Abstract
In many animals, behaviours such as territoriality, mate guarding, navigation and food acquisition rely heavily on spatial memory abilities; this has been demonstrated in diverse taxa, from invertebrates to mammals. However, spatial memory ability in squamate reptiles has been seen as possible, at best, or non-existent, at worst. Of the few previous studies testing for spatial memory in squamates, some have found no evidence of spatial memory while two studies have found evidence of spatial memory in snakes, but have been criticized based on methodological issues. We used the Barnes maze, a common paradigm to test spatial memory abilities in mammals, to test for spatial memory abilities in the side-blotched lizard (Uta stansburiana). We found the existence of spatial memory in this species using this spatial task. Thus, our study supports the existence of spatial memory in this squamate reptile species and seeks to parsimoniously align this species with the diverse taxa that demonstrate spatial memory ability.
Keywords: cognition, lizards, spatial memory, Uta stansburiana
1. Introduction
Higher cognitive abilities, including spatial memory, are known to be important for a variety of ecologically relevant behaviours. In many animals, spatial memory has been explicitly tested for and verified. However, spatial memory capabilities in squamate reptiles have been debated [1–3], even though recent evidence has shown that reptiles may possess other typically higher cognitive processing [4]. Thus, in squamate reptiles, the existence of spatial memory has been contentious, probably owing to the paucity of studies explicitly testing it. Although previous studies have determined that lizards can use sun compass cues for orienting [5], it is unclear whether they can use visual cues as well. Two studies have claimed demonstration of spatial memory using visual cues in snakes [6,7]; however, the methodology of those studies has been criticized [2,3]. Specifically, these studies used only one distal intramaze cue, and snakes were oriented towards that cue during the trials; thus, snakes could have been using egocentric encoding of the cue rather than true spatial memory. Two additional studies found no evidence of spatial memory in three lizard species [3,8]. Because the null hypothesis is typically that squamate reptiles lack spatial memory, the latter studies argue against the existence of spatial memory in this taxonomic group. Here we provide support for spatial memory abilities in squamate reptiles.
The goal in this study was to ascertain if side-blotched lizards, Uta stansburiana, demonstrate the use of spatial memory. Side-blotched lizards are territorial, and thus spatial memory might provide a selective advantage during territory defence. To test for spatial memory, we used the Barnes maze (figure 1), a standard apparatus used to test spatial memory in numerous taxa [9]. The Barnes maze disentangles a non-spatial strategy of navigation, which uses local cues that directly identify a goal, from a spatial strategy of navigation, which requires the use of distal cues that do not directly identify the goal. If lizards do maintain and use spatial memory when navigating to a goal, they should navigate using spatial cues and direct their movement towards a spatially correct goal. Similarly, they should not use local cues to navigate towards a locally correct goal. These expectations are identical to previous studies testing a variety of taxa on the same task.
Figure 1.
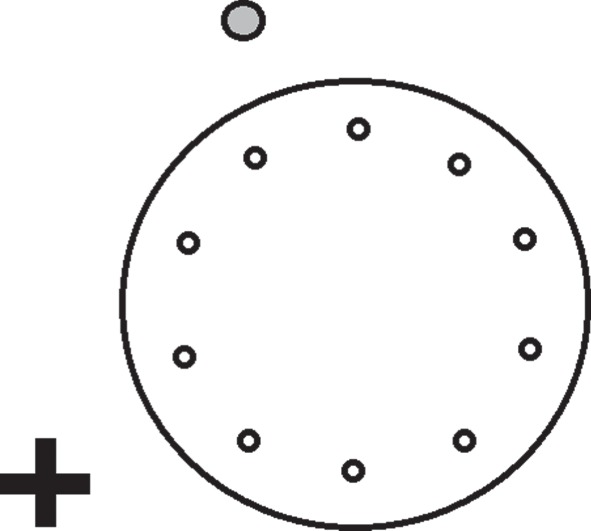
Schematic of the Barnes maze used to test spatial memory ability in the side-blotched lizard (U. stansburiana). To allow for spatial navigation, cues in the form of a circle (grey) and an ‘+’ (black), were located on the adjacent walls (at least 30 cm from the centre of the Barnes maze holes and 15.24 cm above the plane of the maze). The door to the testing room could also have been used as a potential visual cue. Room size was 2.13×1.22 m. Drawing is not to scale.
The lizards first engaged in training trials, where they were trained to enter a predetermined goal hole. The criterion for learning was unassisted descent into the hole on three separate occasions. After lizards reached the learning criterion, the maze was rotated 180°. By rotating all local cues 180°, while leaving the spatial cues throughout the room undisturbed, we could dissociate navigation using local versus distal spatial cues. All other cues, such as olfactory cues, were removed. Therefore, if lizards were using memory of local cues to navigate, they should now direct movements towards the hole 180° opposite of the original location. Alternatively, if lizards were using memory of spatial cues to navigate, they should direct movements towards the original, spatially correct hole.
