Abstract
Rodents are important ecological components of virtually every terrestrial ecosystem. Their success is a result of their gnawing incisors, battery of grinding molars and diastema that spatially and functionally separates the incisors from the molars. Until now these traits defined all rodents. Here, we describe a new species and genus of shrew-rat from Sulawesi Island, Indonesia that is distinguished from all other rodents by the absence of cheek teeth. Moreover, rather than gnawing incisors, this animal has bicuspid upper incisors, also unique among the more than 2200 species of rodents. Stomach contents from a single specimen suggest that the species consumes only earthworms. We posit that by specializing on soft-bodied prey, this species has had no need to process food by chewing, allowing its dentition to evolve for the sole purpose of procuring food. Thus, the removal of functional constraints, often considered a source of evolutionary innovations, may also lead to the loss of the very same traits that fuelled evolutionary diversification in the past.
Keywords: convergence, key innovation, new species, shrew-rat, Sulawesi, vermivory
1. Introduction
Evolutionary innovations leading to major shifts in the morphological, and hence ecological, adaptations of organisms may unlock ecological opportunity and serve as fertile ground for adaptive radiation [1–4]. However, occasionally these innovations are reversed. Examples include the loss of tetrapod limbs in snakes and whales and the loss of flight in penguins, ratites and other birds. In each case, the loss of a key innovation allowed organisms to exploit resources that were not previously available.
Rodents represent a remarkable radiation, comprising over 40 per cent of all extant mammal diversity [5]. They are ubiquitous members of terrestrial mammal communities around the world and are major ecological components of nearly every terrestrial ecosystem, serving key functions such as seed dispersal and soil engineering [6–9]. The broad ecological success of rodents is undoubtedly related to their unique dental mechanics [10]. Indeed the name Rodentia is derived from the Latin roots rodere (to gnaw) and dentis (tooth). Because rodent incisors grow continuously, with enamel on only the labial surface, the lingual surface wears faster and gnawing maintains a chisel-shaped cutting edge. These incisors, combined with the diastema separating them from the cheek teeth, arm rodents with a powerful tool that facilitates access to resources not available to other mammals. The broad ecological success of rodents is a product of the general utility of their dentition. Most rodents are opportunistically omnivorous, feeding on vegetation, seeds and invertebrates. However, some species have specialized diets with major anatomical adaptations to their digestive tract and molar surfaces, but retain the key gnawing and grinding components of the rodent skull.
The most notable exceptions to the gnawing capacity of rodents occur among the shrew-rats endemic to Luzon Island, the Philippines (Archboldomys, Chrotomys and Rhynchomys) and Sulawesi Island, Indonesia (Echiothrix, Melasmothrix, Sommeromys and Tateomys). These species are not a natural (i.e. monophyletic) group [11–13], but rather have independently evolved similar morphological characteristics associated with dietary specialization on earthworms and other invertebrates. Shrew-rats have long faces with a pointed snout, reminiscent of true shrews. Their incisors have either reduced or lost entirely the enamel on the labial surface, thus limiting their capacity to gnaw. The lower incisors are long, slender and procumbent. The upper incisors are more typical of those of other rodents in their overall shape, though generally more delicate. All known species of shrew-rats have molars. Most possess the typical rodent arrangement of three molars per quadrant with complex occlusal surfaces (e.g. Echiothrix and Archboldomys). Others have fewer and simpler molars (Rhynchomys has two molars per quadrant). New Guinea moss-mice have some similar adaptations, including molar loss (e.g. Pseudohydromys ellermani has one molar per quadrant), but they retain typical rodent incisors [14].
Here, we describe a remarkable new shrew-rat that lacks molars and possesses bicuspid upper incisors, both of which are unique among the more than 2200 rodent species.
