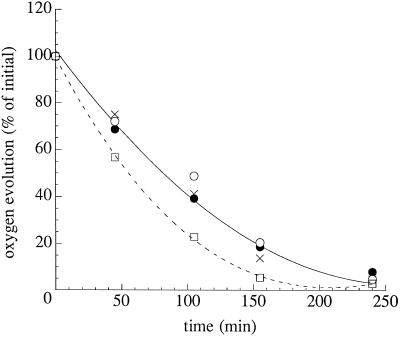
Photoinhibition measurements. Effect of high-light treatment (1000 μmol m−2 s−1) on the light-saturated rate of oxygen evolution from whole cells of the wild type, Nsi16, and the mutants Thr2Ala and Thr2Ser in the presence of 200 μg/mL CAP. Cells were suspended in HSM at 25°C to a chlorophyll concentration of 25 μg/mL. Oxygen evolution was measured using 1 mm DCBQ and 1 mm potassium ferricyanide. The 100% rates of oxygen evolution were approximately 251 μmol O2 mg−1 chlorophyll h−1 for the wild type (•), 356 μmol O2 mg−1 chlorophyll h−1 for Nsi16 (×), 222 μmol O2 mg−1 chlorophyll h−1 for Thr2Ala (○), and 333 μmol O2 mg−1 chlorophyll h−1 for Thr2Ser (□).
In Vitro-Phosphorylation Experiments
The lack of any phenotypic difference between the wild type and the Thr-2 mutants led us to reinvestigate the assignment of D2 as a phosphorylated protein in C. reinhardtii. In vitro-phosphorylation experiments using [32P]ATP were performed to determine if a phosphorylated form of D2 could be detected. The experimental conditions were similar to those that had previously been shown to give rise to a labeled band, which was assigned to phosphorylated D2 (Wollman and Delepelaire, 1984; Delepelaire and Wollman, 1985). The labeling reaction contained 10 mm NaF to inhibit phosphatases and approximately 0.2 mm ATP at a specific activity of approximately 3000 Ci mmol−1. Preliminary experiments in the wild type showed that the best labeling of thylakoid membrane proteins occurred using thylakoids isolated from cultures grown in the dark and labeled in the light (Fig. 5A). Light stimulates the labeling of all of the phosphorylated polypeptides and, in agreement with a study by Wollman and Delepelaire (1984), some phosphorylation occurred in the absence of light.
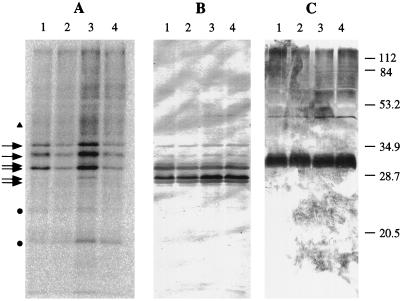
Figure 5.
In vitro labeling of wild-type thylakoid membranes with [γ-32P]ATP. A, Autoradiogram of a 12%, 6 m urea-SDS gel. B and C, Immunoblotting with α-LHCII (B) and α-D2 (C) antibodies. Lanes 1, Cells grown in the light and thylakoids labeled in the light; lanes 2, cells grown in the light and thylakoids labeled in the dark; lanes 3, cells grown in the dark and thylakoids labeled in the light; and lanes 4, cells grown in the dark and thylakoids labeled in the dark. Arrows indicate positions of main LHCII bands picked up by the α-LHCII antibody. The triangle represents the position of CP43. Dots indicate unidentified phosphoproteins. Low-molecular-mass polypeptides have been run off the bottom of the gel. Numbers next to the α-D2 blot represent the bands of the molecular-mass marker in kilodaltons (prestained LMW, Bio-Rad).
In broad detail the pattern of phosphorylation was similar to previously published autoradiograms (Owens and Ohad, 1982; Wollman and Delepelaire, 1984; Delepelaire and Wollman, 1985; Gans and Wollman, 1995). Immunoblotting experiments using antisera specific for higher-plant LHCII and D2 indicated that all of the labeled polypeptides could be assigned to LHCII components (Fig. 5, B and C). The uppermost, heavily labeled band can be assigned to CP29 (Bassi and Wollman, 1991; Allen and Staehelin, 1994). The labeled band migrating below CP29 is probably an LHCII polypeptide, number 11 or 13 (Wollman and Delepelaire, 1984; Allen and Staehelin, 1994). This band migrated in proximity to the upper part of D2 (Fig. 5, compare B and C). Therefore, its identification as the phosphorylated form of D2 cannot be completely excluded. The lightly labeled band (designated with a triangle) in Figure 5A is most probably the phosphorylated form of CP43.
