Abstract
Social network analysis is an ideal quantitative tool for advancing our understanding of complex social behaviour. However, this approach is often limited by the challenges of accurately characterizing social structure and measuring network heterogeneity. Technological advances have facilitated the study of social networks, but to date, all such work has focused on large vertebrates. Here, we provide proof of concept for using proximity data-logging to quantify the frequency of social interactions, construct weighted networks and characterize variation in the social behaviour of a lek-breeding bird, the wire-tailed manakin, Pipra filicauda. Our results highlight how this approach can ameliorate the challenges of social network data collection and analysis by concurrently improving data quality and quantity.
Keywords: coded nanotag, proximity data-loggers, social networks
1. Introduction
Non-random and heterogeneous social interactions are the foundation of animal social groups [1], and the dynamic structure of these social networks can have important implications for evolutionary processes [2,3]. Social network analysis provides a rigorous statistical framework for understanding the functional and evolutionary implications of social interactions, because it documents how individual-level patterns of association scale-up to produce population-level social structure [1,2]. Theoretical and empirical applications of social network theory have advanced our understanding of how selection acts on specific behaviours (e.g. cooperation), and how social connectivity influences individual fitness [4–6].
Despite the power of network analyses, models of animal social structure in free-living populations often lack replication and are limited in temporal scope, resulting in simplified binary networks (0/1 matrices) [7,8]. As such, a number of investigators have advocated the use of weighted networks that account for the frequency of interactions and thus better characterize complex social dynamics [7–9]. Data collection is the most prominent challenge associated with measuring social structure in free-living animals, because interactions can be difficult to quantify and observer effects, including sampling biases and error, can influence data reliability [10]. Moreover, observational approaches are spatially and temporally constrained by observer number and inherent limitations on sampling time. Recent advances in proximity logging technology that use either fixed receivers (coded nanotags) or interactive tags (Sirtrack [11,12] and Encounternet [13]) have begun to revolutionize the way we collect social network data. Proximity logging can increase both the quality and quantity of data that are essential for constructing weighted social networks. Yet, to date, the few applications have focused solely on large vertebrate taxa [11,12,14]. Research testing the efficacy of proximity data-loggers for small vertebrates is a necessary step for advancing our understanding of animal sociality.
Avian social systems have become indispensible models for exploring the evolutionary implications of social network structure because of their diversity and complexity. For example, wire-tailed manakins are cooperative lek-breeding birds that exhibit dynamic social behaviour in which territory males perform courtship displays alone, in a coalition with a non-territorial partner (floater), or in a coalition with another territory holder. Previous work using observational approaches has shown that display coalitions form the basis for complex networks and that network connectivity is a strong predictor of male fitness (social rise and reproductive success) [5,6]. Here, we test proximity data-loggers to quantify the frequency of social interactions, construct weighted networks and characterize variation in wire-tailed manakin social behaviour.
2. Material and methods
We studied wire-tailed manakins at Tiputini Biological Station in the Orellana province of eastern Ecuador (0°38′ S, 76°08′ W) during the peak reproductive period (January 2012). To ensure that we captured variation in social behaviour, we replicated sampling within each social class (definitive territorial, n = 11; definitive floater, n = 3; and pre-definitive floater, n = 2; females, n = 2; see the electronic supplementary material). Individuals were fitted with coded nanotags (NTQB-2, 0.35 g, Lotek Wireless) that use a single very high frequency (166.340 MHz) and emit a unique code (see the electronic supplementary material). Tags were monitored with a digitally encoded proximity data-logger (SRX-DL1; Lotek Wireless). The data-logger notes the time and signal strength (an index related to the log of the received signal in decibel) for each tag detection, and from these data, we can infer when individuals are in close proximity and their distance from the data-logger.