2. Material and methods
Seven male side-blotched lizards, U. stansburiana, were used in this study (eight to 10 months); all were hatched and raised in identical laboratory conditions. Previous spatial memory studies in squamates have been criticized on methodological issues. Thus, we used the Barnes maze, a standard apparatus and methodology used to test spatial memory in many taxa [9]. The Barnes maze was a circular platform (73.66 cm high, 105.74 cm diameter) with 10 holes, equidistant from each other (26.04 cm); each hole was 15.54 cm from the edge of the maze. Holes were large enough to allow a lizard to pass through comfortably (2.54 cm diameter). To allow for spatial navigation, cues in the form of a circle and an ‘+’ on the wall, were located at least 30 cm from the Barnes maze holes and 15.24 cm above the plane of the table (figure 1); cues measured between 15 and 20 cm in diameter. By positioning cues in this way, none of the cues could serve as a local cue that directly identified the goal. The door of the testing room could also have been used as a visual cue. The room measured 2.13 × 1.22 m. The maze was brightly lit by an overhead light.
Subjects were randomly assigned different goal holes and subjected to training trials and a probe trial to ascertain spatial ability. During training trials, the subject's home enclosure was mounted underneath the assigned goal hole to provide an escape; all other holes were open. A subject was placed in the middle of the maze and allowed to explore for 10 min. If the subject did not go into his hole within 10 min, he was gently guided to the hole and allowed to descend. Once inside the home enclosure, we allowed him to sit undisturbed for 10 min. Some previous studies have used aversive stimuli (loud noises, lights) to motivate subjects to the goal; however, stimuli such as those can cause lizards to freeze and not perform (L. Ladage 2010, personal observation). Thus, all subjects in this study were self-motivated to enter the goal hole during the 10 min training trials.
Subjects participated in not more than four training trials per day. Criterion for progression into the probe trial was unassisted entry into the goal, during three different training trials, indicating that the subject had learned the location of the goal. All subjects eventually reached criterion, although they took a variable number of training trials to reach criterion (range: 17–81 training trials; average: 48.29).
During the probe trial, the home cage was removed from under the table, all olfactory cues were eliminated with a dilute bleach solution, and the maze was rotated 180° to preclude the use of local cues when navigating. The spatial cues in the room were not moved. Thus, if a subject was using spatial cues to navigate, he should investigate the spatially correct location. If the subject was using local cues, such as marks or discolorations associated with a particular hole, to navigate, he should investigate the locally correct location which was now rotated 180°. A subject was introduced into the maze and covered with a cup for 30 s to avoid experimental bias of positioning the animal in one particular direction. After 30 s, the cup was lifted by pulling on a string attached to the cup and threaded through to the adjoining observation room. The subject was allowed 10 min to explore. We scored an animal as investigating a hole when the subject's snout was within 1 cm of a hole. All seven subjects reached criterion in the training trials and logged at least one exploration of a hole during the probe trials.
All probe trials were videotaped with a webcam mounted above the maze. The recorded trials were scored by using custom computer vision software written for Matlab and the Image Processing toolbox (v. R2010b, The Mathworks, Inc., Natick, MA, USA). After importing videos, the 10 holes and the maze field were manually identified. Doing so eliminated any potential error in registering the maze and holes owing to small changes in the maze position over the experiment. Every 15th frame was processed via point processing and morphological operations to isolate the lizard against the background of the maze. After a final blob detection step, the position of the lizard was reported twice per second. To process the position versus time data reported by the vision software, an Excel worksheet was created to count the number of errors, as well as track total time spent in the four quadrants before reaching the goal hole in the probe trials (see electronic supplementary material, S1).
3. Results
During training trials, individuals exhibited a decrease in latency to arrive at the spatially correct goal (F1,27 = 3.196, p = 0.042), most noticeably between the first and last quartile of training trials (p = 0.012), indicating that lizards took less time to discover the spatially correct goal hole over the training trials. The number of errors during the training trials did not reflect a similar decrease (F1,27 = 0.532, p = 0.665), probably because our more conservative criterion for acceptance into the probe trial allowed animals to make a correct choice during earlier trials, yet continue making errors in later trials.
During probe trials, all subjects found the spatially correct goal and none investigated the locally correct goal. Four of seven individuals went to the correct spatial goal on the first try (Binomial test; p = 0.003). When exploring, lizards non-randomly chose the correct spatial location (t6 = −15.0, p < 0.001) and, when computing for sampling without replacement, they still non-randomly chose the correct spatial location (t6 = −10.33, p < 0.001), indicating that the lizards were not choosing the correct spatial location randomly and were using spatial memory to locate the correct location. Finally, all subjects spent a disproportionate amount of time in the correct spatially based quadrant (t6 = 18.354, p < 0.001).