2. Material and methods
We collected small mammals during 2011 on Mounts Latimojong and Gandangdewata, Sulawesi, Indonesia (see the electronic supplementary material, figure S1). We made qualitative comparisons between specimens of the new taxon and all other known shrew-rat genera from Sulawesi (Echiothrix centrosa, Melasmothrix naso, Sommeromys macrorhinos, Tateomys macrocercus and Tateomys rhinogradoides) and Rhynchomys soricoides, an ecologically similar shrew-rat endemic to Luzon, Philippines. We took standard morphometric data from adult specimens of the earlier-named species (see the electronic supplementary material). We removed the stomach from one specimen of the new taxon and examined its contents.
3. Results
We collected one specimen of the new taxon on Mount Latimojong and another approximately 100 km to the northwest on Mount Gandangdewata (see the electronic supplementary material, figure S1). Both were taken in pitfall traps in mature forest. Morphometric data are presented in the electronic supplementary materials.
(a). New genus and species
Paucidentomys vermidax
(b). Holotype
FMNH 213102/MZB 35000 (figures 1 and 2; electronic supplementary material, figures S2 and S3), an adult male collected on 3 March 2011, fixed in buffered 10 per cent formalin and later transferred to 70 per cent ethanol. The skull was removed and cleaned. This specimen will be transferred from the Field Museum (FMNH) to the Museum Zoologicum Bogoriense (MZB).
Figure 1.

Holotype of P. vermidax.
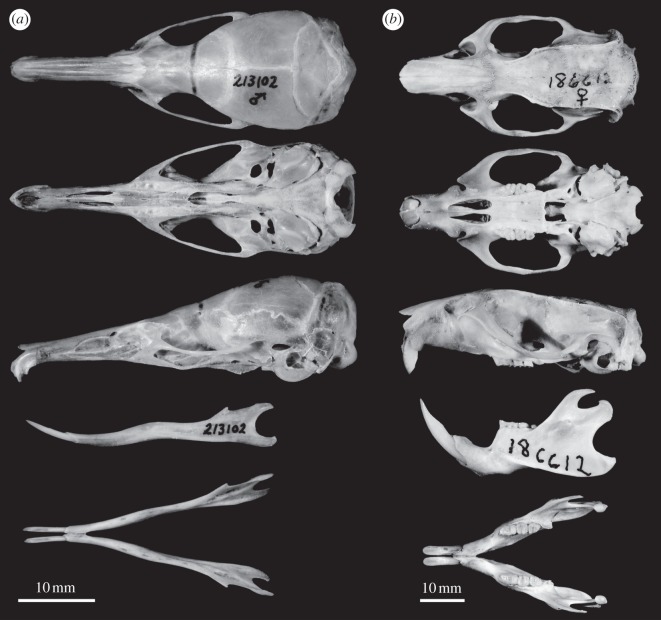
Figure 2.
Skulls (dorsal, ventral and lateral views) and mandibles (lateral and dorsal) of (a) P. vermidax (FMNH 213102) and (b) Rattus norvegicus (FMNH 186612), representing the typical murid rodent morphology.
(c). Type locality
Mount Latimojong (3.40755° S, 120.00780° E, 2050 m), Bantanase, Karangan, Desa Latimojong, Buntu Batu, Enrekang, Sulawesi Selatan, Indonesia.
(d). Paratype
We collected one additional specimen (MZB 35001) on Mount Gandangdewata (2.88289° S, 119.38644° E, 1571 m), Rantepangko, Mamasa, Sulawesi Barat, Indonesia. It was prepared as a dried skin, cleaned skull and fluid-preserved carcass.
(e). Distribution
Paucidentomys is known only from montane forest on Mount Latimojong and the transition between lowland and montane forest on Mount Gandangdewata, but probably occurs more broadly in mid- to high-elevation areas. The species may be endemic to the southwestern mountains of central Sulawesi [15].