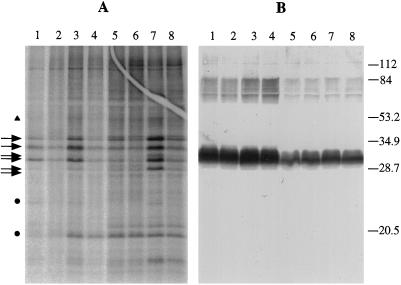
The profile obtained using thylakoids isolated from the Thr2Ala mutant was very similar to the one obtained with wild-type membranes (Fig. 6A). However, immunoblotting using an α-D2 antibody, shown in Figure 6B, reveals differences between wild-type and Thr2Ala samples. The Thr2Ala samples appear to lack the upper part of the immunodecorated D2 band seen in the wild type, regardless of the growth or labeling conditions. This part of D2 does not correspond to any labeled band in the autoradiogram and therefore cannot be attributed to the phosphorylated form of D2. In the experiment shown in Figure 6B, the reduced level of D2 in the Thr2Ala thylakoids may reflect fewer PSII centers compared with the wild type.
Figure 6.
In vitro labeling of wild-type (lanes 1–4) and Thr2Ala (lanes 5–8) thylakoid membranes with [γ-32P]ATP. A, Autoradiogram of a 12%, 6 m urea-SDS gel. B, Immunoblotting with α-D2 antibody. Lanes 1 and 5, Cells grown in the light and thylakoids labeled in the light; lanes 2 and 6, cells grown in the light and thylakoids labeled in the dark; lanes 3 and 7, cells grown in the dark and thylakoids labeled in the light; lanes 4 and 8, cells grown in the dark and thylakoids labeled in the dark. Arrows indicate positions of main LHCII bands picked up by the α-LHCII antibody as shown in Figure 5. The triangle represents the position of CP43. Dots indicate unidentified phosphoproteins. Numbers next to the α-D2 blot represent the bands of the molecular-mass marker in kilodaltons (prestained LMW, Bio-Rad).
In Vivo Phosphorylation Experiments
One of the main lines of evidence supporting D2 phosphorylation in C. reinhardtii comes from the work of de Vitry and co-workers (1987, 1991), who used the double mutant F54-14 (strain CC2655) in in vivo 32P-labeling experiments. After labeling whole cells with 32Pi, these investigators isolated PSII-containing particles, which they then examined by SDS-PAGE and autoradiography. The upper part of a protein band, assigned to D2 (D2.1), comigrated with a phosphorylated band. However, LHCII contamination within the core complex makes it difficult to rule out LHCII as the cause of the phosphorylated band. Recently the PSI− ATPase− strain used by de Vitry and co-workers was used for the preparation of a PSII RC containing D1, D2, Cyt b559, and PsbI (Alizadeh et al., 1995). Therefore, by labeling thylakoid membrane proteins with 32Pi and subsequently isolating PSII RCs, one should be able to detect phosphorylated D2 in a preparation containing minimal LHCII. Analysis of phosphorylated PSII RCs has been previously used successfully to show that D1 and D2 are both phosphorylated in vitro in pea (Pisum sativum) (Telfer et al., 1987).
