Abstract
Bat echolocation is primarily used for orientation and foraging but also holds great potential for social communication. The communicative function of echolocation calls is still largely unstudied, especially in the wild. Eavesdropping on vocal signatures encoding social information in echolocation calls has not, to our knowledge, been studied in free-living bats so far. We analysed echolocation calls of the polygynous bat Saccopteryx bilineata and found pronounced vocal signatures encoding sex and individual identity. We showed experimentally that free-living males discriminate approaching male and female conspecifics solely based on their echolocation calls. Males always produced aggressive vocalizations when hearing male echolocation calls and courtship vocalizations when hearing female echolocation calls; hence, they responded with complex social vocalizations in the appropriate social context. Our study demonstrates that social information encoded in bat echolocation calls plays a crucial and hitherto underestimated role for eavesdropping conspecifics and thus facilitates social communication in a highly mobile nocturnal mammal.
Keywords: eavesdropping, sex-specific signature, individual signature, Saccopteryx bilineata, vocal communication, Chiroptera
1. Introduction
Social animals rely on a sophisticated communication system to facilitate interactions with conspecifics [1]. In many taxa, acoustic signals encode social information about the sender, such as sex [2], age [3], size [4], reproductive status [5], group affiliation [6], or individual identity [7]. However, it is often unclear whether social information encoded in vocal signatures is voluntarily or involuntarily shared with conspecifics [1], and whether shared information is actually used by receivers [8,9]. Vocal signals that are solely used for social communication constitute an active form of information transfer from signaller to receiver, even though passive information transfer exploited by eavesdropping conspecifics might occur simultaneously [1].
In echolocating animals, such as dolphins and bats, certain acoustic signals are not primarily used for communication but for orientation in space and foraging [10]. Echolocation signals are not directed at an external receiver per se; nevertheless, they can be used as an information source by eavesdroppers [10–12]. Echolocation calls indicate both the presence and the feeding activity of bats; therefore, they can inform other bats in hearing distance about good hunting grounds [13,14], new foraging tasks [15] or roost sites [16]. A growing body of evidence indicates that social information about the calling bat can be encoded in echolocation calls as well (reviewed in [11,17]). We distinguish personal information (e.g. individual identity, sex and age) from information about the bat's activity (e.g. hunting and roosting) and refer to the former as ‘social information’. Vocal signatures in bat echolocation calls can encode information ranging from species identity [18,19], age [20,21] and sex [22,23] to group affiliation [21,24] and individual identity [25–27] of the calling bat. However, it is still controversial as to what extent this social information is actually exploited by eavesdropping conspecifics [11,17,28,29].
Only a few laboratory studies provide experimental evidence for vocal signatures in echolocation calls (species identity [19], sex [30] and individuality [25–27]) and to our knowledge no study so far has tested whether and to what extent free-living, naturally behaving bats use vocal signatures to gain social information about conspecifics. This is owing to the fact that free-flying bats are hard to identify individually and their behavioural responses to conspecifics' echolocation calls are often difficult to quantify. Moreover, it is challenging to identify a particular social situation in which eavesdropping on vocal signatures in conspecifics' echolocation calls is presumably occurring naturally and in which it is feasible to conduct experiments under field conditions. For our investigation, we therefore selected the greater sac-winged bat, Saccopteryx bilineata, whose social behaviour allowed us to overcome the above-mentioned difficulties.
Saccopteryx bilineata is a highly social Neotropical bat exhibiting resource-defence polygyny (reviewed in [31]). Colony composition is characterized by male philopatry and female-biased natal dispersal [32]. Both males and females exhibit high fidelity to their day-roost colony throughout their lives. Males become territorial as they mature and vigorously defend small areas of suitable roosting space in the day-roost against other males [33,34]. Territorial males try to attract females to their respective territories and, if they succeed, defend harems of two to eight females against other males [33,34]. However, males cannot monopolize females [33,35]. Some males, especially young ones, may defend a territory but fail to attract females to it; those males are called non-harem males [31,34]. Day-roost colonies may contain several different harem territories adjacent to one another [34]. Although mating is restricted to only a few days in December, males defend territories and court females throughout the whole year [31]. Each female usually mates with a resident male from her day-roost colony but not necessarily with her own harem male [35]. Males produce complex courtship songs to woo females [36] and different types of aggressive vocalizations during territorial defence, the most remarkable of which is called territorial song [36–38].
Saccopteryx bilineata is an aerial hawking insectivorous bat and uses echolocation calls for orientation in space and prey capture [39,40]. The echolocation calls of S. bilineata are multi-harmonic and consist of a central, narrowband component accompanied by one or two short frequency-modulated sweeps [39–41]. During foraging flights, S. bilineata regularly alternates between two call frequencies, resulting in echolocation call duplets of a low and a high call [40–42]. When entering the day-roost at dawn, S. bilineata also produces echolocation calls, even though its eyesight is probably well developed, and there is sufficient ambient light available for vision [33]. Harem males are normally the first to arrive at the day-roost at dawn, while females and non-harem males return a few minutes later [33]. This behaviour provided an excellent opportunity to test whether roosting harem males used vocal signatures in the echolocation calls to discriminate between approaching colony members, thus facilitating social interactions.
We used a statistical and an experimental approach to assess the occurrence of vocal signatures in echolocation calls of S. bilineata. Both the social structure [31–33,43] and the vocal repertoire [36–38,44,45] of S. bilineata are exceptionally complex for a bat; we thus expected that echolocation calls serve an additional communicative function and differ in their acoustic properties between individuals and sexes. Furthermore, we hypothesized that these differences can be used by roosting harem males to determine the sex of approaching conspecifics. We expected harem males to produce aggressive vocalizations in response to approaching males and benign vocalizations in response to approaching females.
