Abstract
Local species extinctions may lead to, often unexpected, secondary extinctions. To predict these, we need to understand how indirect effects, within a network of interacting species, affect the ability of species to persist. It has been hypothesized that the persistence of some predators depends on other predator species that suppress competitively dominant prey to low levels, allowing a greater diversity of prey species, and their predators, to coexist. We show that, in experimental insect communities, the absence of one parasitoid wasp species does indeed lead to the extinction of another that is separated by four trophic links. These results highlight the importance of a holistic systems perspective to biodiversity conservation and the necessity to include indirect population dynamic effects in models for predicting cascading extinctions in networks of interacting species.
Keywords: aphids, indirect effects, food web, parasitoid, resource competition, secondary extinctions
1. Introduction
Owing to the interdependence of species in ecosystems, biodiversity loss as a result of human activities may lead to cascades of secondary extinctions [1–4]. Predicting these extinctions is often difficult and requires an understanding of the mechanisms by which the effects of the loss of one species are transmitted through the network of interactions among species [5]. Models of secondary extinctions in food webs (networks of trophically interacting species) can be divided into two classes: (i) topological models that only consider network structure, and in which extinctions happen when species no longer have any resources and (ii) dynamic models in which population dynamics of all species are governed by their interactions. The limitations of the first class are commonly acknowledged, chief among these being that they do not allow for top-down effects and competitive interactions [6,7]. Nevertheless, they are still often applied to test the robustness of natural food webs [8], even though dynamic models clearly indicate the importance of top-down and horizontal indirect effects among species in propagating extinction cascades [9–11]. Currently, there is little empirical evidence for secondary extinctions being caused by the loss of positive indirect interactions following a primary extinction, despite evidence that indirect interactions play a dominant role in structuring ecological communities [12].
Species at higher trophic levels (carnivores) are particularly vulnerable to extinction [1], and positive indirect interactions among these species have long been considered a potentially important mechanism for the maintenance of species diversity [13–16]. Competition between resource species can lead to an indirect mutualism between their consumers [16,17], because a consumer that reduces the density of its prey also reduces competition at the prey's trophic level with positive effects on other prey species and, thereby, their consumers. Consequently, the extinction of one carnivore species could lead to cascading extinctions of other carnivores whose prey are outcompeted by the prey that is released from top-down control. Indirect mutualism can occur when consumer species are sufficiently specialized, with each feeding predominantly on different food resources [13]. Despite the potential importance of this effect, empirical evidence for it is scarce and either based on observations of co-occurrence patterns of aquatic predators [15] or experimental studies of interactions among herbivores on a rocky shore [14] and granivores in a desert ecosystem [18], both of which have problems associated with their experimental design [19,20].
Communities of plants, insect herbivores and insect parasitoids account for a large part of the Earth's species and are characterized by a high degree of diet specialization [21]. Furthermore, they have proved to be excellent experimental models for food web ecology [22–24]. We assembled replicate communities consisting of plants as a shared resource for two aphid species and two parasitoid wasps each of which attacks only one of the aphid species. We hypothesized that exclusion of either parasitoid species would have a negative effect on the population of the other as a result of increased resource competition between their respective host species. We show that the absence of one parasitoid species leads to the extinction of the other and that this effect is transmitted through four trophic links.
2. Material and methods
Experimental communities were assembled from Vicia faba (L) (var. the Sutton) plants as a shared resource for the herbivorous aphids Aphis fabae (Scopoli) and Acyrthosiphon pisum (Harris) and the parasitoid wasps Lysiphlebus fabarum (Marshall) and Aphidius ervi (Haliday), each of which attacks only one of the aphid species. Experimental cages were arranged in seven blocks, each containing one cage of each experimental treatment (figure 1a): (i) both the parasitoid species L. fabarum and Aphidius ervi present, (ii) only L. fabarum present, and (iii) only Aphidius ervi present. The plant V. faba and the aphid species Acyrthosiphon pisum and Aphis fabae were present in all treatments.
Figure 1.
Population dynamics in the experimental insect communities. (a) Compositions of the three experimental community treatments (seven replicates of each). (b–e) Densities of the four insect species during the eight weeks of the experiment in treatments with both parasitoid species (purple diamonds), L. fabarum only (red circles) and Aphidius ervi only (blue triangles). Values are the means of the seven replicates in each treatment and error bars represent ± s.e.m.