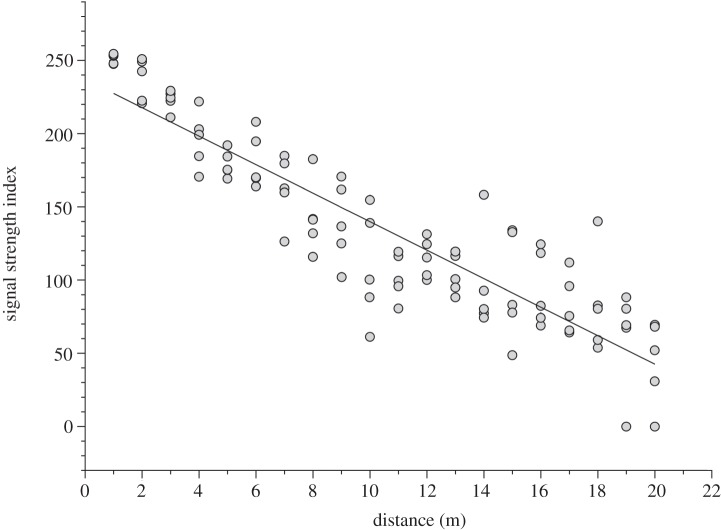
To monitor the social interactions of territorial males and their visitors, we placed the data-logger centrally in each territory from 06.00 to 16.30. The data-logger was rotated daily between 11 male territories for a total of 26 sampling occasions (24.32 ± 1.39 h per territorial male). To ensure we recorded within-territory social interactions, we used a short whip antenna and optimized receiver settings (receiver gain = 10 dB) to constrain detections within a radius of 25 m. To test how signal strength varied with vegetation density and distance from the data-logger, we walked 20 m transects and collected replicated signal strength data (n = 10) at 1 m increments in five independent territories.
Given the extensive knowledge of social interactions (cooperative display coalitions) in our system, we used proximity data from nanotags to quantify weighted social networks that accounted for the frequency of interactions. First, we defined territories using the observed range of signal strength for each territory holder (see the electronic supplementary material, table S1). Second, we quantified joint detections as social interactions if the males were detected less than 10 s apart and within the range of signal strength for the defined territory. Third, the duration of each interaction was measured as the time elapsed, in seconds, between the first joint detection and the last joint detection. Fourth, the frequency of interactions was tabulated using only joint detections that were spaced greater than 5 min apart. Because monitoring took place in each male's territory, the directionality of social interactions was defined with visitors (regardless of status) as the initiator and the territory holder as the targets. When two males were simultaneously detected as visitors in a third male's territory, the relationship between the visiting males was considered reciprocal. While not all joint detections represent male–male display coalitions, here we present non-filtered data, because many partnerships were confirmed by direct observation and frequent non-random social interactions are well documented in our system [15].
We used the R package tnet v. 3.0.5 [16] and a program Ucinet v. 6.0 [17] to calculate weighted and unweighted network metrics (see the electronic supplementary material). We visualized our network using program Gephi v. 0.8 with nodes arranged using a force-based algorithm. The emergent properties of the observed network were compared with a random graph with the same number of nodes and edges (see the electronic supplementary material, figure S1). Network metrics were compared between male-status classes using t-tests in the program JMP v. 8.0. Means and s.e. are reported.