2. Material and methods
The study was conducted in March 2010 at three different locations in Costa Rica: Santa Rosa National Park (10°50′ N, 86°22′ W), Curú Wildlife Refuge (09°47′ N, 85°04′ W) and Villa Lapas Eco-Resort (09°45′ N, 85°23′ W). Bats were captured with mist nets (Ultrathin Mist Nets M-14; Ecotone, Gdynia, Poland), sexed and measurements of their forearm length (to the nearest 0.05 mm) were taken as an indicator of body size. To discriminate individuals within the colony, we banded them with split plastic bands (A.C. Hughes, Middlesex, UK; size XCL). All bats were subsequently released one by one, and we recorded their echolocation calls when they circled at the respective capture site.
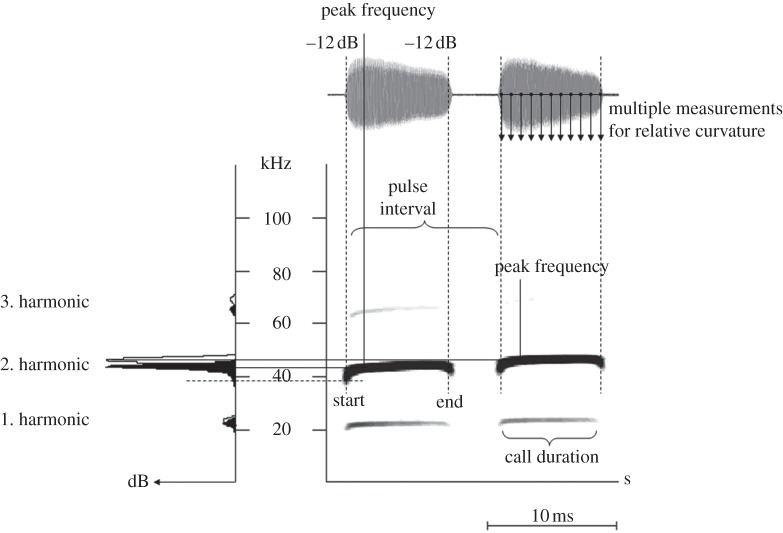
Recordings were made in real time with a handheld ultrasound detector (Petterson D1000X; Pettersson Elektronik AB, Uppsala, Sweden; 500 kHz sampling rate, 16 bit resolution) and originated from several capture sites at a given location. We only used high-quality recordings with good signal-to-noise ratio, and thus obtained data for 27 bats (13 males and 14 females, out of 57 individuals in total) and a total of 206 echolocation call duplets for acoustic analysis with Avisoft-SASLab Pro v. 4.52 (R. Specht, Avisoft Bioacoustics, Berlin, Germany). At both Santa Rosa and Curú, five males and five females each were included in the analysis, whereas we only gained good quality recordings of seven individuals at Villa Lapas (three males and four females). Measurements were taken from spectrograms using a FlatTop window with 1024 fast Fourier transform and 98 per cent overlap, which resulted in a frequency resolution of 488 Hz and a time resolution of 0.032 ms. To characterize echolocation call duplets, we measured seven echolocation call parameters separately for both the low and high call of a duplet (figure 1; peak frequency, start and end frequency of the call (−12 dB relative to the peak frequency of the signal), call duration and three principal components describing call curvature). An estimate of call curvature was obtained by measuring peak, minimum and maximum frequency (−12 dB relative to the peak) as well as bandwidth at 11 points distributed evenly over the entire call. We performed a principal component analysis with varimax rotation on these parameters and extracted principal components describing relative call curvature. For further analyses, we used the first three principal components which explained 78.9 per cent and 78.4 per cent of the variance in the low and high calls, respectively. The principal component analyses fulfilled Kaiser-Meyer-Olkin and Bartlett's test criteria.
Figure 1.
Acoustic measurement points of an echolocation call doublet from S. bilineata. Oscillogram, spectrogram and power spectrum are depicted. Measurements were taken from the second harmonic only, in which most of the energy was concentrated.
We performed a discriminant function analysis (DFA) to test for an individual signature in echolocation calls. A cross-validation procedure was used to correctly assign echolocation call duplets to the respective individuals. We estimated the significance of the classification success by using a two-tailed binomial test (following [46]). In order to test for sex- and location-specific signatures in echolocation calls, we used two different statistical approaches. First, we used the Mahalanobis distances between individual bats in the signal space defined by the DFA as an indicator of acoustic similarity [45,47,48]. Similar sounding individuals cluster together in signal space. If bats of the same sex or from the same location produced echolocation calls similar to one another, they had shorter Mahalanobis distances to one another than to bats of the other sex or from a different location. The Mahalanobis distance (i.e. acoustic similarity) between bats of the same or other sex and bats from the same or a different location were compared using paired samples t-tests and a sequential Bonferroni correction following [49]. We calculated mean distance values for each bat and (i) all same-sex bats and (ii) all opposite-sex bats in the analysis. Accordingly, we calculated mean distance values for each bat and (i) all bats from the same location and (ii) all bats from different locations in the analysis. Thus, we obtained two mean distance values per individual that we used for estimating the sex-specific signature (‘same sex distance’ versus ‘opposite sex distance’) and two mean distance values that we used for estimating the location-specific signature (‘same location distance’ versus ‘other location distance’). Second, we used a multivariate general linear model (GLM) with echolocation call parameters as variables and sex and location as fixed effects to test for sex- and location-specific differences in all call parameters. For the GLM, we calculated mean values per individual for each acoustic parameter.
Additionally, we performed an independent sample t-test to test for differences in echolocation call rate between the sexes and Spearman rank correlations to test for a relation between peak frequency of echolocation calls and the bats' forearm length as an indicator of body size. For both tests, we used mean values per individual for each acoustic parameter. In the Spearman rank correlations, males and females were tested separately; we applied a sequential Bonferroni correction following the study of Holm [49].
