Abstract
Personality traits, such as exploration–avoidance, are expected to be adaptive in a given context (e.g. low-risk environment) but to be maladaptive in others (e.g. high-risk environment). Therefore, it is expected that personality traits are flexible and respond to environmental fluctuations, given that consistency across different contexts is maintained, so that the relative individual responses in relation to others remains the same (i.e. although the magnitude of the response varies the differences between high and low responders are kept). Here, we tested the response of male cichlid fish (Oreochromis mossambicus) to a novel object (NO) in three different social contexts: (i) social isolation, (ii) in the presence of an unfamiliar conspecific, and (iii) in the presence of a familiar conspecific. Males in the familiar treatment exhibited more exploratory behaviour and less neophobia than males in either the unfamiliar or the social isolation treatments. However, there were no overall correlations in individual behaviour across the three treatments, suggesting a lack of consistency in exploration–avoidance as measured by the NO test in this species. Moreover, there were no differences in cortisol responsiveness to an acute stressor between the three treatments. Together, these results illustrate how behavioural traits usually taken as measures of personality may exhibit significant flexibility and lack the expected consistency across different social contexts.
Keywords: exploratory behaviour, neophobia, boldness, personality, phenotypic plasticity, stress
1. Introduction
The novel object (NO) test has been widely used in personality research to assess neophobia (i.e. fear of novelty) and exploratory behaviour (i.e. curiosity towards novelty) both in humans and in other animals, including fishes [1–3]. Novelty usually elicits a conflict between avoidance and exploration tendencies, and the relative expression of these two competing behavioural systems is considered to be modulated by anxiety [4]. According to their reaction to a NO, usually measured by the latency to approach the object and/or by the time spent near the object, individuals are classified on an exploration–avoidance scale that used to be equated with a shy–bold continuum [3], until a recent review of the terminology proposed boldness and exploration–avoidance to be independent temperament traits [5]. However, in most of these studies, animals are tested alone, and potential effects of social context on exploration–avoidance have been largely ignored. This is particularly relevant in highly social or gregarious species where behavioural responses to tests in non-social conditions may not reflect the natural response that would be given in a more naturalistic social setting. Indeed, social context is known to influence an individual's behaviour and its consistency in various ways [6,7]. Consistency in behaviour across social contexts depends on the nature of the social relationship and on the personality of the individuals involved [8–10]. One key characteristic of the social context that may influence the direction of the response is the level of familiarity between the participants, which is also known to promote social learning [11] and to reduce aggression [12] and the response to stressors [13]. The effect of the presence of a conspecific on NO tests may either facilitate or inhibit NO exploration, depending on whether it acts as an anxiolytic or an anxiogenic, respectively. It is thus hypothesized that familiar conspecifics should reduce anxiety and therefore promote exploratory behaviour and reduce neophobia, whereas the presence of stranger conspecifics is expected to increase anxiety and hence inhibit exploratory behaviour and enhance neophobia. Therefore, the presence of conspecifics is expected to influence the results of NO tests in a way that is moderated by the familiarity that the focal subject has with them.
We tested this hypothesis using a highly social cichlid fish, the Mozambique tilapia, Oreochromis mossambicus [14]. Moreover, we also assessed the individual consistency in exploratory behaviour and neophobia, that is, if individuals remain more or less explorative in relation to others across treatments. Finally, we collected data on the cortisol response to an acute stressor in the three treatments in order to use cortisol responsiveness as a proxy for anxiety state.
2. Material and methods
All animals were housed in stock aquaria of 240 l in groups of eight animals each (three males and five females) at 26 ± 2°C, with a photoperiod of 12 L : 12 D. Fish were daily fed ad libitum with commercial cichlid sticks (ASTRA).
A paired design with three treatments was used: (i) male in visual contact with a familiar female, (ii) male in visual contact with an unfamiliar female, and (iii) male in social isolation. We used 12 adult males (weight = 99 ± 3.1 g) as focal individuals and 12 adult females (weight = 50 ± 2.7 g) as bystanders to create the different social contexts of the experiment. Focal males were placed individually in the experimental tanks (40 l tanks with all the remaining conditions the same as described earlier for the stock) during 4 days for acclimatization. In the familiar and unfamiliar treatments, males spent these days in visual contact with familiar females (i.e. from the same stock tank), whereas in the social isolation treatment males remained isolated. On the fifth day, 1 h before the trial (which started between 10.30 and 14.30), the female of the unfamiliar treatment was replaced, without disturbance of the male, by an unfamiliar female (i.e. from a different stock tank). The same focal individual was exposed to each treatment every other week and spent the week in between tests in its home stock tank. Treatment order was balanced between males. Each conspecific female was used only once to avoid pseudoreplication. Females were chosen for use as conspecifics in order to promote a more affiliative and less competitive social context; because in a previous study, isolated males have expressed a high motivation, comparable to the motivation for obtaining food, to come in close contact with females [15].