3. Results
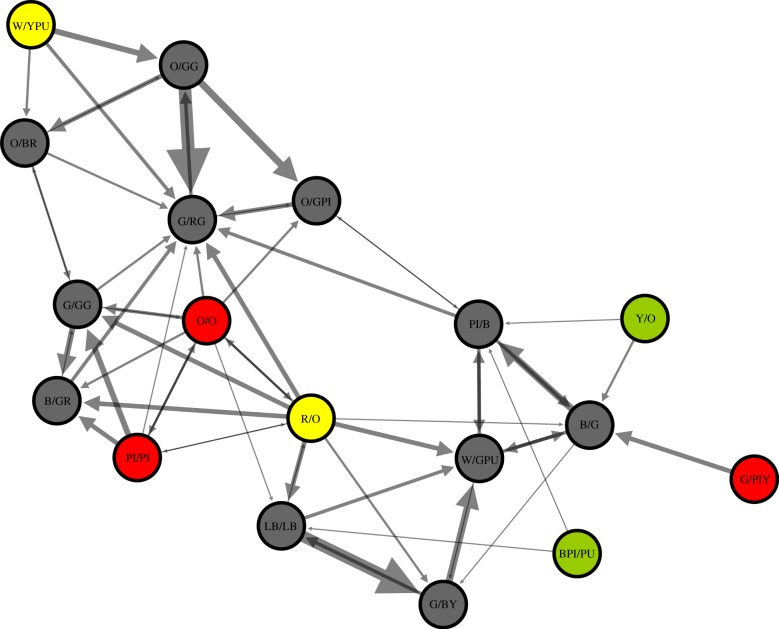
During our sampling, we logged 92 040 tag detections with territorial males being detected more frequently (8238±1446 detections per male) than floater males (345±169 detections per male; see the electronic supplementary material, table S1). Tag signal strength was negatively correlated with distance from receiver (F1,99 = 441.96, p < 0.0001, r2 = 0.82; figure 1), yet there was variation among male territories, presumably as a result of vegetation density. We quantified 170 independent dyadic social interactions for the construction of our weighted network (2.83 ± 0.26 interactions per dyad; range: 1–11; figure 2). Joint detections varied from brief interactions to longer territorial visits; the latter probably represented coordinated display bouts (148.93± 18.53 s; range: 5–1020). Key emergent properties of the network included a high, generalized clustering coefficient (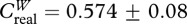 ;
; 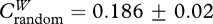 ; p<0.0001) and short average path length (Lreal = 1.98; Lrandom = 2.33) when compared with null expectations. Finally, we found significant status-related differences in weighted network metrics that quantified male social connectivity (table 1 and figure 2).
; p<0.0001) and short average path length (Lreal = 1.98; Lrandom = 2.33) when compared with null expectations. Finally, we found significant status-related differences in weighted network metrics that quantified male social connectivity (table 1 and figure 2).
Figure 1.
The relationship between signal strength and distance from the proximity data-logger for five male wire-tailed manakin territories. Points represent the mean of 10 replicate signal strength measures at each distance.
Figure 2.
A weighted social network of wire-tailed manakin social interactions. Individuals are represented by nodes (black, territorial; red, definitive floater; yellow, pre-definitve floater; green, female) and social interactions are depicted with directional lines that are weighted by interactions frequency (range: 1–11).
Table 1.
Weighted network metrics for wire-tailed manakin males of different status calculated using a spatial proximity approach. (Bold values denote significant differences in network metrices between male-status classes.)
| territorial (n = 11) | floater (n = 5) | ||
|---|---|---|---|
| network metric | mean ± s.e. | mean ± s.e. | p |
| in-strength | 14.18 ± 2.33 | 2.20 ± 0.92 | <0.001 |
| out-strength | 8.82 ± 0.53 | 13.00 ± 3.87 | 0.359 |
| in-degree | 4.73 ± 0.57 | 1.40 ± 0.60 | 0.002 |
| out-degree | 2.73 ± 0.19 | 5.00 ± 1.41 | 0.184 |
| wbetweennessa | 47.10 ± 8.83 | 9.40 ± 9.15 | 0.013 |
| eigenvector centrality | 0.24 ± 0.04 | 0.18 ± 0.05 | 0.340 |
aWeighted betweenness.