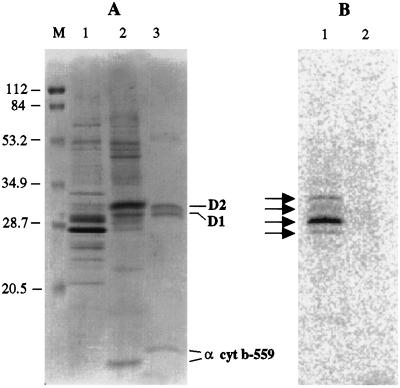
Figure 7 shows the SDS-PAGE analysis and autoradiogram of thylakoid proteins and a PSII-RC preparation isolated from C. reinhardtii strain CC2655 after in vivo phosphorylation. Because of the small amounts involved, the PSII-RC preparation from C. reinhardtii contained some protein contamination not seen in the pea PSII RC. The labeling pattern of the thylakoid membranes was similar to that observed after in vitro labeling of wild-type thylakoids (Fig. 5). The four labeled bands with apparent molecular masses between 25 and 35 kD (Fig. 7B) were similar in size to corresponding in vivo-labeled phosphoproteins reported in the literature using either wild-type cells (Delepelaire and Wollman, 1985; Gans and Wollman, 1995) or a PSI− mutant of C. reinhardtii (Delosme et al., 1996). These bands are probably the LHC polypeptides designated in C. reinhardtii as 10, 9, 13/11, and 17 (in descending order of molecular mass, starting from approximately 35 kD). No phosphorylation of D2 or any other PSII subunit was detected in the PSII-RC preparation (Fig. 7B, lane 2).
Figure 7.
In vivo labeling of C. reinhardtii CC2655 cells with 32Pi using 10 to 17% Coomassie blue-stained, 6 m urea-SDS gel (A) and autoradiography (B). Lane M, Molecular-mass marker in kilodaltons (prestained LMW, Bio-Rad); lanes 1, thylakoid membranes; lanes 2, isolated PSII RCs; lane 3, PSII RCs from pea. The autoradiogram was obtained by exposing the gel to a Phosphor Imager screen (Molecular Dynamics) for 7 d. The amount of chlorophyll was 5 μg for the CC2655 thylakoids, 1 μg for the CC2655 PSII RCs, and 0.2 μg for pea PSII RCs.
Anti-phospho-Thr antibodies were used to probe PSII-RC preparations isolated from pea and C. reinhardtii. Although the signals were weak, only D1 and D2 in the higher-plant preparation gave a cross-reaction (data not shown).
In Vivo [14C]Acetate-Labeling Experiments
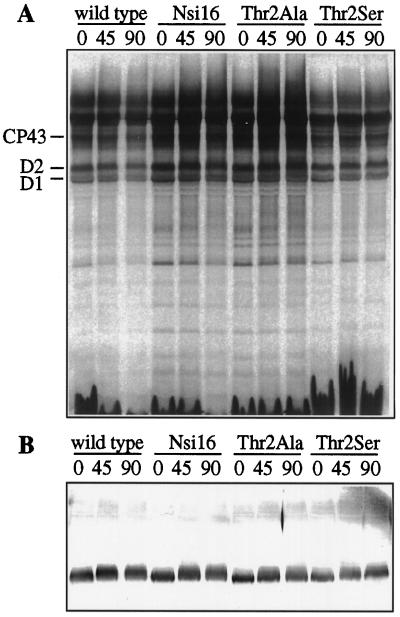
Cells of the wild type, the control strain Nsi16, and the mutants Thr2Ala and Thr2Ser were radiolabeled with [14C]acetate as described by Kuras and Wollman (1994) and analyzed by urea-SDS-PAGE under conditions that have been reported to resolve D2 into the nonphosphorylated (D2.2) and phosphorylated (D2.1) forms. As shown in Figure 8, both the immunodetectable D2 band and the radiolabeled D2 band migrated as diffuse bands.
Figure 8.
Pulse-chase labeling of whole cells with [14C]acetate. Autoradiogram (A) and α-D2 immunoblot of a 14%, 8 m urea-SDS gel (B) of thylakoid membranes isolated from wild-type, Nsi16, Thr2Ala, and Thr2Ser cells pulse labeled for 5 min with [14C]acetate at an incident light intensity of 100 μmol m−2 s−1 and then chased for 45 and 90 min in the absence of label. Both pulse and chase were carried out in the presence of 10 μg/mL cycloheximide.