We experimentally verified the sex-specific signature in echolocation calls by testing the ability of males to sex conspecifics solely based on their echolocation calls. Experiments were conducted at the same locations at which we recorded echolocation calls. We released bats of both sexes one by one under controlled circumstances in the vicinity of their colony and recorded the subsequent vocal responses of resident males to the echolocation calls of the released conspecifics. Individual bats were released after all conspecifics had entered the colony day-roost. All bats flew directly into the day-roost in a matter of seconds, none circled the roost or landed, e.g. in a nearby tree, before entering the roost. Therefore, we are confident that both sexes approached the roost in a similar manner and that the approach behaviour could not have been used as a cue by the resident males. In the absence of wind (there was no discernible breeze during our experiments), air particles are not moving ahead of a flying bat but are getting pushed aside and impart a wake pattern to the air behind it [50,51], indicating that scent could not have travelled ahead of the flying bat and be used as a cue by the males in the roost. An experiment was successful when male vocal responses complied with three strict criteria: first, the vocal response was uttered when the approaching bat was still at least 5 m away from the colony, ensuring that only echolocation calls, not odour, behaviour or visual appearance, could be used to sex conspecifics; second, simultaneous behavioural observations of all individuals in the colony ensured that the vocal response was actually directed at the approaching bat and not at other colony members; and third, the vocal response either interrupted the echolocation series or, if the approaching bat ceased echolocating, succeeded the last echolocation call with an interval of less than 500 ms. We analysed the vocal responses of eight males to the echolocation calls of 20 released conspecifics (12 females and eight males). We used a Fisher's exact test to analyse the count data (aggressive vocalizations: absent/present; benign vocalizations: absent/present) on vocal responses to echolocation calls of conspecifics. Each male was presented with one approaching male and one to three approaching females (only one female per day; see the electronic supplementary material for details). For the males that were presented with more than one female, the respective vocal responses to approaching females were identical, which is why we averaged the count data for approaching females per male. This yielded one response to an approaching female and one response to an approaching male per subject (n = 8 males). All statistical tests were two-tailed (α = 0.05 if not indicated otherwise) and performed with SPSS v. 20.0 or R v. 2.15.0 [52].
3. Results
Our statistical approach assessing vocal signatures revealed a strong individual signature in echolocation calls. A DFA classified 70.9 per cent of echolocation call duplets to the correct individual (cross-validation procedure; see table 1 for a summary of results). The obtained classification success was significantly better than expected in a random classification (binomial test: p < 0.0001; random classification success: 3.7%), suggesting that echolocation calls encode enough information for individual recognition.
Table 1.
Statistical evidence for an individual signature in echolocation calls of S. bilineata.
| assessment of model fit | DF1 | DF2 | DF3 |
|---|---|---|---|
| eigenvalue | 22.810 | 6.426 | 3.331 |
| explained variation (%) | 61.4 | 17.3 | 9.0 |
| Wilk's λ | 0.0001 | 0.001 | 0.007 |
| χ2 (all p < 0.0001) | 1874.532 | 1289.651 | 919.730 |
Furthermore, our results indicate sex- and location-specific signatures in echolocation calls of S. bilineata (figure 2). Individuals clustered significantly closer with members of the same sex than with members of the other sex in the DFA signal space (paired t-test; t26 = −3.822, p = 0.001; α = 0.025). Moreover, individual bats from the same location showed a trend to cluster closer together than individuals from different locations (paired t-test; t26 = 1.754, p = 0.091; α = 0.05). In accordance, a multivariate model revealed a significant effect of location, a strong trend for sex and a significant effect for their interaction (GLM with sex and location as fixed factors; location: F28,16 = 5.545, p < 0.0001, partial η2 = 0.907; sex: F14,8 = 3.069, p = 0.058, partial η2 = 0.843; sex × location: F28,16 = 2.739, p = 0.019, partial η2 = 0.827) on echolocation call parameters. Results of between-subject effects for each acoustic parameter are summarized in table 2.
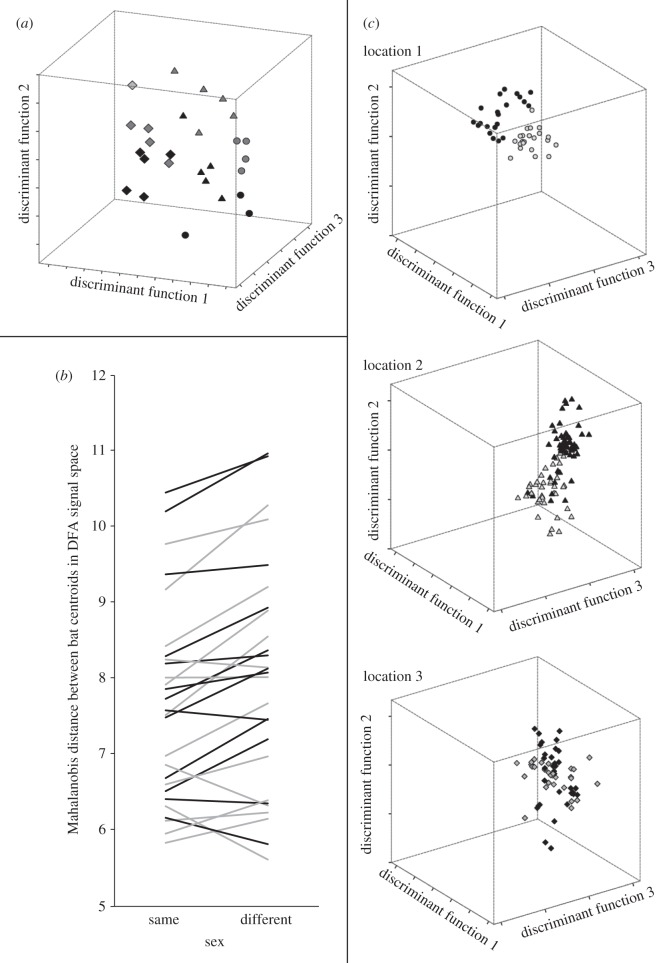
Figure 2.