4. Discussion
Researchers applying social network theory to animal populations must be aware of the challenges associated with both data collection (representative sampling) and analysis [9]. Our study provides proof of concept for a relatively new technology that addresses these challenges by maximizing both the quality and quantity of data needed to characterize biological networks and analyse network heterogeneity. The benefits of nanotags and proximity data-loggers include: (i) increased quantity and quality of network data; (ii) minimized observer effects on focal animals and reduced sampling bias; (iii) the ability to collect longitudinal data on the frequency and duration of social interactions; (iv) versatile tag configurations with 500+ unique codes (weight: 0.29–2.6 g, pulse rate: 1–40 s and battery life with 12 h on/off programming: 45–928 days); (v) flexible data collection (variable scan time and number of frequencies, multiple antenna, gain/detection range: 5 m–20 km); and (vi) waterproof data-loggers that can run autonomously (approx. two weeks) and record more than 250 000 detections.
Despite the clear benefits of using proximity approaches to collect social network data, there are also a number of important limitations to consider. First, researchers must realize that proximity data cannot differentiate types of interactions (cooperation versus aggression) and that associations based on spatial proximity (gambit of the group) must use biologically relevant criteria [2]. As such, researchers must apply species-specific knowledge of social behaviour to interpret and analyse networks generated from spatial proximity data. Here, our detailed knowledge of the manakin social system, frequent social interactions and consistent use of space facilitated the use of spatial proximity approaches to quantify social networks. Second, tags (199 US dollars per tag) and data-loggers (2495 US dollars per logger) are expensive, and thus the costs as well as the benefits of proximity logging must be weighed against those of observational approaches. Third, proximity logging may yield yet unknown sources of bias and/or error (false-positive joint detections and tag-induced behavioural changes), although we did not experience these problems with our data and study species. Finally, while improved data collection techniques will advance our understanding of complex social systems, increased data quantity and quality alone do not negate other analytical challenges (non-independence, data filtering and management; see the electronic supplementary material) and the importance of using null models for testing network hypotheses [9].
Here, we advance our previous work on manakin social networks [5,6] by using a proximity logging approach to characterize weighted social networks. Two complementary measures of average network structure and topology suggest that males are both closely connected (path length) and highly clustered (clustering coefficient), with leks probably serving as densely interconnected neighbourhoods. Our weighted network data also suggest that social status is a strong predictor of a male's centrality and the extent to which he initiates display partnerships. Ultimately, the ability to measure individual variation in social behaviour using weighted network metrics will begin to advance our understanding of how behavioural phenotypes influences dominance and subsequent fitness.
The technique and data presented here suggest that proximity approaches can be used to streamline social network data collection in small vertebrates when there is sufficient a priori knowledge of a species social system. Proximity technology greatly enhanced our ability to quantify social networks in the manakin system by reducing sampling time (see the electronic supplementary material, table S2), detecting previously unrecognized complexity in the frequency and directionality of male–male interactions, and capturing rare social interactions between territorial males at different leks. We believe that proximity approaches can also advance data collection in other areas of behavioural ecology (parental care, territorial intrusions, flocking behaviour and cooperative breeding). One particularly promising application would be the study of reproductive behaviours (female mate searching, extra-pair behaviour) that are often cryptic yet drive patterns of male reproductive success. While limited in scope, our data suggest proximity tags can successfully be used to collect data on patterns of female visitation in lekking birds (also see Mennill et al. [13]). Ultimately, the ability to autonomously collect longitudinal data on social networks is a promising starting point for understanding the mechanisms that drive social network structure and selection on social behaviour.
Acknowledgements
This research followed Smithsonian Institutions ACUC guidelines.
We thank Tiputini and Universidad San Francisco de Quito for logistical support and NSF for funding to ITM (IOS 0545735).