DISCUSSION
Our results show that the replacement of Thr-2 of the D2 protein with either Ala or Ser does not have any dramatic effect on PSII function. Photoautotrophic growth rates, fluorescence decay, fluorescence-induction measurements, and thermoluminescence measurements all indicate that the Thr2Ala and Thr2Ser mutants behave similarly to the wild type. Wild-type levels of steady-state oxygen evolution were also obtained for the Thr2Ser mutant, whereas the Thr2Ala mutant exhibited rates of oxygen evolution around 55 to 100% of those of the wild type, possibly because of the variability of PSII content in different cultures.
It was speculated by Michel et al. (1988) that phosphorylation of the N terminus of D1 or D2 in higher plants may lead to an interaction between the phosphoryl group and the nonheme iron atom located between the QA- and QB-binding sites. Such an interaction may provide pH buffering, which would affect protonation of D1 and D2 and rates of QA-to-QB electron transfer (Michel et al., 1988). However, no significant perturbation to electron transfer between QA and QB was detected in the Thr-2 mutants described here (Fig. 3A).
One of the aims of the construction of the two Thr mutants was to study the relationship between the phosphorylation of PSII proteins and photoinhibition (for review, see Allen, 1992). Our results indicate that Thr-2 of D2 does not have a crucial role either in protecting PSII from photoinactivation or in regulating the degradation of the D2 polypeptide (data not shown). Therefore, phosphorylation of Thr-2, if it exists, plays only a minor role in these processes.
Attempts to interpret the results obtained from the phenotypic analysis of the mutants have been based on the assumption that D2 can be phosphorylated in wild-type C. reinhardtii. The evidence for D2 phosphorylation in C. reinhardtii in the literature is based on autoradiograms of 14C- and 32P-labeled cells and thylakoid membranes (Delepelaire and Wollman, 1985; de Vitry et al., 1987, 1991; de Vitry and Wollman, 1988), which were not accompanied by immunoblots to identify D2. In an effort to obtain satisfactory data concerning the state of D2 phosphorylation in the wild type and in mutant strains, different approaches were followed, including in vitro and in vivo 32P labeling of thylakoid membrane proteins, identification of labeled bands using a monoclonal antibody against phospho-Thr, and pulse-chase labeling of whole cells with [14C]acetate.
The in vitro-phosphorylation pattern of thylakoid membrane polypeptides from the wild type was similar to previous results (Owens and Ohad, 1982; Wollman and Delepelaire, 1984; Delepelaire and Wollman, 1985; Gans and Wollman, 1995). However, all of the radiolabeled bands could be assigned to LHC polypeptides using a specific antibody. This is particularly relevant to the assignment of the radiolabeled band that migrates close to the upper part of D2, since this band has most commonly been attributed to phosphorylated D2 in previous studies (Lemaire et al., 1987; de Vitry and Wollman, 1988; de Vitry et al., 1991; Gans and Wollman, 1995).
The profile obtained in the in vitro-phosphorylation experiments for the Thr2Ala mutant was similar to that for the wild type. At first sight this would indicate that D2 is not phosphorylated in C. reinhardtii. However, other possible explanations are that (a) D2 can still be phosphorylated in the Thr2Ala mutant, possibly at alternative Thr residues such as those present at positions 7 and 13 (Erickson et al., 1986); (b) phosphorylated D2 migrates as a very diffuse band (Ikeuchi et al., 1987b), making identification difficult; (c) D2 cannot be labeled in the wild type in in vitro-labeling experiments because D2 is already phosphorylated or because the kinase has been inactivated; or (d) D2 is readily dephosphorylated by phosphatases during sample preparation. Although the latter three explanations cannot be ruled out, the experimental conditions used were identical to conditions in other studies that assigned a phosphorylated D2 band.
Immunoblotting with D2-specific antibodies revealed that the upper part of the D2 band is apparently missing in the Thr2Ala mutant (Fig. 6). Although suggestive of the absence of the slower-migrating, phosphorylated form of D2 in this mutant, it is possible that the Thr-to-Ala change alters the mobility of the protein on SDS-PAGE, as in the case of the Pro161Leu mutation of D2 in Synechocystis PCC 6803 (Tommos et al., 1993).