Communities were contained in 30 × 30 × 30 cm Plexiglas cages with a fine mesh sleeve for ventilation and access. Each cage contained eight 9 cm diameter pots with four V. faba plants each. The plants were renewed by twice a week replacing the two oldest pots with fresh ones containing two-week old seedlings, taking care to return all insects to the cage. This procedure allows the long-term maintenance of experimental aphid populations, with a carrying capacity in the order of 2000–3000 individuals, but does not prevent interspecific competition [24]. The experimental cages were kept in a controlled environment room at 20°C, relative humidity 75 per cent, with a 16 L : 8 D cycle. Under these conditions, the generation times are 9 and 12 days for the aphids and parasitoids, respectively.
At the start of the experiment, five wingless adult females of each aphid species were introduced (both species reproduce asexually by giving birth to live female young under summer conditions). Ten days after the aphids, three mated female parasitoids were released for each species as per experimental treatment. The parasitoid females lay eggs in immature aphids and the wasp larvae feed internally on their host before killing it and pupating inside the dried skin of the host, forming a so-called ‘mummy’. Only a single parasitoid individual can develop per host individual so the number of mummies in a cage can be used as a measure of population density. Once a week, during the eight weeks of the experiment, the number of aphids and parasitoid mummies of each species was counted on half of the plants in each cage.
The responses of aphid and parasitoid populations to the experimental treatments were analysed using linear mixed-effects models with log transformed cumulative (over time) density data as response variable. The effect of experimental treatment on the population size of each species was tested as fixed effect with ‘block’ included as random effect. Significance levels were determined by comparing models with and without the treatment effect using maximum-likelihood methods. Analysis was carried out using the open source software R v. 2.13.2 [25].
3. Results
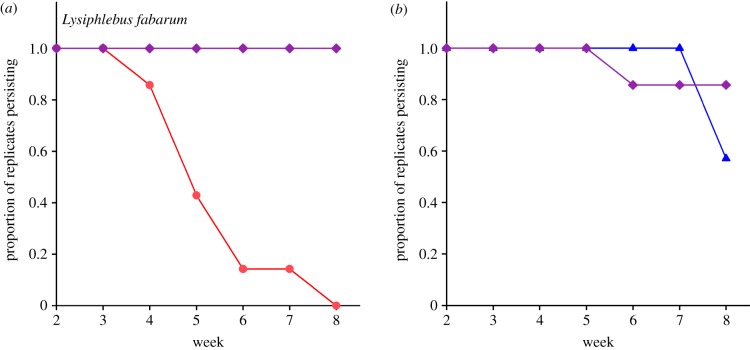
The parasitoid L. fabarum showed lower population densities (figure 1b; likelihood-ratio (LR) = 9.44, p < 0.01), and went extinct in all seven replicates within six weeks (two to three generations; figure 2a), when Aphidius ervi was omitted from the community. In contrast, L. fabarum persisted in all replicates in the presence of Aphidius ervi. These differences were associated with lower population densities, and eventually extinction, of Aphis fabae (the host of L. fabarum) (figure 1d, LR = 9.73, p < 0.01) and markedly higher population densities of Acyrthosiphon pisum (figure 1e, LR = 17.18, p < 0.0001) in the absence of the parasitoid Aphidius ervi.
Figure 2.
Persistence of experimental parasitoid populations. (a) L. fabarum in the presence (purple diamonds) and absence (red circles) of Aphidius ervi. (b) Aphidius ervi in the presence (purple diamonds) and absence (blue triangles) of L. fabarum.
We found no evidence for a reciprocal positive effect of L. fabarum on Aphidius ervi (figure 1c, LR = 3.29, p > 0.05) and there was no difference in population persistence of the latter between the treatments (figure 2b). There was a clear effect of the presence of L. fabarum on the population of its host Aphis fabae (figure 1d, LR = 7.32, p < 0.01), but this did not affect the density of Acyrthosiphon pisum (figure 1e, LR = 2.63, p > 0.1).
4. Discussion
We found a clear positive indirect effect of the parasitoid Aphidius ervi on another parasitoid, L. fabarum, via four trophic links (figure 1a), such that populations of L. fabarum could only persist in the presence of Aphidius ervi. The extinction of L. fabarum was a result of the extinction of its host, Aphis fabae, through competitive exclusion by the aphid Acyrthosiphon pisum, when the latter species was not controlled by its parasitoid. Our experiment demonstrated that consumer species whose prey compete for a shared resource can have a strong positive indirect effect on each other, promoting species persistence at the resource and the consumer level. Therefore, when considering this mechanism, species losses can indeed lead to secondary extinctions of indirectly linked species.