References
- 1.Krause J., Ruxton G. D. 2002. Living in groups. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Croft D. P., James R., Krause J. 2008. Exploring animal social networks. Princeton, NJ: Princeton University Press [Google Scholar]
- 3.Wey T., Blumstein D. T., Shen W., Jordan F. 2008. Social network analysis of animal behavior: a promising tool for the study of sociality. Anim. Behav. 75, 333–344 10.1016/j.anbehav.2007.06.020 (doi:10.1016/j.anbehav.2007.06.020) [DOI] [Google Scholar]
- 4.Ohtsuki H., Hauert C., Lieberman E., Nowak M. A. 2006. A simple rule for the evolution of cooperation on graphs and social networks. Nature 441, 502–505 10.1038/nature04605 (doi:10.1038/nature04605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryder T. B., McDonald D. B., Blake J. G., Parker P. G., Loiselle B. A. 2008. Social networks in the lek-mating wire-tailed manakin (Pipra filicauda). Proc. R. Soc. B 275, 1367–1374 10.1098/rspb.2008.0205 (doi:10.1098/rspb.2008.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryder T. B., Parker P. G., Blake J. G., Loiselle B. A. 2009. It takes two to tango: reproductive skew and social correlates of male mating success in a lek-breeding bird. Proc. R. Soc. B 276, 2377–2384 10.1098/rspb.2009.0208 (doi:10.1098/rspb.2009.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lusseau D., Whitehead H., Gero S. 2008. Incorporating uncertainty into the study of animal social networks. Anim. Behav. 75, 1809–1815 10.1016/j.anbehav.2007.10.029 (doi:10.1016/j.anbehav.2007.10.029) [DOI] [Google Scholar]
- 8.James R., Croft D. P., Krause J. 2009. Potential banana skins in animal social network analysis. Behav. Ecol. Sociobiol. 63, 989–997 10.1007/s00265-009-0742-5 (doi:10.1007/s00265-009-0742-5) [DOI] [Google Scholar]
- 9.Croft D., Madden J., Franks D. W., James R. 2011. Hypothesis testing in animal social networks. Trends Ecol. Evol. 26, 502–507 10.1016/j.tree.2011.05.012 (doi:10.1016/j.tree.2011.05.012) [DOI] [PubMed] [Google Scholar]
- 10.Krause J., Wilson A. D., Croft D. P. 2011. New technology facilitates the study of social networks. Trends Ecol. Evol. 26, 5–6 10.1016/j.tree.2010.10.004 (doi:10.1016/j.tree.2010.10.004) [DOI] [PubMed] [Google Scholar]
- 11.Bohm M., Hutchings M. R., White P. C. L. 2009. Contact networks in a wildlife-livestock host community: indentifying high-risk individuals in the transmission of bovine TB among badgers and cattle. PLoS ONE 4, e5016. 10.1371/journal.pone.0005016 (doi:10.1371/journal.pone.0005016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsh M. K., McLeod S. R., Hutchings M. R., White P. C. L. 2011. Use of proximity loggers and network analysis to quantify social interactions in free-ranging wild rabbit populations. Wildl. Res. 38, 1–12 10.1071/WR10150 (doi:10.1071/WR10150) [DOI] [Google Scholar]
- 13.Mennill D. J., Doucet S. M., Ward K. A., Maynard D. F., Otis B., Burt J. M. 2012. A novel digital telemetry system for studying mate choice in a lekking tropical bird. Methods Ecol. Evol. 10.1111/j.2041-210X.2012.00206.x (doi:10.1111/j.2041-210X.2012.00206.x) [DOI] [Google Scholar]
- 14.Gutteridge T. L., Gruber S. H., Krause J., Sims D. W. 2010. Novel acoustic technology for studying free-ranging shark social behavior by recording individual interactions. PLoS ONE 5, e9324. 10.1371/journal.pone.0009324 (doi:10.1371/journal.pone.0009324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryder T. B., Blake J. G., Loiselle B. A., Parker P. G. 2011. The composition, stability and kinship of reproductive coalitions in a lekking bird. Behav. Ecol. 22, 282–290 10.1093/beheco/arq213 (doi:10.1093/beheco/arq213) [DOI] [Google Scholar]
- 16.Opshal T. 2009. Structure and evolution of weighted networks. London, UK: University of London [Google Scholar]
- 17.Borgatti S. P., Everet M. G., Freeman L. C. 2002. Ucinet for Windows: software for social network analysis. Harvard, MA: Analytic Technologies [Google Scholar]