A more revealing experiment regarding D2 phosphorylation came from the in vivo labeling of whole cells with 32Pi and the subsequent isolation of PSII RCs. The labeling pattern obtained for the thylakoid membranes was quite similar to the in vitro-labeling profile. No labeled band could be observed in the PSII RC, implying that none of the PSII-RC proteins in C. reinhardtii was phosphorylated. Immunoblots using a monoclonal antibody specific for phospho-Thr also suggested that D1 and D2 were unphosphorylated in the PSII-RC sample. Again, these results cannot be interpreted unambiguously, for the reasons outlined above.
Attempts to identify the two bands that constitute the D2 doublet (D2.1 and D2.2) (Delepelaire, 1984) were also carried out by pulse labeling with [14C]acetate and chasing in the absence of radioactive label. In these experiments, D2 always appeared as one diffuse band. Some broadening of the immunodetected D2 band during the chase is ascribed to D2 oxidation upon exposure to high light, as has been observed in in vitro systems (e.g. Ponticos et al., 1993). It should also be noted that in higher plants phosphorylated and nonphosphorylated D2 cannot be separated by SDS-PAGE (Telfer et al., 1987).
In summary, replacement of Thr-2 of D2 with either Ala or Ser did not result in any dramatic changes in PSII function. We cannot, however, conclude that phosphorylation of D2 is not important for PSII function, because our data did not provide us with conclusive evidence concerning D2 phosphorylation in either the wild type or the mutant strains. Most of the labeled bands observed after in vitro and in vivo labeling with 32P could be attributed to polypeptides belonging to the LHCII complex or to minor antenna polypeptides (CP29). We must therefore seriously consider the possibility that the band that has been reported in the literature as the phosphorylated form of D2 (designated as D2.1) is in fact a phosphorylated form of a LHCII polypeptide. This possibility is particularly pertinent to the assignment of 32P-labeled bands in PSII core complexes that contain trace amounts of LHC polypeptides (de Vitry et al., 1987, 1991). Therefore, final confirmation that D2 is indeed phosphorylated in C. reinhardtii awaits the application of more refined techniques such as MS.
ACKNOWLEDGMENTS
We thank Jean-David Rochaix (University of Geneva, Switzerland) for plasmid pH3, Michel Goldschmidt-Clermont (University of Geneva) for plasmid pUC-atpX-aadA, and Howard Thomas (Institute of Grassland and Environmental Research, Aberystwyth, UK) for the LHCII antiserum.
Abbreviations:
- CAP
chloramphenicol
- DCBQ
1 mm 2,6-dichloro-p-benzoquinone
- Fm
maximal level of chlorophyll fluorescence when all PSII centers are closed
- Fo
minimal level of chlorophyll fluorescence when all PSII centers are open
- Fv
variable chlorophyll fluorescence (Fm − Fo)
- HSM
high-salt minimal medium
- LHCII
light-harvesting antenna of PSII
- QA and QB
primary and secondary electron-accepting plastoquinones of PSII
- RC
reaction center
- TAP
Tris-acetate phosphate
Footnotes
This research was supported by The Royal Society (P.J.N.) and the Hungarian granting agency OTKA (nos. F017454 and T017049). C.A. was supported by a fellowship from the State Scholarship Foundation of Greece.