We found no evidence for a reciprocal effect of L. fabarum on Aphidius ervi. Acyrthosiphon pisum (the host of Aphidius ervi) populations were only regulated by their parasitoid and not by interspecific resource competition, and hence there was no scope for indirect effects to be transmitted in this direction. This asymmetry in competition among the two aphid species led to an indirect commensalism between the parasitoid species, rather than the expected indirect mutualism. We have observed such strongly asymmetric competition among aphids before [24] but the underlying mechanisms remain unclear. In general, competition is typically asymmetric [26] so that indirect commensalism should be more frequently found than indirect mutualism.
We conclude that secondary consumer species whose prey compete for a shared resource can indeed have a strong positive indirect effect on each other, promoting population persistence and the maintenance of biodiversity. Loss of this positive indirect effect, through the removal of one species, can lead to the extinction of another species that is four trophic links removed. Our experimental communities were very simple and in natural diverse and complex communities there are likely a number of other indirect pathways that either reduce or strengthen the effect identified here. Moreover, in spatially structured communities the degree of competition could vary in space and allow for recolonization following local extinctions. These results emphasize the necessity for including population dynamic effects in food web models aimed at testing the robustness of natural multitrophic communities to primary extinctions [27]. Current species extinctions are biased towards higher order consumers [1] which, by the mechanism demonstrated here, could lead to a cascade of secondary extinctions.
Acknowledgements
D.S. was supported by Deutsche Forschungsgemeinschaft grant no. SA 2003/1-1. University of Exeter BIO2407 (2011) students assisted with data collection.
References
- 1.Borrvall C., Ebenman B. 2006. Early onset of secondary extinctions in ecological communities following the loss of top predators. Ecol. Lett. 9, 435–442 10.1111/j.1461-0248.2006.00893.x (doi:10.1111/j.1461-0248.2006.00893.x) [DOI] [PubMed] [Google Scholar]
- 2.Borrvall C., Ebenman B., Jonsson T. 2000. Biodiversity lessens the risk of cascading extinction in model food webs. Ecol. Lett. 3, 131–136 10.1046/j.1461-0248.2000.00130.x (doi:10.1046/j.1461-0248.2000.00130.x) [DOI] [Google Scholar]
- 3.Montoya J. M., Pimm S. L., Sole R. V. 2006. Ecological networks and their fragility. Nature 442, 259–264 10.1038/nature04927 (doi:10.1038/nature04927) [DOI] [PubMed] [Google Scholar]
- 4.Paine R. T. 1966. Food web complexity and species diversity. Am. Nat. 100, 65–75 10.1086/282400 (doi:10.1086/282400) [DOI] [Google Scholar]
- 5.Ives A. R., Cardinale B. J. 2004. Food-web interactions govern the resistance of communities after non-random extinctions. Nature 429, 174–177 10.1038/nature02515 (doi:10.1038/nature02515) [DOI] [PubMed] [Google Scholar]
- 6.Brose U. 2011. Extinctions in complex, size-structured communities. Basic Appl. Ecol. 12, 557–561 10.1016/j.baae.2011.09.010 (doi:10.1016/j.baae.2011.09.010) [DOI] [Google Scholar]
- 7.Ebenman B. 2011. Response of ecosystems to realistic extinction sequences. J. Anim. Ecol. 80, 307–309 10.1111/j.1365-2656.2011.01805.x (doi:10.1111/j.1365-2656.2011.01805.x) [DOI] [PubMed] [Google Scholar]
- 8.de Visser S. N., Freymann B. P., Olff H. 2011. The Serengeti food web: empirical quantification and analysis of topological changes under increasing human impact. J. Anim. Ecol. 80, 484–494 10.1111/j.1365-2656.2010.01787.x (doi:10.1111/j.1365-2656.2010.01787.x) [DOI] [PubMed] [Google Scholar]
- 9.Eklof A., Ebenman B. 2006. Species loss and secondary extinctions in simple and complex model communities. J. Anim. Ecol. 75, 239–246 10.1111/j.1365-2656.2006.01041.x (doi:10.1111/j.1365-2656.2006.01041.x) [DOI] [PubMed] [Google Scholar]