A NO (table tennis ball filled with sand) was carefully placed in a predetermined point of the focal male tank, after which the trial started. The following behaviours were sampled using focal instantaneous sampling (10 min per individual) [16]: freezing exploring the NO, and time spent in the NO area. To infer the level of social interest in females, two other behavioural variables were sampled: male touches the glass wall of the adjacent aquarium with the snout; and being in the area close to the adjacent aquarium (i.e. male head within 2 cm from the glass wall of the adjacent aquarium). After the test, the NO was removed and focal males were exposed to an acute stressor (confinement stress = lowering of the water level) after which, a blood sample was taken. Anaesthesia and blood sampling procedures followed Galhardo et al. [17]. Intra- and inter-assay variability for the cortisol radioimunnoassay were respectively 5.8 and 6.5 per cent. All animals were placed back in their original stock tanks after each trial, where social and physical conditions had remained the same.
Given the small sample sizes (n = 12), the effects of the social treatments were tested using a non-parametric Friedman ANOVA. Wilcoxon matched-pairs tests were used as post hoc tests with a Bonferroni correction, so that significant effects are reported for a p < 0.0167. A value of p < 0.05 was used in all other statistical tests. The consistency of behaviour across treatments was assessed using Spearman correlations. All statistical analyses were conducted on Statistica v. 8 (StatSoft Inc., USA, 1984–2008).
3. Results
Males in the familiar context explored the NO more frequently (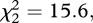 p < 0.001), spent more time close to it (
p < 0.001), spent more time close to it (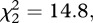 p < 0.001) and spent less time in freezing behaviour (
p < 0.001) and spent less time in freezing behaviour (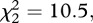 p < 0.01) than either the males of unfamiliar context or in social isolation (figure 1). Males in both social treatments (i.e. familiar or unfamiliar) spent more time in the area close to the adjacent aquarium than males in social isolation, where the adjacent aquarium was empty (
p < 0.01) than either the males of unfamiliar context or in social isolation (figure 1). Males in both social treatments (i.e. familiar or unfamiliar) spent more time in the area close to the adjacent aquarium than males in social isolation, where the adjacent aquarium was empty (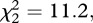 p < 0.01). There were no differences in the time spent close to the female's aquarium wall (t = 31, p = 0.53) or in the frequency of touches in it (t = 22.5, p = 0.61) between familiar and unfamiliar males.
p < 0.01). There were no differences in the time spent close to the female's aquarium wall (t = 31, p = 0.53) or in the frequency of touches in it (t = 22.5, p = 0.61) between familiar and unfamiliar males.
Figure 1.
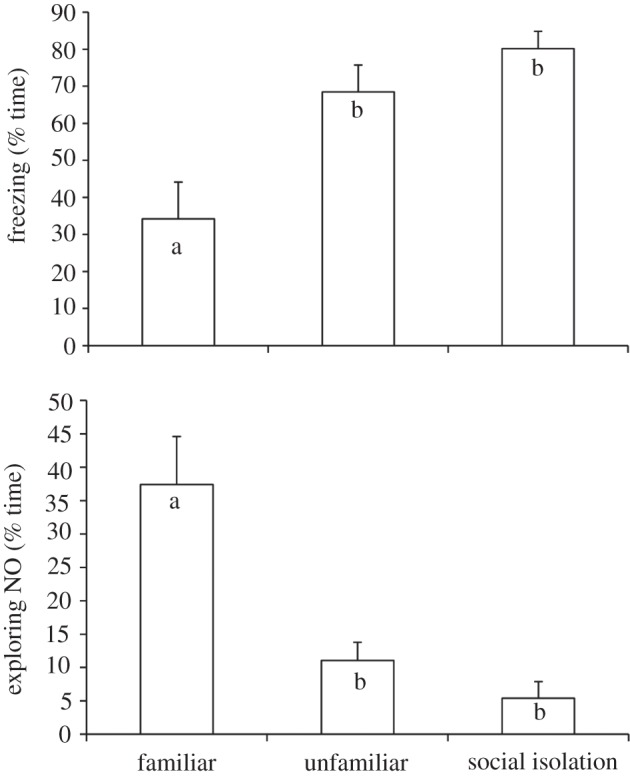
Novel object (NO) test in cichlid fish. Time spent freezing and exploring the NO in the three social contexts; different letters represent significant differences between treatments (Wilcoxon matched-pairs test with Bonferroni correction, p < 0.0167).