LITERATURE CITED
- Alizadeh S, Nixon PJ, Telfer A, Barber J. Isolation and characterisation of the photosystem two reaction centre complex from a double mutant of Chlamydomonas reinhardtii. Photosynth Res. 1995;43:165–171. doi: 10.1007/BF00042974. [DOI] [PubMed] [Google Scholar]
- Allen JF. Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta. 1992;1098:275–335. doi: 10.1016/s0005-2728(09)91014-3. [DOI] [PubMed] [Google Scholar]
- Allen JF. Thylakoid protein phosphorylation, state 1-state 2 transitions, and photosystem stoichiometry adjustment: redox control at multiple levels of gene expression. Physiol Plant. 1995;93:196–205. [Google Scholar]
- Allen JF, Bennett J, Steinback KE, Arntzen CJ. Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature. 1981;291:25–29. [Google Scholar]
- Allen KD, Staehelin LA. Polypeptide composition, assembly and phosphorylation patterns of the photosystem II antenna system of Chlamydomonas reinhardtii. Planta. 1994;194:42–54. [Google Scholar]
- Aro E-M, Kettunen R, Tyystjarvi E. ATP and light regulate D1 protein modification and degradation: role of D1* in photoinhibition. FEBS Lett. 1992;297:29–33. doi: 10.1016/0014-5793(92)80320-g. [DOI] [PubMed] [Google Scholar]
- Aro E-M, Virgin I, Andersson B. Photoinhibition of photosystem II: inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Bassi R, Wollman F-A. The chlorophyll-a/b proteins of photosystem II in Chlamydomonas reinhardtii. Planta. 1991;183:423–433. doi: 10.1007/BF00197742. [DOI] [PubMed] [Google Scholar]
- Bennett J. Phosphorylation of chloroplast membrane proteins. Nature. 1977;269:344–346. [Google Scholar]
- Bennett J. Protein phosphorylation in green plant chloroplasts. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:281–311. [Google Scholar]
- Bennett J, Steinback KE, Arntzen CJ. Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membrane polypeptides. Proc Natl Acad Sci USA. 1980;77:5253–5257. doi: 10.1073/pnas.77.9.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton JE, Gillham NW. Chloroplast transformation in Chlamydomonas. Methods Enzymol. 1993;217:510–536. doi: 10.1016/0076-6879(93)17087-l. [DOI] [PubMed] [Google Scholar]
- Boynton JE, Gillham NW, Harris EH, Hosler JP, Johnson AM, Jones AR, Randolph-Anderson BL, Robertson D, Klein TD, Shark KB and others. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988;240:1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- Delepelaire P. Partial characterization of the biosynthesis and integration of the photosystem II reaction centres in the thylakoid membrane of Chlamydomonas reinhardtii. EMBO J. 1984;3:701–706. doi: 10.1002/j.1460-2075.1984.tb01872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepelaire P, Wollman F-A. Correlations between fluorescence and phosphorylation changes in thylakoid membranes of Chlamydomonas reinhardtii in vivo: a kinetic analysis. Biochim Biophys Acta. 1985;809:277–283. [Google Scholar]
- Delosme R, Olive J, Wollman F-A. Changes in light energy distribution upon state transitions: an in vivo photoacoustic study of the wild type and photosynthesis mutants from Chlamydomonas reinhardtii. Biochim Biophys Acta. 1996;1273:150–158. [Google Scholar]
- de Vitry C, Diner BA, Lemoine Y. Chemical composition of photosystem II reaction centres (PS II): phosphorylation of PS II polypeptides. In: Biggins J, editor. Progress in Photosynthesis Research, Vol II. Dordrecht, The Netherlands: Martinus Nijhoff; 1987. pp. 105–108. [Google Scholar]
- de Vitry C, Diner BA, Popot J-L. Photosystem II particles from Chlamydomonas reinhardtii. J Biol Chem. 1991;266:16614–16621. [PubMed] [Google Scholar]
- de Vitry C, Olive J, Drapier D, Recouvreur M, Wollman F-A. Posttranslational events leading to the assembly of photosystem II protein complex: a study using photosynthesis mutants from Chlamydomonas reinhardtii. J Cell Biol. 1989;109:991–1006. doi: 10.1083/jcb.109.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vitry C, Wollman F-A. Changes in phosphorylation of thylakoid membrane proteins in light-harvesting complex mutants from Chlamydomonas reinhardtii. Biochim Biophys Acta. 1988;933:444–449. [Google Scholar]