(a) Relative position (i.e. centroids) of 27 S. bilineata individuals based on their echolocation call parameters in a signals space obtained by a DFA (three of 26 dimensions are shown). Centroids of 13 males and 14 females from three different locations are depicted. Locations and sexes are encoded by different symbols and shading, respectively (males in black and females in grey). Individuals with similar echolocation calls clustered together in signal space. Please note that bats from different locations were mainly separated by discriminant function 1, whereas different sexes were mainly separated by discriminant functions 2 and 3. (b) Mahalanobis distances between 27 S. bilineata individuals in signal space as an indicator of acoustic similarity of echolocation calls. For the majority of individuals, the mean Mahalanobis distance to same sex individuals was smaller than to opposite sex individuals, which indicates the existence of a sex-specific signature in echolocation calls of S. bilineata. Males (13) are depicted with black lines, females (14) with grey lines. (c) Relative position of 206 echolocation call duplets from 27 S. bilineata individuals based on their acoustic parameters in a signal space obtained by a DFA (three of 26 dimensions are shown). All locations were analysed together and only plotted separately for clarity. Calls from males (location 1, three; location 2, five; location 3, five) are depicted in black, calls from females (location 1, four; location 2, five; location 3, five) in grey. Centroids of individual bats are not shown.
Table 2.
Test of between-subjects effects for acoustic parameters of S. bilineata echolocation calls in a GLM with sex and location as fixed factors. (Values in bold were significant at α < 0.05. PC, principal component.)
| acoustic parameters | sex |
location |
sex × location |
|||
|---|---|---|---|---|---|---|
| p-value | partial η2 | p-value | partial η2 | p-value | partial η2 | |
| duration (low call) | 0.0037 | 0.3366 | 0.0004 | 0.5245 | 0.0297 | 0.2846 |
| start frequency (low call) | 0.0763 | 0.1420 | 0.0001 | 0.7966 | 0.2259 | 0.1321 |
| end frequency (low call) | 0.8576 | 0.0016 | 0.0001 | 0.7438 | 0.2222 | 0.1335 |
| peak frequency (low call) | 0.3158 | 0.0479 | 0.0001 | 0.8111 | 0.7293 | 0.0296 |
| PC1 call curvature (low call) | 0.6965 | 0.0074 | 0.0001 | 0.8208 | 0.4928 | 0.0652 |
| PC2 call curvature (low call) | 0.2242 | 0.0695 | 0.0238 | 0.2995 | 0.8535 | 0.0150 |
| PC3 call curvature (low call) | 0.8899 | 0.0009 | 0.0240 | 0.2989 | 0.8381 | 0.0167 |
| duration (high call) | 0.0081 | 0.2898 | 0.0006 | 0.5045 | 0.0544 | 0.2422 |
| start frequency (high call) | 0.3447 | 0.0426 | 0.0012 | 0.4718 | 0.3906 | 0.0856 |
| end frequency (high call) | 0.6937 | 0.0075 | 0.0033 | 0.4193 | 0.8458 | 0.0158 |
| peak frequency (high call) | 0.4814 | 0.0239 | 0.0001 | 0.6030 | 0.3103 | 0.1055 |
| PC1 call curvature (high call) | 0.8486 | 0.0018 | 0.0001 | 0.6575 | 0.6295 | 0.0431 |
| PC2 call curvature (high call) | 0.4170 | 0.0316 | 0.0017 | 0.4549 | 0.9297 | 0.0069 |
| PC3 call curvature (high call) | 0.2338 | 0.0668 | 0.8217 | 0.0185 | 0.0854 | 0.2089 |
We found no significant difference in call rate between the sexes (independent sample t-tests; low calls: T25 = 0.411, p = 0.684; high calls: T25 = 0.297, p = 0.769). At all locations, females produced slightly higher echolocation calls than males; however, there was considerable overlap in call frequency between the sexes (females: 45.5 ± 0.3 kHz (low calls) and 47.0 ± 0.3 kHz (high calls); males: 45.4 ± 0.3 kHz (low calls) and 46.9 ± 0.3 kHz (high calls); mean ± s.e. for peak frequency). In addition, females produced shorter echolocation calls than males (females: 5.3 ± 0.4 ms (low calls) and 5.5 ± 0.3 ms (high calls); males: 6.5 ± 0.6 ms (low calls) and 6.6 ± 0.5 ms (high calls); mean ± s.e. for call duration). A selection of original call parameter values can be found in the electronic supplementary material, table S1. Within each sex, several spectral parameters of echolocation calls were negatively correlated with forearm length as an indicator of body size (see the electronic supplementary material, table S2). Peak frequency of the low and high calls was significantly negatively correlated with forearm length in both females and males (Spearman rank correlation; females (n = 14): rS = −0.656, p = 0.011, α = 0.167 (low calls), rS = −0.556, p = 0.039, α = 0.05 (high calls); males (n = 13): rS = −0.767, p = 0.001, α = 0.0125 (low calls), rS = −0.660, p = 0.014, α = 0.025 (high calls)), suggesting that, within each sex, larger bats produced echolocation calls with lower peak frequencies. Nevertheless, females, as the larger sex, produced echolocation calls with higher peak frequencies than males.
Our experimental approach revealed that male S. bilineata discriminated between males and females approaching the colony, and thus were able to sex conspecifics based on echolocation calls alone. All males produced aggressive vocalizations (territorial songs and, in some cases, additional barks) in response to approaching males whereas they exclusively uttered benign vocalizations (courtship songs) in response to approaching females (Fisher's exact test; n = 8, p < 0.0001; figure 3). Details on the vocal responses of males to the echolocation calls of conspecifics can be found in the electronic supplementary material, table S3. To our knowledge, this is the first experimental evidence of bats producing complex social vocalizations in response to conspecifics' echolocation calls.
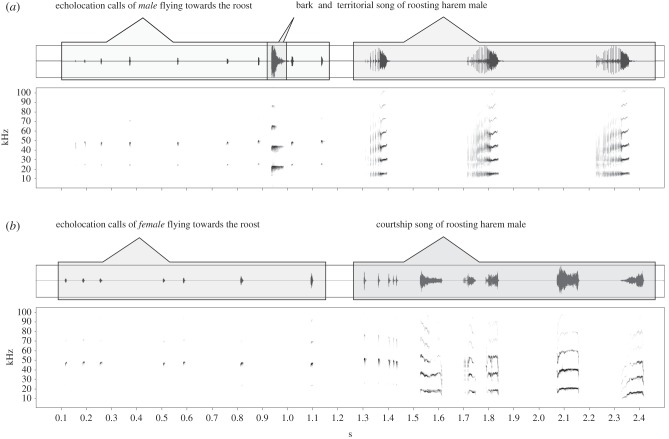
Figure 3.