Exploratory behaviour (i.e. % time exploring the NO) was positively correlated between isolated and familiar treatments (n = 12, rs = 0.742, p = 0.005), but not between familiar and unfamiliar treatments (n = 12, rs = 0.406, p = 0.19), or isolated and unfamiliar treatments (n = 12, rs = 0.311, p = 0.32). Similarly, freezing behaviour was not significantly correlated across treatments (familiar versus unfamiliar: rs = 0.335, p = 0.29; familiar versus isolated: rs = 0.173, p = 0.59; isolated versus unfamiliar: rs = 0.149, p = 0.64).
There were also no differences in patterns of cortisol release among treatments (isolation = 36.9 ± 2.5 ng ml−1; familiar = 31.6 ± 1.8 ng ml−1; non-familiar 32.0 ± 2.2 ng ml−1; 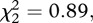 p = 0.64).
p = 0.64).
4. Discussion
Males in a familiar context exhibited less neophobia and more exploratory behaviour than those in an unfamiliar context or in isolation. Given the fact that the patterns of social interest were not different between the familiar and unfamiliar treatments, it is unlikely that the avoidance of the NO in the unfamiliar treatment was owing to a redirected motivation to interact with the female. Thus, the presence of an unfamiliar female induced as much neophobia as social isolation.
These results are potentially explained by differential effects of the three social contexts on state anxiety during the NO test. The higher neophobia expressed in isolated fish is in line with the described isolation-induced anxiety and stress responses in social species of higher vertebrates [13]. Thus, the increase in exploratory behaviour induced by the presence of a familiar conspecific is most probably owing to a social buffering effect that reduces state anxiety during the NO test [18], which does not occur in the presence of an unfamiliar conspecific, because the latter is most probably also acting as a novel social stimulus, and hence contributes to a heightened anxiety state.
Cortisol levels elicited by an acute stressor did not vary across social contexts. These levels are within those previously reported for tilapia (e.g. ca 43 ng ml−1 in social isolation; [17]), and are much higher than those observed in individuals kept in familiar groups (ca 15 ng ml−1; L. Galhardo & R. F. Oliveira 2010, unpublished data). The lack of social buffering of the cortisol response by familiar conspecifics, already described for other fish species [19], may be owing to a carry-over effect from the NO test to the stress test, such that the higher exploratory behaviour expressed in this condition increased cortisol, as other authors have also noticed in new and enriched environments [20]. Thus, cortisol does not seem to be a reliable measure of anxiety because it is not possible to disentangle effects owing to state anxiety from those owing to exploratory behaviour.
The overall lack of correlations across the three treatments for neophobia and exploratory behaviour indicates a lack of consistency in these measures, which are usually taken as measures of a temperament trait and as such should covary in different contexts. Therefore, the NO test lacks convergent validity across social contexts: high responders in a familiar context are not necessarily high responders in the isolated context. Thus, a practical implication of this study is that NO tests in highly social species should be run in the presence of familiar conspecifics that mimic the natural social settings.
In conclusion, personality traits may vary with social context and when this happens, it is crucial to assess their consistency across different social environments and to use the most naturalistic social setting when assessing them.
Acknowledgements
The experiments reported here have been approved by the national authorities (ethical permit 30489 from the Portuguese Veterinary Office).
We thank Tânia Oliveira for running the cortisol analyses and Nadia Aubin-Horth for helpful comments on a preliminary version of the manuscript. This study was funded by the Pluriannual Programme of Fundação para a Ciência e a Tecnologia (FCT, UI&D 331/2001) and by the FCT research grant no. PTDC/MAR/72117/2006. L.G. was supported by a PhD fellowship from FCT (SFRH/BD/16162/2004).