(f). Etymology
The generic name combines the Latin ‘paucus’ (few) with ‘dentis’ (tooth) and the Greek ‘mys’ (mouse) in reference to the lack of molars. The epithet is a hybrid of ‘vermi’ (worm) and ‘edax’ (devourer), in reference to the animal's diet.
(g). Diagnosis
Generic and species diagnoses are the same. Paucidentomys has a medium body size and a very long rostrum (relative to other Sulawesi shrew-rats), small eyes, large ears, a soft pelage and a long, thick, hairy and dorsoventrally bicoloured tail (figure 1). The mouth contains one incisor per quadrant, but no other teeth (figure 2). Upper incisors are short with an anterior cusp and slightly inferior posterior cusp; these cusps are connected by a sharp, concave cutting edge at the lateral margin of the tooth (see the electronic supplementary material, figure S3). Palate is very long (see the electronic supplementary material, table S1). Vestiges of molar alveoli are visible under a thin layer of semi-translucent bone below the zygomatic process of the maxilla (figure 2). Pterygoid plate is absent (figure 2). Dentary is long and delicate, lacking significant muscle attachment points (figure 2). Lower incisors are unicuspid, procumbent, sharp and delicate.
(h). Brief description and comparisons
Paucidentomys vermidax is larger than Melasmothrix naso, Sommeromys macrorhinos and Tateomys macrocercus, similar in size to T. rhinogradoides, smaller than Rhynchomys soricoides and substantially smaller than species of Echiothrix. The face is more elongate than that of any other Sulawesi shrew-rat, but similar in this regard to Rhynchomys. Paucidentomys has bicuspid upper incisors and no molars (figure 2; see the electronic supplementary material, figures S2 and S3), both unique characters among rodents. Some rodents have a posterior shelf on the upper incisor (e.g. Mus and Musseromys), but only Paucidentomys has a prominent posterior projection on the upper incisor. Paucidentomys lacks pterygoid plates, distinguishing it from all Sulawesi shrew-rats except Echiothrix (see the electronic supplementary material, figure S2). Additional comparisons are provided in the electronic supplementary material.
(i). Ecology
The stomach of MZB 35001 was distended by segments of earthworms, each 5–10 mm long. No other contents were found. Paucidentomys probably eats only soft animal tissues and perhaps only earthworms. The incisors probably serve to cut or tear earthworms into segments before they are swallowed. Paucidentomys is probably a terrestrial (i.e. not scansorial or arboreal) earthworm specialist restricted to moist forests above ca 1500 m.
4. Discussion
Paucidentomys vermidax has an extremely long face, bicuspid upper incisors and a lack of chewing teeth, suggesting it is a specialist vermivore. It shares several features with Echiothrix, including the lack of pterygoid plates, very small coronoid processes and the general conformation of the basicranial region (see the electronic supplementary material, figure S2). Therefore, we suspect that Echiothrix and Paucidentomys are sister taxa. Ecologically, Paucidentomys is probably very similar to Rhynchomys. However, Rhynchomys is nested within an endemic radiation of Philippine shrew-rats [16] and is only a distant relative of Melasmothrix [13], the only Sulawesi shrew-rat yet included in phylogenetic studies. Therefore, it is unlikely that Rhynchomys and Paucidentomys are close relatives; their similarity is almost certainly the result of convergence. Both lineages are found in moist, high-elevation forests on tropical islands ([17,18], this study). They share a long, slender rostrum, procumbent lower incisors and the inability to gnaw. Rhynchomys retains molars, but they are greatly reduced and probably are of little functional consequence. As such, these genera appear to be approximate ecological equivalents, the result of convergent evolution in isolated but similar environments, suggesting a role for ecological opportunity in shaping morphological evolution.
On both Luzon and Sulawesi, potential competitors are present in the form of other shrew-rats and true shrews belonging to the genus Crocidura. Only one species of Crocidura is known from Luzon, but as many as nine species have been reported from Sulawesi [19]. However, it remains an open question whether shrews and shrew-rats interact competitively on either island.