Vocal responses of male S. bilineata to conspecifics' echolocation calls. Oscillograms and spectrograms depict vocalizations of a roosting S. bilineata harem male in response to the echolocation calls of conspecifics approaching the roost. Echolocation calls of approaching males always triggered aggressive vocalizations ((a) territorial songs and barks), whereas echolocation calls of approaching females triggered benign vocalizations ((b) courtship songs). This demonstrates that harem males were able to sex conspecifics solely based on their echolocation calls and to respond with vocalizations in the appropriate social context.
4. Discussion
Our study provides strong statistical evidence for vocal signatures encoding sex and individual identity in echolocation calls of S. bilineata and unequivocal experimental evidence that free-living harem males are capable of discriminating between conspecifics' sexes. This novel finding is intriguing because the transfer of social information about the sender through vocal signatures in echolocation calls has been considered difficult [53,54]. During flight, sensory constraints imposed by the foraging habitat [55,56] and foraging task [57] are the main selection pressures affecting echolocation call design and bats constantly have to adjust their echolocation call parameters in response to their environment. Presumably, these adjustments make it difficult to encode social information in echolocation calls in a constant and reliable fashion [53,54]. Nevertheless, we found pronounced vocal signatures in echolocation calls of S. bilineata, suggesting that bats are apparently capable of maintaining vocal signatures even when adjusting echolocation call parameters according to their flight situation (but see [23]).
We are aware of only one other experimental study on sex-specific vocal signatures in bat echolocation calls [30] which revealed that captive female big brown bats, Eptesicus fuscus, discriminated between conspecifics' sexes based on echolocation calls. However, the authors could not identify the acoustic parameter that allowed them to do so. A later study found sex-specific vocal signatures in echolocation calls of big brown bats when roosting but not when flying, suggesting that acoustic differences between the sexes are present only when socializing but not when foraging [23]. When tested in the laboratory, female big brown bats responded more strongly to female than to male echolocation calls but the biological significance of this reaction remained unclear [30]. This highlights the importance of testing free-living, naturally behaving bats whenever possible in order to assess vocal signature in echolocation calls and reveal the functional significance of cognitive discriminative abilities.
It is important to note that our statistical evidence for an individual signature does not necessarily imply individual recognition in S. bilineata since we verified only the sex-specific signature experimentally. Moreover, we recorded only one echolocation call series per individual that ultimately resulted in an enhanced individual classification success compared with using multiple call series per individual [54]. Consequently, the actual strength of the individual vocal signature is probably somewhat lower than reported here. The strength of the sex-specific signature, however, is not statistically overestimated since we avoided pseudoreplication by using only mean values per individual (see [46] for details).
Interestingly, our results indicated that female S. bilineata which are approximately 15 per cent larger than males [31] tended to call at higher frequencies with shorter call duration compared with males. When considering intraspecific and interspecific differences in echolocation call frequencies, larger bats are usually known to call at lower frequencies compared with smaller bats [58,59]; correspondingly, we found that within each sex, larger bats called with lower peak frequencies than smaller bats. These seemingly contradicting results indicate that sexual dimorphism in echolocation call frequencies of S. bilineata is independent of morphological constraints [59,60]. Considering the complex social system of S. bilineata [31], the sex-specific signatures in echolocation calls might have evolved specifically in order to facilitate male territorial defence and courtship behaviour.
The sex-specific vocal signature in conspecifics' echolocation calls prompted S. bilineata harem males to respond with complex social vocalizations referring to unequivocal social intentions [36]. We are certain that the harem males used only spectral and temporal parameters of echolocation calls to distinguish between approaching male and female conspecifics for two reasons. First, both the approach behaviour and the calling rate did not differ between the sexes. Second, odour could not have been used as a cue because bats do not project odour ahead of them during normal flight [50,51]; harem males responded to approaching conspecifics when the latter were still 5 m or more away, indicating that they could not have used odour cues to discriminate between male and female conspecifics. Visual cues, such as size or appearance, are also extremely unlikely to have played a role, since visibility over a distance of more than 5 m is poor at dusk and dawn.
Even though it is probably impossible for S. bilineata males to monitor the movement of foraging females based on echolocation calls while foraging throughout the night [61], the sex-specific vocal signature in echolocation calls plays a crucial role for social communication in the vicinity of the day-roost: it allows harem males to greet approaching females with courtship songs and rebuff approaching male rivals with aggressive vocalizations. Male eavesdropping on the echolocation calls of approaching conspecifics most probably mediates instantaneous and appropriate vocal reactions, and thus facilitates social communication in the polygynous S. bilineata.
In summary, our study demonstrates that social communication in free-living bats is facilitated through a sex-specific vocal signature in echolocation calls. To our knowledge, this is the first experimental evidence of bats exploiting social information encoded in conspecifics' echolocation calls in the context of courtship and territorial defence. This indicates that echolocation calls have a pronounced communicative function in addition to orientation and prey capture. Through passive information transfer and eavesdropping, echolocation calls play a crucial and hitherto underestimated role for social communication in a highly mobile and gregarious nocturnal mammal. Future studies focusing on the dual role of bat echolocation will undoubtedly enhance our understanding of adaptive sensory constraints on communicative signals and the functional significance of passive information transfer.
Acknowledgements
All fieldwork was approved by the Costa Rican Ministerio del Ambiente y Energía (MINAE) and adhered to the ASAB/ABS Guidelines for the Use of Animals in Research.
We are indebted to the owners of Curú Wildlife Refuge and Villa Lapas Eco-Resort for their logistic support. The Costa Rican authorities, especially Javier Guevara and the National System of Conservation Areas (SINAC), allocated both support and research permissions. Funding was provided by a start-up grant from the University of Ulm, Germany, to M.K.