4. Discussion
After rotation of the maze, all seven subjects investigated the spatially correct goal; none investigated the locally correct goal. Four of seven individuals went directly to the spatial goal first, two individuals found the spatial goal within two attempts, and the final individual found the spatial goal within three attempts. When exploring the maze, lizards did better than chance when locating the correct spatial goal, indicating that lizards were not locating the correct spatial goal randomly and returning to that goal via spatial memory. Further, we can eliminate the possibility of navigating via local cues during this task, as none of the subjects went to the locally correct goal location.
We found that side-blotched lizards do possess the ability to engage spatial memory when navigating to a goal. Using a standard paradigm to test for spatial memory in a variety of taxa, we feel confident that we have precluded some of the methodological criticisms encountered by previous studies that have sought to explicitly test for spatial memory ability in squamates. Our design included several spatial cues and we did not orient the animals towards any particular cue. We argue that by standardizing methodology across these studies, as has been done in other taxa, we can have more confidence that our results are not due to methodological issues, and we are actually tapping into spatial memory ability. Our findings refute previous assertions that squamate reptiles do not have spatial memory [1–3]. However, the paucity of studies on spatial memory via visual cues precludes meaningful comparisons among these studies. For instance, previous studies have used different species than the current study; it may be that different species experience differential demands on their spatial memory abilities and this may be reflected in their dependence on spatial memory during navigation. Thus, previous study species may not depend on spatial memory via visual cues during navigation whereas side-blotched lizards do. Also, all of these studies employed different methodologies. An alternative testing paradigm could also produce differing results depending on the ecology of the species. Unfortunately, at this point, we cannot determine if our differing results is an aspect of species-typical differences or methodological differences. Regardless, we can at least parsimoniously align this species of squamate reptile to the broad taxa of animals that possess spatial memory during navigation, albeit the mechanisms underlying this navigation may differ significantly among various taxonomic groups. Finally, we also add to the growing body of evidence that exothermic taxa also possess some cognitive abilities which may be on par with mammals and birds [4].
Acknowledgements
We thank M. Forney, R. Maged and K. Hellwinkle for data collection. This research was supported by NSF (IOS-0918268) to L.D.L. All procedures were approved by UNR IACUC (2009-00434).
References
- 1.Jacobs L. F. 2003. The evolution of the cognitive map. Brain Behav. Evol. 62, 128–139 10.1159/000072443 (doi:10.1159/000072443) [DOI] [PubMed] [Google Scholar]
- 2.Jacobs L. F., Schenk F. 2003. Unpacking the cognitive map: the parallel map theory of hippocampal function. Psychol. Rev. 110, 285–315 10.1037/0033-295X.110.2.285 (doi:10.1037/0033-295X.110.2.285) [DOI] [PubMed] [Google Scholar]
- 3.Day L. B., Crews D., Wilczynski W. 1999. Spatial and reversal learning in congeneric lizards with different foraging strategies. Anim. Behav. 57, 393–408 [DOI] [PubMed] [Google Scholar]
- 4.Leal M., Powell B. J. 2012. Behavioural flexibility and problem-solving in a tropical lizard. Biol. Lett. 23, 28–30 10.1098/rsbl.2011.0480 (doi:10.1098/rsbl.2011.0480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foa A., Basaglia F., Beltrami G., Carnacina M., Moretto E., Bertolucci C. 2009. Orientation of lizards in a Morris water-maze: roles of the sun compass and the parietal eye. J. Exp. Biol. 212, 2918–2924 10.1242/jeb.032987 (doi:10.1242/jeb.032987) [DOI] [PubMed] [Google Scholar]
- 6.Holtzman D. A., Harris T. W., Aranguren G., Bostock E. 1999. Spatial learning of an escape task by young corn snakes, Elaphe guttata guttata. Anim. Behav. 57, 51–60 10.1006/anbe.1998.0971 (doi:10.1006/anbe.1998.0971) [DOI] [PubMed] [Google Scholar]
- 7.Holtzman D. A. 1998. From slither to hither: orientation and spatial learning in snakes. Integr. Biol. 1, 81–89 (doi:10.1002/(SICI)1520-6602(1998)1:3<81::AID-INBI2>3.0.CO;2-V) [DOI] [Google Scholar]
- 8.Day L. B., Crews D., Wilczynski W. 2001. Effects of medial and dorsal cortex lesions on spatial memory in lizards. Behav. Brain Res. 118, 27–42 10.1016/S0166-4328(00)00308-9 (doi:10.1016/S0166-4328(00)00308-9) [DOI] [PubMed] [Google Scholar]
- 9.Sunyer B., Patil S., Höger H., Lubec G. 2007. Barnes maze, a useful task to assess spatial reference memory in the mice. Protoc. Exch. 10.1038/nprot.2007.390 (doi:10.1038/nprot.2007.390) [DOI] [Google Scholar]