Both Rhynchomys and Paucidentomys have lost the evolutionary innovations associated with gnawing, which presumably unlocked new ecological opportunity by allowing the efficient exploitation of soft-bodied prey in moist forests. The extreme facial elongation, with reduction or loss of molars and muscle attachment points associated with chewing, suggests a functional shift in the way food is procured and processed by the rodent mouth [20]. With the transition to soft-bodied prey, the mouth was relieved of the need to process food by chewing, and therefore was free to evolve according to the pressures of food acquisition. Many morphological features of animals are constrained by balancing selection because they perform multiple tasks. For example, the evolution of the pharyngeal jaws in cichlid fish relieved the mouth of the need to chew, perhaps facilitating diversification in oral jaws [1]. Paucidentomys and Rhynchomys use the mouth primarily for procuring food, not processing it, perhaps reducing the constraining influences of balancing selection and allowing the evolution of their unusual morphologies. These strange animals highlight the role of convergence in producing similar body plans under similar ecological circumstances, and the opportunistic nature of evolution, allowing the loss of previously successful evolutionary innovations.
Acknowledgements
We thank Mardin Sarkam, Rohim, Papa Daud, Gherzon, Jhon, Joni and Pailin for assistance with fieldwork, and Richard Marchant for help identifying stomach contents. The Ontario Ministry of Research and Innovation, National Science Foundation (OISE 0965856) and Jim Patton provided financial support. Rafe Brown, Erich Fitzgerald, Lawrence Heaney, Guy Musser, Jim Patton and three anonymous reviewers provided helpful comments and discussion. Sean Maher and Rebecca Banasiak helped generate figures. We thank the staff of AMNH, FMNH and MZB for support and access to specimens.
References
- 1.Hunter J. P. 1998. Key innovations and the ecology of macroevolution. Trends Ecol. Evol. 13, 31–36 10.1016/S0169-5347(97)01273-1 (doi:10.1016/S0169-5347(97)01273-1) [DOI] [PubMed] [Google Scholar]
- 2.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University [Google Scholar]
- 3.Losos J. B. 2010. Adaptive radiation, ecological opportunity, and evolutionary determinism. Am. Nat. 175, 623–639 10.1086/652433 (doi:10.1086/652433) [DOI] [PubMed] [Google Scholar]
- 4.Kassen R., Llewellyn M., Rainey P. B. 2004. Ecological constraints on diversification in a model adaptive radiation. Nature 431, 984–988 10.1038/nature02923 (doi:10.1038/nature02923) [DOI] [PubMed] [Google Scholar]
- 5.Carleton M. D., Musser G. G. 2005. Order Rodentia In Mammal species of the world, 3rd edn (eds Wilson D. E., Reeder D. M.), pp 745–1600 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 6.Brown J. H., Heske E. J. 1990. Control of a desert-grassland transition by a keystone rodent guild. Science 250, 1705–1707 10.1126/science.250.4988.1705 (doi:10.1126/science.250.4988.1705) [DOI] [PubMed] [Google Scholar]
- 7.Davidson A. D., Lightfoot D. C. 2007. Interactive effects of keystone rodents on the structure of desert grassland arthropod communities. Ecography 30, 515–525 10.1111/j.0906-7590.2007.05032.x (doi:10.1111/j.0906-7590.2007.05032.x) [DOI] [Google Scholar]