References
- 1.Bradbury J. W., Vehrencamp S. L. 1998. Principles of animal communication. Sunderland, MA: Sinauer [Google Scholar]
- 2.Charlton B. D., Zhihe Z., Snyder R. J. 2009. The information content of giant panda, Ailuropoda melanoleuca, bleats: acoustic cues to sex, age and size. Anim. Behav. 78, 893–898 10.1016/j.anbehav.2009.06.029 (doi:10.1016/j.anbehav.2009.06.029) [DOI] [Google Scholar]
- 3.Ey E., Hammerschmidt K., Seyfarth R. M., Fischer J. 2007. Age- and sex-related variations in clear calls of Papio ursinus . Int. J. Primatol. 28, 947–960 10.1007/s10764-007-9139-3 (doi:10.1007/s10764-007-9139-3) [DOI] [Google Scholar]
- 4.Pfefferle D., Fischer J. 2006. Sounds and size: identification of acoustic variables that reflect body size in hamadryas baboons, Papio hamadryas . Anim. Behav. 72, 43–51 10.1016/j.anbehav.2005.08.021 (doi:10.1016/j.anbehav.2005.08.021) [DOI] [Google Scholar]
- 5.Leong K. M., Ortolani A., Graham L. H., Savage A. 2003. The use of low-frequency vocalizations in African elephant (Loxodonta africana) reproductive strategies. Horm. Behav. 43, 433–443 10.1016/S0018-506X(03)00025-4 (doi:10.1016/S0018-506X(03)00025-4) [DOI] [PubMed] [Google Scholar]
- 6.Riesch R., Ford J. B. K., Thomsen F. 2006. Stability and group specificity of stereotyped whistles in resident killer whales, Orcinus orca, off British Columbia. Anim. Behav. 71, 79–91 10.1016/j.anbehav.2005.03.026 (doi:10.1016/j.anbehav.2005.03.026) [DOI] [Google Scholar]
- 7.Lemasson A., Boutin A., Boivin S., Blois-Heulin C., Hausberger M. 2009. Horse (Equus caballus) whinnies: a source of social information. Anim. Cogn. 12, 693–704 10.1007/s10071-009-0229-9 (doi:10.1007/s10071-009-0229-9) [DOI] [PubMed] [Google Scholar]
- 8.Schibler F., Manser M. B. 2007. The irrelevance of individual discrimination in meerkat alarm calls. Anim. Behav. 74, 1259–1268 10.1016/j.anbehav.2007.02.026 (doi:10.1016/j.anbehav.2007.02.026) [DOI] [Google Scholar]
- 9.Townsend S. W., Hollén L. I., Manser M. B. 2010. Meerkat close calls encode group-specific signatures, but receivers fail to discriminate. Anim. Behav. 80, 133–138 10.1016/j.anbehav.2010.04.010 (doi:10.1016/j.anbehav.2010.04.010) [DOI] [Google Scholar]
- 10.Thomas J. A., Moss C. F., Vater M. 2004. Echolocation in bats and dolphins. Chicago, IL: University of Chicago Press [Google Scholar]
- 11.Fenton M. B. 2003. Eavesdropping on the echolocation and social calls of bats. Mamm. Rev. 33, 193–204 10.1046/j.1365-2907.2003.00019.x (doi:10.1046/j.1365-2907.2003.00019.x) [DOI] [Google Scholar]
- 12.Götz T., Verfuß U. K., Schnitzler H.-U. 2006. ‘Eavesdropping’ in wild rough-toothed dolphins (Steno bredanensis)? Biol. Lett. 2, 5–7 10.1098/rsbl.2005.0407 (doi:10.1098/rsbl.2005.0407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillam E. H. 2007. Eavesdropping by bats on the feeding buzzes of conspecifics. Can. J. Zool. 85, 795–801 10.1139/Z07-060 (doi:10.1139/Z07-060) [DOI] [Google Scholar]
- 14.Dechmann D. K. N., Heucke S. L., Giuggioli L., Safi K., Voigt C. C., Wikelski M. 2009. Experimental evidence for group hunting via eavesdropping in echolocating bats. Proc. R. Soc. B 276, 2721–2728 10.1098/rspb.2009.0473 (doi:10.1098/rspb.2009.0473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright G. S., Wilkinson G. S., Moss C. F. 2011. Social learning of a novel foraging task by big brown bats, Eptesicus fuscus . Anim. Behav. 82, 1075–1083 10.1016/j.anbehav.2011.07.044 (doi:10.1016/j.anbehav.2011.07.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruczynski I., Kalko E. K. V., Siemers B. M. 2007. The sensory basis of roost finding in a forest bat, Nyctalus noctula . J. Exp. Biol. 210, 3607–3615 10.1242/jeb.009837 (doi:10.1242/jeb.009837) [DOI] [PubMed] [Google Scholar]
- 17.Jones G., Siemers B. M. 2010. The communicative potential of bat echolocation pulses. J. Comp. Physiol. A 197, 447–457 10.1007/s00359-010-0565-x (doi:10.1007/s00359-010-0565-x) [DOI] [PubMed] [Google Scholar]
- 18.Russo D., Mucedda M., Bello M., Biscardi S., Pidinchedda E., Jones G. 2007. Divergent echolocation call frequencies in insular rhinolophids (Chiroptera): a case of character displacement? J. Biogeography 34, 2129–2138 10.1111/j.1365-2699.2007.01762.x (doi:10.1111/j.1365-2699.2007.01762.x) [DOI] [Google Scholar]
- 19.Schuchmann M., Siemers B. M. 2010. Behavioral evidence for community-wide species discrimination from echolocation calls in bats. Am. Nat. 176, 72–82 10.1086/652993 (doi:10.1086/652993) [DOI] [PubMed] [Google Scholar]
- 20.Jones G., Ransome R. D. 1993. Echolocation calls of bats are influenced by maternal effects and change over a lifetime. Proc. R. Soc. Lond. B 252, 125–128 10.1098/rspb.1993.0055 (doi:10.1098/rspb.1993.0055) [DOI] [PubMed] [Google Scholar]