- Diner BA, Wollman F-A. Isolation of highly active photosystem II particles from a mutant of Chlamydomonas reinhardtii. Eur J Biochem. 1980;110:521–526. doi: 10.1111/j.1432-1033.1980.tb04894.x. [DOI] [PubMed] [Google Scholar]
- Dunn SD. Effect of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on western blots by monoclonal antibodies. Anal Biochem. 1986;157:144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- Elich TD, Edelman M, Mattoo AK. Identification, characterization, and resolution of the in vivo phosphorylated form of the D1 photosystem II reaction centre protein. J Biol Chem. 1992;267:3253–3259. [PubMed] [Google Scholar]
- Erickson JM, Rahire M, Malnoe P, Girard-Bascou J, Pierre Y, Bennoun P, Rochaix J-D. Lack of the D2 protein in a Chlamydomonas reinhardtii psbD mutant affects photosystem II stability and D1 expression. EMBO J. 1986;5:1745–1754. doi: 10.1002/j.1460-2075.1986.tb04422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans P, Wollman F-A. The effect of cyanide on state transitions in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1995;1228:51–57. [Google Scholar]
- Giardi MT. Phosphorylation and disassembly of the photosystem II core as an early stage of photoinhibition. Planta. 1993;190:107–113. [Google Scholar]
- Goldschmidt-Clermont M. Transgenic expression of aminoglycoside adenine transferase in the chloroplast: a selectable marker for site-directed transformation of Chlamydomonas. Nucleic Acids Res. 1991;19:4083–4089. doi: 10.1093/nar/19.15.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankamer B, Nield J, Zheleva D, Boekema E, Jansson S, Barber J. Isolation and biochemical characterisation of monomeric and dimeric photosystem II complexes from spinach and their relevance to the organisation of photosystem II in vivo. Eur J Biochem. 1997;243:422–429. doi: 10.1111/j.1432-1033.1997.0422a.x. [DOI] [PubMed] [Google Scholar]
- Harris EH (1989) The Chlamydomonas Sourcebook. Academic Press, New York
- Hilditch P. Immunological quantification of the chlorophyll a/b binding protein in senescing leaves of Festuca pratensis Huds. Plant Sci. 1986;45:95–99. [Google Scholar]
- Ikeuchi M, Plumley FG, Inoue Y, Schmidt GW. Phosphorylation of photosystem II components, CP43 apoprotein, D1, D2, and 10 to 11 kilodalton protein in chloroplast thylakoids of higher plants. Plant Physiol. 1987a;85:638–642. doi: 10.1104/pp.85.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M, Plumley FG, Inoue Y, Schmidt GW. Identification of phosphorylated reaction centre polypeptides in thylakoids of Chlamydomonas reinhardtii and Pisum sativum. In: Biggins J, editor. Progress in Photosynthesis Research, Vol II. Dordrecht, The Netherlands: Martinus Nijhoff; 1987b. pp. 805–808. [Google Scholar]
- Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:313–349. [Google Scholar]
- Kruse O, Zheleva D, Barber J. Stabilization of photosystem II dimers by phosphorylation: implications for the regulation of the turnover of D1 protein. FEBS Lett. 1997;408:276–280. doi: 10.1016/s0014-5793(97)00439-0. [DOI] [PubMed] [Google Scholar]
- Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-directed mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kuras R, Wollman F-A. The assembly of cytochrome b6/f complexes: an approach using genetic transformation of the green alga Chlamydomonas reinhardtii. EMBO J. 1994;13:1019–1027. doi: 10.1002/j.1460-2075.1994.tb06350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire C, Girard-Bascou J, Wollman F-A. Characterisation of the b6/f complex subunits and studies on the LHC-kinase in Chlamydomonas reinhardtii using mutant strains altered in the b6/f complex. In: Biggins J, editor. Progress in Photosynthesis Research, Vol IV. Dordrecht, The Netherlands: Martinus Nijhoff; 1987. pp. 655–658. [Google Scholar]
- Mattoo AK, Marder JB, Edelman M. Dynamics of the photosystem II reaction centre. Cell. 1989;56:241–246. doi: 10.1016/0092-8674(89)90897-0. [DOI] [PubMed] [Google Scholar]
- Michel H, Hunt DF, Shabanowitz J, Bennett J. Tandem mass spectrometry reveals that three photosystem II proteins of spinach chloroplasts contain N-acetyl-O-phosphothreonine at their NH2 termini. J Biol Chem. 1988;3:1123–1130. [PubMed] [Google Scholar]