- 8.Grinnell J. 1923. The burrowing rodents of California as agents in soil formation. J. Mammal. 4, 200–205 10.2307/1373562 (doi:10.2307/1373562) [DOI] [Google Scholar]
- 9.Reichman O. J., Seabloom E. W. 2002. The role of pocket gophers as subterranean ecosystem engineers. Trends Ecol. Evol. 17, 44–49 10.1016/S0169-5347(01)02329-1 (doi:10.1016/S0169-5347(01)02329-1) [DOI] [Google Scholar]
- 10.Ungar P. S. 2010. Mammal teeth: origin, evolution, and diversity. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 11.Musser G. G. 1982. Results of the Archbold expeditions. no. 110. Crunomys and the small-bodied shrew-rats native to the Philippine Islands and Sulawesi (Celebes). Bull. Am. Mus. Nat. Hist. 174, 1–95 [Google Scholar]
- 12.Musser G. G., Durden L. A. 2002. Sulawesi rodents: description of a new genus and species of Murinae (Muridae, Rodentia) and its parasitic new species of sucking louse (Insecta, Anoplura). Am. Mus. Nov. 3368, 1–50 (doi:10.1206/0003-0082(2002)368<0001:SRDOAN>2.0.CO;2) [DOI] [Google Scholar]
- 13.Rowe K. C., Reno M. L., Richmond D. M., Adkins R. M., Steppan S. J. 2008. Pliocene colonization and adaptive radiations in Australia and New Guinea (Sahul): Multilocus systematics of the old endemic rodents (Muroidea: Murinae). Mol. Phylogenet. Evol. 47, 84–101 10.1016/j.ympev.2008.01.001 (doi:10.1016/j.ympev.2008.01.001) [DOI] [PubMed] [Google Scholar]
- 14.Helgen K. M., Helgen L. E. 2009. Biodiversity and biogeography of the moss-mice of New Guinea: A taxonomic revision of Pseudohydromys (Muridae: Murinae). Bull. Am. Mus. Nat. Hist. 331, 230–313 10.1206/582-8.1 (doi:10.1206/582-8.1) [DOI] [Google Scholar]
- 15.Musser G. G., Durden L. A., Holden M. E., Light J. E. 2009. Systematic review of endemic Sulawesi squirrels (Rodentia, Sciuridae), with descriptions of new species of associated sucking lice (Insecta, Anoplura), and phylogenetic and zoogeographic assessments of sciurid lice. Bull. Am. Mus. Nat. Hist. 339, 1–260 10.1206/695.1 (doi:10.1206/695.1) [DOI] [Google Scholar]
- 16.Jansa S. A., Barker F. K., Heaney L. R. 2006. The pattern and timing of diversification of Philippine endemic rodents: evidence from mitochondrial and nuclear gene sequences. Syst. Biol. 55, 73–88 10.1080/10635150500431254 (doi:10.1080/10635150500431254) [DOI] [PubMed] [Google Scholar]
- 17.Balete D. S., Rickart E. A., Rosell-Ambal R. G., Jansa S., Heaney L. R. 2007. Descriptions of two new species of Rhynchomys Thomas (Rodentia: Muridae: Murinae) from Luzon Island, Philippines. J. Mammal. 88, 287–301 10.1644/06-MAMM-A-090R.1 (doi:10.1644/06-MAMM-A-090R.1) [DOI] [Google Scholar]
- 18.Rickart E. A., Heaney L. R., Balete D. S., Tabaranza B. R., Jr 2011. Small mammal diversity along an elevational gradient in northern Luzon, Philippines. Mammal. Biol. 76, 12–21 10.1016/j.mambio.2010.01.006 (doi:10.1016/j.mambio.2010.01.006) [DOI] [Google Scholar]
- 19.Esselstyn J. A., Timm R. M., Brown R. M. 2009. Do geological or climatic processes drive speciation in dynamic archipelagos? The tempo and mode of diversification in Southeast Asian shrews. Evolution 63, 2595–2610 10.1111/j.1558-5646.2009.00743.x (doi:10.1111/j.1558-5646.2009.00743.x) [DOI] [PubMed] [Google Scholar]
- 20.Samuels J. X. 2009. Cranial morphology and dietary habits of rodents. Zool. J. Linn. Soc. 156, 864–888 10.1111/j.1096-3642.2009.00502.x (doi:10.1111/j.1096-3642.2009.00502.x) [DOI] [Google Scholar]