- 21.Masters W. M., Raver K. A. S., Kazial K. A. 1995. Sonar signals of big brown bats, Eptesicus fuscus, contain information about individual identity, age and family affiliation. Anim. Behav. 50, 1243–1260 10.1016/0003-3472(95)80041-7 (doi:10.1016/0003-3472(95)80041-7) [DOI] [Google Scholar]
- 22.Jones G., Gordon T., Nightingale J. 1992. Sex and age differences in the echolocation calls of the horseshoe bat, Rhinolophus hipposideros. Mammalia 56, 189–193 [Google Scholar]
- 23.Grilliot M. E., Burnett S. C., Mendoça M. T. 2009. Sexual dimorphism in big brown bat (Eptesicus fuscus) ultrasonic vocalizations is context dependent. J. Mammal. 90, 203–209 10.1644/07-MAMM-A-161.1 (doi:10.1644/07-MAMM-A-161.1) [DOI] [Google Scholar]
- 24.Jameson J. W., Hare J. F. 2009. Group-specific signatures in the echolocation calls of female little brown bats (Myotis lucifugus) are not an artefact of clutter at the roost entrance. Acta Chiropterologica 11, 163–172 10.3161/150811009X465785 (doi:10.3161/150811009X465785) [DOI] [Google Scholar]
- 25.Kazial K. A., Kenny T. L., Burnett S. C. 2008. Little brown bats (Myotis lucifugus) recognize individual identity of conspecifics using sonar calls. Ethology 114, 469–478 10.1111/j.1439-0310.2008.01483.x (doi:10.1111/j.1439-0310.2008.01483.x) [DOI] [Google Scholar]
- 26.Yovel Y., Melcon M. L., Franz M. O., Denzinger A., Schnitzler H.-U. 2009. The voice of bats: how greater mouse-eared bats recognize individuals based on their echolocation calls. PLoS Comput. Biol. 5, e1000400. 10.1371/journal.pcbi.1000400 (doi:10.1371/journal.pcbi.1000400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voigt-Heucke S. L., Taborsky M., Dechmann D. N. K. 2010. A dual function of echolocation: bats use echolocation calls to identify familiar and unfamiliar individuals. Anim. Behav. 80, 59–67 10.1016/j.anbehav.2010.03.025 (doi:10.1016/j.anbehav.2010.03.025) [DOI] [Google Scholar]
- 28.Barclay R. M. R. 1982. Interindividual use of echolocation calls: eavesdropping by bats. Behav. Ecol. Sociobiol. 10, 271–275 10.1007/BF00302816 (doi:10.1007/BF00302816) [DOI] [Google Scholar]
- 29.Balcombe J. P., Fenton M. B. 1988. Eavesdropping by bats: the influence of echolocation call design and foraging strategy. Ethology 79, 158–166 10.1111/j.1439-0310.1988.tb00708.x (doi:10.1111/j.1439-0310.1988.tb00708.x) [DOI] [Google Scholar]
- 30.Kazial K. A., Masters W. M. 2004. Female big brown bats, Eptesicus fuscus, recognize sex from a caller's echolocation signals. Anim. Behav. 67, 855–863 10.1016/j.anbehav.2003.04.016 (doi:10.1016/j.anbehav.2003.04.016) [DOI] [Google Scholar]
- 31.Voigt C. C., Behr O., Caspers B. A., von Helversen O., Knörnschild M., Mayer F., Nagy M. 2008. Songs, scents, and senses: sexual selection in the greater sac-winged bat, Saccopteryx bilineata. J. Mammal. 89, 1401–1410 10.1644/08-MAMM-S-060.1 (doi:10.1644/08-MAMM-S-060.1) [DOI] [Google Scholar]
- 32.Nagy M., Heckel G., Voigt C. C., Mayer F. 2007. Female-biased dispersal and patrilocal kin groups in a mammal with resource-defence polygyny. Proc. R. Soc. B 274, 3019–3025 10.1098/rspb.2007.1008 (doi:10.1098/rspb.2007.1008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tannenbaum R. 1975. Reproductive strategies in the white-lined bat. PhD dissertation, Cornell University, Ithaca, NY, USA [Google Scholar]
- 34.Bradbury J. W., Emmons L. 1974. Social organization of some Trinidad bats. I. Emballonuridae. Zeit. Tierpsychol. 36, 137–183 10.1111/j.1439-0310.1974.tb02130.x (doi:10.1111/j.1439-0310.1974.tb02130.x) [DOI] [PubMed] [Google Scholar]
- 35.Heckel G., von Helversen O. 2003. Genetic mating system and the significance of harem associations in the bat Saccopteryx bilineata. Mol. Ecol. 12, 219–227 10.1046/j.1365-294X.2003.01722.x (doi:10.1046/j.1365-294X.2003.01722.x) [DOI] [PubMed] [Google Scholar]
- 36.Behr O., von Helversen O. 2004. Bat serenades: complex courtship songs of the sac-winged bat (Saccopteryx bilineata). Behav. Ecol. Sociobiol. 56, 106–115 10.1007/s00265-004-0768-7 (doi:10.1007/s00265-004-0768-7) [DOI] [Google Scholar]
- 37.Behr O., von Helversen O., Heckel G., Nagy M., Voigt C. C., Mayer F. 2006. Territorial songs indicate male quality in the sac-winged bat Saccopteryx bilineata (Chiroptera, Emballonuridae). Behav. Ecol. 17, 810–817 10.1093/beheco/arl013 (doi:10.1093/beheco/arl013) [DOI] [Google Scholar]
- 38.Behr O., Knörnschild M., von Helversen O. 2009. Territorial counter-singing in male sac-winged bats (Saccopteryx bilineata): low-frequency songs trigger a stronger response. Behav. Ecol. Sociobiol. 63, 433–442 10.1007/s00265-008-0677-2 (doi:10.1007/s00265-008-0677-2) [DOI] [Google Scholar]
- 39.Barclay R. M. R. 1983. Echolocation calls of emballonurid bats from Panama. J. Comp. Physiol. A 151, 515–520 10.1007/BF00605468 (doi:10.1007/BF00605468) [DOI] [Google Scholar]
- 40.Kalko E. K. V. 1995. Echolocation signal design, foraging habitats and guild structure in six neotropical sheath-tailed bats (Emballonuridae). Symp. Zool. Soc. Lond. 67, 259–273 [Google Scholar]