- 10.Petchey O. L., Eklof A., Borrvall C., Ebenman B. 2008. Trophically unique species are vulnerable to cascading extinction. Am. Nat. 171, 568–579 10.1086/587068 (doi:10.1086/587068) [DOI] [PubMed] [Google Scholar]
- 11.Curtsdotter A., Binzer A., Brose U., de Castro F., Ebenman B., Eklöf A., Riede J. O., Thierry A., Rall B. C. 2011. Robustness to secondary extinctions: comparing trait-based sequential deletions in static and dynamic food webs. Basic Appl. Ecol. 12, 571–580 10.1016/j.baae.2011.09.008 (doi:10.1016/j.baae.2011.09.008) [DOI] [Google Scholar]
- 12.Bukovinszky T., van Veen F. J. F., Jongema Y., Dicke M. 2008. Direct and indirect effects of resource quality on food web structure. Science 319, 804–807 10.1126/science.1148310 (doi:10.1126/science.1148310) [DOI] [PubMed] [Google Scholar]
- 13.Abrams P. A., Nakajima M. 2007. Does competition between resources change the competition between their consumers to mutualism? Variations on two themes by Vandermeer. Am. Nat. 170, 744–757 10.1086/522056 (doi:10.1086/522056) [DOI] [PubMed] [Google Scholar]
- 14.Dethier M. N., Duggins D. O. 1984. An indirect commensalism between marine herbivores and the importance of competitive hierarchies. Am. Nat. 124, 205–219 10.1086/284264 (doi:10.1086/284264) [DOI] [Google Scholar]
- 15.Dodson S. I. 1970. Complementary feeding niches sustained by size-selective predation. Limnol. Oceanogr. 15, 131–137 [Google Scholar]
- 16.Vandermeer J. 1980. Indirect mutualism: variations on a theme by Levine. Am. Nat. 116, 441–448 10.1086/283637 (doi:10.1086/283637) [DOI] [Google Scholar]
- 17.Levine S. H. 1976. Competitive interactions in ecosystems. Am. Nat. 110, 903–910 10.1086/283116 (doi:10.1086/283116) [DOI] [Google Scholar]
- 18.Davidson D. W., Inouye R. S., Brown J. H. 1984. Granivory in a desert ecosystem: experimental evidence for indirect facilitation of ants by rodents. Ecology 65, 1780–1786 10.2307/1937774 (doi:10.2307/1937774) [DOI] [Google Scholar]
- 19.Brown J. H., Davidson D. W. 1986. Do desert rodent populations increase when ants are removed reply. Ecology 67, 1423–1425 10.2307/1938698 (doi:10.2307/1938698) [DOI] [Google Scholar]
- 20.Galindo C. 1986. Do desert rodent populations increase when ants are removed. Ecology 67, 1422–1423 10.2307/1938697 (doi:10.2307/1938697) [DOI] [Google Scholar]
- 21.van Veen F. J. F., Morris R. J., Godfray H. C. J. 2006. Apparent competition, quantitative food webs, and the structure of phytophagous insect communities. Ann. Rev. Entomol. 51, 187–208 10.1146/annurev.ento.51.110104.151120 (doi:10.1146/annurev.ento.51.110104.151120) [DOI] [PubMed] [Google Scholar]
- 22.Harmon J. P., Moran N. A., Ives A. R. 2009. Species response to environmental change: impacts of food web interactions and evolution. Science 323, 1347–1350 10.1126/science.1167396 (doi:10.1126/science.1167396) [DOI] [PubMed] [Google Scholar]
- 23.Morris R. J., Lewis O. T., Godfray H. C. J. 2004. Experimental evidence for apparent competition in a tropical forest food web. Nature 428, 310–313 10.1038/nature02394 (doi:10.1038/nature02394) [DOI] [PubMed] [Google Scholar]
- 24.van Veen F. J. F., van Holland P. D., Godfray H. C. J. 2005. Stable coexistence in insect communities due to density- and trait-mediated indirect effects. Ecology 86, 3182–3189 10.1890/04-1590 (doi:10.1890/04-1590) [DOI] [Google Scholar]
- 25.R Development Core Team. 2009. R: a language and environment for statistical computing. Vienna, Austria: R foundation for statistical computing.
- 26.Lawton J. H., Hassell M. P. 1981. Asymmetrical competition in insects. Nature 289, 793–795 10.1038/289793a0 (doi:10.1038/289793a0) [DOI] [Google Scholar]
- 27.Ebenman B., Jonsson T. 2005. Using community viability analysis to identify fragile systems and keystone species. Trends Ecol. Evol. 20, 568–575 10.1016/j.tree.2005.06.011 (doi:10.1016/j.tree.2005.06.011) [DOI] [PubMed] [Google Scholar]