- Michel HP, Bennett J. Identification of the phosphorylation site of an 8.3 kDa protein from photosystem II of spinach. FEBS Lett. 1987;212:103–108. [Google Scholar]
- Newman SJ, Boynton JE, Gillham NW, Randolph-Anderson BL, Jonhson AM, Harris EH. Transformation of chloroplast ribosomal RNA genes in Chlamydomonas: molecular and genetic characterisation of integration events. Genetics. 1990;126:875–888. doi: 10.1093/genetics/126.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon PJ, Metz JG, Roegner M, Diner BA. A Synechocystis PCC 6803 psbA deletion mutant and its transformation with a psbA gene from a higher plant. In: Baltscheffsky M, editor. Current Research in Photosynthesis, Vol I. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 471–474. [Google Scholar]
- Owens GC, Ohad I. Phosphorylation of Chlamydomonas reinhardtii chloroplast membrane proteins in vivo and in vitro. J Cell Biol. 1982;93:712–718. doi: 10.1083/jcb.93.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens GC, Ohad I. Changes in thylakoid polypeptide phosphorylation during membrane biogenesis in Chlamydomonas reinhardtii y-1. Biochim Biophys Acta. 1983;722:234–241. [Google Scholar]
- Ponticos M, Shipton CA, De Las Rivas J, Barber J. Two D1 protein degradation patterns in isolated photosystem 2 core and reaction centre complexes. Photosynthetica. 1993;28:215–224. [Google Scholar]
- Rintamaki E, Kettunen R, Aro E-M. Differential D1 dephosphorylation in functional and photodamaged photosystem II centers. J Biol Chem. 1996;271:14870–14875. doi: 10.1074/jbc.271.25.14870. [DOI] [PubMed] [Google Scholar]
- Rintamaki E, Kettunen R, Tyystjarvi E, Aro E-M. Light-dependent phosphorylation of D1 reaction centre protein of photosystem II: hypothesis for the functional role in vivo. Physiol Plant. 1995;93:191–195. [Google Scholar]
- Robinson HH, Crofts AR. Kinetics of the oxidation-reduction reactions of the photosystem II quinone acceptor complex, and the pathways for deactivation. FEBS Lett. 1983;153:221–226. [Google Scholar]
- Roffey RA, Golbeck JH, Russ Hille C, Sayre RT. Photosynthetic electron transport in genetically altered photosystem II reaction centers of chloroplasts. Proc Natl Acad Sci USA. 1991;88:9122–9126. doi: 10.1073/pnas.88.20.9122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford JC, Smith FD, Russell JA. Optimizing the biolistic process for different biological applications. Methods Enzymol. 1993;217:483–509. doi: 10.1016/0076-6879(93)17086-k. [DOI] [PubMed] [Google Scholar]
- Summer EJ, Schmid VHR, Bruns BU, Schmidt GW. Requirement for the H phosphoprotein in photosystem II of Chlamydomonas reinhardtii. Plant Physiol. 1997;113:1359–1368. doi: 10.1104/pp.113.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer A, Marder JB, Barber J. Photosystem II reaction centres isolated from phosphorylated pea thylakoids carry phosphate on the D1 and D2 polypeptide subunits. Biochim Biophys Acta. 1987;893:557–563. [Google Scholar]
- Tommos C, Davidsson L, Svensson B, Madsen C, Vermaas WFJ, Styring S. Modified EPR spectra of the tyrosine D radical in photosystem II in site-directed mutants of Synechocystis sp. PCC 6803: identification of side chains in the immediate vicinity of tyrosine D on the D2 protein. Biochemistry. 1993;32:5436–5441. doi: 10.1021/bi00071a020. [DOI] [PubMed] [Google Scholar]
- Vass I, Horvath G, Herczeg T, Demeter S. Photosynthetic energy conversion investigated by thermoluminescence: activation energies and half-lives of thermoluminescence bands of chloroplasts determined by mathematical resolution of glow curves. Biochim Biophys Acta. 1981;634:140–152. doi: 10.1016/0005-2728(81)90134-1. [DOI] [PubMed] [Google Scholar]
- Whitelegge JP, Koo D, Diner BA, Domian I, Erickson JM. Assembly of the photosystem II oxygen-evolving complex is inhibited in psbA site-directed mutants of Chlamydomonas reinhardtii. J Biol Chem. 1995;270:225–235. doi: 10.1074/jbc.270.1.225. [DOI] [PubMed] [Google Scholar]
- Wollman F-A, Delepelaire P. Correlation between changes in light energy distribution and changes in thylakoid membrane polypeptide phosphorylation in Chlamydomonas reinhardtii. J Cell Biol. 1984;98:1–7. doi: 10.1083/jcb.98.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]