- 41.Jung K., Kalko E. K. V., von Helversen O. 2007. Echolocation calls in Central American emballonurid bats: signal design and call frequency alternation. J. Zool. 272, 125–137 10.1111/j.1469-7998.2006.00250.x (doi:10.1111/j.1469-7998.2006.00250.x) [DOI] [Google Scholar]
- 42.Ratcliffe J. M., Jakobsen L., Kalko E. K. V., Surlykke A. 2011. Frequency alternation and an offbeat rhythm indicate foraging behavior in the echolocating bat, Saccopteryx bilineata. J. Comp. Physiol. A 197, 413–423 10.1007/s00359-011-0630-0 (doi:10.1007/s00359-011-0630-0) [DOI] [PubMed] [Google Scholar]
- 43.Nagy M., Knörnschild M., Voigt C. C., Mayer F. 2012. Male greater sac-winged bats gain direct fitness benefits when roosting in multi-male colonies. Behav. Ecol. 23, 597–606 10.1093/beheco/ars003 (doi:10.1093/beheco/ars003) [DOI] [Google Scholar]
- 44.Knörnschild M., Behr O., von Helversen O. 2006. Babbling behaviour in the sac-winged bat (Saccopteryx bilineata). Naturwissenschaften 93, 451–454 [DOI] [PubMed] [Google Scholar]
- 45.Knörnschild M., Nagy M., Metz M., Mayer F., von Helversen O. 2010. Complex vocal imitation during ontogeny in a bat. Biol. Lett. 6, 156–159 10.1098/rsbl.2009.0685 (doi:10.1098/rsbl.2009.0685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mundry R., Sommer C. 2007. Discriminant function analysis with nonindependent data: consequences and an alternative. Anim. Behav. 74, 965–976 10.1016/j.anbehav.2006.12.028 (doi:10.1016/j.anbehav.2006.12.028) [DOI] [Google Scholar]
- 47.Boughman J. 1998. Vocal learning by greater spear-nosed bats. Proc. R. Soc. Lond. B 265, 227–233 10.1098/rspb.1998.0286 (doi:10.1098/rspb.1998.0286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knörnschild M., von Helversen O., Mayer F. 2007. Twin siblings sound alike: isolation call variation in the noctule bat, Nyctalus noctula. Anim. Behav. 74, 1055–1063 10.1016/j.anbehav.2006.12.024 (doi:10.1016/j.anbehav.2006.12.024) [DOI] [Google Scholar]
- 49.Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70 [Google Scholar]
- 50.Hedenström A., Johansson L. C., Wolf M., von Busse R., Winter Y., Spedding G. R. 2007. Bat flight generates complex aerodynamic tracks. Science 316, 894–897 10.1126/science.1142281 (doi:10.1126/science.1142281) [DOI] [PubMed] [Google Scholar]
- 51.Riskin D. K., Willis D. J., Iriarte-Díaz J., Hedrick T. L., Kostandov M., Chen J., Laidlaw D. H., Breuer K. S., Swartz S. M. 2008. Quantifying the complexity of bat wing kinematics. J. Theoret. Biol. 254, 604–615 10.1016/j.jtbi.2008.06.011 (doi:10.1016/j.jtbi.2008.06.011) [DOI] [PubMed] [Google Scholar]
- 52.R Development Core Team 2008. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org) [Google Scholar]
- 53.Siemers B. M., Beedholm K., Dietz C., Dietz I., Ivanova T. 2005. Is species identity, sex, age or individual quality conveyed by echolocation call frequency in European horseshoe bats? Acta Chiropterologica 7, 259–274 10.3161/1733-5329(2005)7[259:ISISAO]2.0.CO;2 (doi:10.3161/1733-5329(2005)7[259:ISISAO]2.0.CO;2) [DOI] [Google Scholar]
- 54.Siemers B. M., Kerth G. 2006. Do echolocation calls of wild colony-living Bechstein's bats (Myotis bechsteinii) provide individual-specific signatures? Behav. Ecol. Sociobiol. 59, 443–454 10.1007/s00265-005-0068-x (doi:10.1007/s00265-005-0068-x) [DOI] [Google Scholar]
- 55.Neuweiler G. 1989. Foraging ecology and audition in echolocating bats. Trends Ecol. Evol. 4, 160–166 10.1016/0169-5347(89)90120-1 (doi:10.1016/0169-5347(89)90120-1) [DOI] [PubMed] [Google Scholar]
- 56.Schnitzler H.U., Kalko E. K. V. 2001. Echolocation behavior of insect-eating bats. BioScience 51, 557–569 10.1641/0006-3568(2001)051[0557:EBIEB]2.0.CO;2 (doi:10.1641/0006-3568(2001)051[0557:EBIEB]2.0.CO;2) [DOI] [Google Scholar]
- 57.Schnitzler H.-U., Moss C. F., Denzinger A. 2003. From spatial orientation to food acquisition in echolocating bats. Trends Ecol. Evol. 18, 386–394 10.1016/S0169-5347(03)00185-X (doi:10.1016/S0169-5347(03)00185-X) [DOI] [Google Scholar]
- 58.Barclay R. M. R., Fullard J. H., Jacobs D. S. 1999. Variation in the echolocation calls of the hoary bat (Lasiurus cinereus): influence of body size, habitat structure, and geographic location. Can. J. Zool. 77, 530–534 [Google Scholar]
- 59.Jones G. 1999. Scaling of echolocation call parameters in bats. J. Exp. Biol. 202, 3359–3367 [DOI] [PubMed] [Google Scholar]
- 60.Pye J. D. 1979. Why ultrasound? Endeavour 3, 57–62 10.1016/0160-9327(79)90067-X (doi:10.1016/0160-9327(79)90067-X) [DOI] [Google Scholar]
- 61.Hoffmann F. F., Hejduk J., Caspers B., Siemers B. M., Voigt C. C. 2007. In bat mating systems auditory constraints prevent efficient female surveillance by eavesdropping male bats. Can. J. Zool. 85, 863–872 10.1139/Z07-069 (doi:10.1139/Z07-069) [DOI] [Google Scholar]