References
- 1.Burns J. G. 2008. The validity of three tests of temperament in guppies (Poecilia reticulata). J. Comp. Psychol. 122, 344–356 10.1037/0735-7036.122.4.344 (doi:10.1037/0735-7036.122.4.344) [DOI] [PubMed] [Google Scholar]
- 2.Sneddon L. U., Braithwaite V. A., Gentle M. J. 2003. Novel object test: examining nociception and fear in the rainbow trout. J. Pain 4, 431–440 10.1067/S1526-5900(03)00717-X (doi:10.1067/S1526-5900(03)00717-X) [DOI] [PubMed] [Google Scholar]
- 3.Wilson D. S., Clark A. B., Coleman K., Dearstyne T. 1994. Shyness and boldness in humans and other animals. Trends Ecol. Evol. 9, 442–446 10.1016/0169-5347(94)90134-1 (doi:10.1016/0169-5347(94)90134-1) [DOI] [PubMed] [Google Scholar]
- 4.Belzung C., Le Pape G. 1994. Comparison of different behavioral test situations used in psychopharmacology for measurement of anxiety. Physiol. Behav. 56, 623–628 10.1016/0031-9384(94)90311-5 (doi:10.1016/0031-9384(94)90311-5) [DOI] [PubMed] [Google Scholar]
- 5.Réale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318 10.1111/j.1469-185X.2007.00010.x (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 6.Webster M. M., Ward A. J. W. 2011. Personality and social context. Biol. Rev. 86, 759–773 10.1111/j.1469-185X.2010.00169.x (doi:10.1111/j.1469-185X.2010.00169.x) [DOI] [PubMed] [Google Scholar]
- 7.Ward A. J. W. 2012. Social facilitation of exploration in mosquitofish (Gambusia holbrooki). Behav. Ecol. Sociobiol. 66, 223–230 10.1007/s00265-011-1270-7 (doi:10.1007/s00265-011-1270-7) [DOI] [Google Scholar]
- 8.Schuett W., Dall S. R. X. 2009. Sex differences, social context and personality in zebra finches, Taeniopygia guttata. Anim. Behav. 77, 1041–1050 10.1016/j.anbehav.2008.12.024 (doi:10.1016/j.anbehav.2008.12.024) [DOI] [Google Scholar]
- 9.Stöwe M., Bugnyar T., Loretto M. C., Schloegl C., Range F., Kotrschal K. 2006. Novel object exploration in ravens (Corvus corax): effects of social relationships. Behav. Proc. 73, 68–75 10.1016/j.beproc.2006.03.015 (doi:10.1016/j.beproc.2006.03.015) [DOI] [PubMed] [Google Scholar]
- 10.Stöwe M., Kotrschal K. 2007. Behavioural phenotypes may determine whether social context facilitates or delays novel object exploration in ravens (Corvus corax). J. Ornithol. 148, 179–184 10.1007/s10336-007-0145-1 (doi:10.1007/s10336-007-0145-1) [DOI] [Google Scholar]
- 11.Swaney W., Kendal J., Capon H., Brown C., Laland K. N. 2001. Familiarity facilitates social learning of foraging behaviour in the guppy. Anim. Behav. 62, 591–598 10.1006/anbe.2001.1788 (doi:10.1006/anbe.2001.1788) [DOI] [Google Scholar]
- 12.Temeles E. J. 1994. The role of neighbours in territorial systems: when are they ‘dear enemies’? Anim. Behav. 47, 339–350 10.1006/anbe.1994.1047 (doi:10.1006/anbe.1994.1047) [DOI] [Google Scholar]
- 13.DeVries A. C., Glasper E. R., Detillion C. E. 2003. Social modulation of stress responses. Physiol. Behav. 79, 399–407 10.1016/s0031-9384(03)00152-5 (doi:10.1016/s0031-9384(03)00152-5) [DOI] [PubMed] [Google Scholar]
- 14.Oliveira R. F. 2009. Social behavior in context: hormonal modulation of behavioral plasticity and social competence. Integr. Comp. Biol. 49, 423–440 10.1093/icb/icp055 (doi:10.1093/icb/icp055) [DOI] [PubMed] [Google Scholar]
- 15.Galhardo L., Almeida O., Oliveira R. F. 2011. Measuring motivation in a cichlid fish: an adaptation of the push-door paradigm. Appl. Anim. Behav. Sci. 130, 60–70 10.1016/j.applanim.2010.12.008 (doi:10.1016/j.applanim.2010.12.008) [DOI] [Google Scholar]
- 16.Martin P., Bateson P. 2007. Measuring behaviour: an introductory guide. Cambridge, UK: Cambridge University Press [Google Scholar]
- 17.Galhardo L., Vital J., Oliveira R. F. 2011. The role of predictability in the stress response of a cichlid fish. Physiol. Behav. 102, 367–372 10.1016/j.physbeh.2010.11.035 (doi:10.1016/j.physbeh.2010.11.035) [DOI] [PubMed] [Google Scholar]
- 18.van Oers K., Klunder M., Drent P. J. 2005. Context dependence of personalities: risk-taking behavior in a social and a nonsocial situation. Behav. Ecol. 16, 716–723 10.1093/beheco/ari045 (doi:10.1093/beheco/ari045) [DOI] [Google Scholar]
- 19.Allen P. J., Barth C. C., Peake S. J., Abrahams M. V., Anderson W. G. 2009. Cohesive social behaviour shortens the stress response: the effects of conspecifics on the stress response in lake sturgeon Acipenser fulvescens. J. Fish Biol. 74, 90–104 10.1111/j.1095-8649.2008.02112.x (doi:10.1111/j.1095-8649.2008.02112.x) [DOI] [PubMed] [Google Scholar]
- 20.von Krogh K., Sorensen C., Nilsson G. E., Overli O. 2010. Forebrain cell proliferation, behavior, and physiology of zebrafish, Danio rerio, kept in enriched or barren environments. Physiol. Behav. 101, 32–39 10.1016/j.physbeh.2010.04.003 (doi:10.1016/j.physbeh.2010.04.003) [DOI] [PubMed] [Google Scholar]


