Abstract
Immobilization of insects is necessary for various experimental purposes, and CO2 exposure remains the most popular anaesthetic method in entomological research. A number of negative side effects of CO2 anaesthesia have been reported, but CO2 probably brings about metabolic modifications that are poorly known. In this work, we used GC/MS-based metabolic fingerprinting to assess the effect of CO2 anaesthesia in Drosophila melanogaster adults. We analysed metabolic variation of flies submitted to acute CO2 exposure and assessed the temporal metabolic changes during short- and long-term recovery. We found that D. melanogaster metabotypes were significantly affected by the anaesthetic treatment. Metabolic changes caused by acute CO2 exposure were still manifested after 14 h of recovery. However, we found no evidence of metabolic alterations when a long recovery period was allowed (more than 24 h). This study points to some metabolic pathways altered during CO2 anaesthesia (e.g. energetic metabolism). Evidence of short-term metabolic changes indicates that CO2 anaesthesia should be used with utmost caution in physiological studies when a short recovery is allowed. In spite of this, CO2 treatment seems to be an acceptable anaesthetic method provided that a long recovery period is allowed (more than 24 h).
Keywords: Drosophila, anaesthesia, GC–MS, metabolic fingerprinting, recovery
1. Introduction
To paralyse insects for various purposes, several anaesthetic methods are used, including chilling, etherization and carbon dioxide (CO2). Among these, CO2 anaesthesia is routinely used for colony maintenance, identification of specimens, surgery, dsRNA injection and sexing of flying insects [1,2]. A number of negative side effects of CO2 have been reported on thermal, reproductive and behavioural traits, some being manifested several hours or days after the CO2 exposure [2–4]. However, the impact of this anaesthetic method on physiological traits remains poorly known [2,5].
CO2 anaesthesia constitutes a fairly stressful treatment because it increases haemolymph acidity and causes the heartbeat to stop [2,6]. Exposure to pure CO2 thus impairs oxygen delivery to the tissues, compromising oxidative phosphorylation and ATP production in cell mitochondria [7]. During the recovery, the heart rate accelerates rapidly to a maximum value above that measured before anaesthesia [2]. Rapid paralysis results from a failure in synaptic transmission at the skeletal neuromuscular junction due to CO2 [6]. In addition, acute CO2 exposure likely brings about modifications in metabolic pathways whose interactions are poorly known [2]. Compared with the amount of work describing the negative side effects of CO2 on insect's performance and behaviour, little effort has been made to determine the metabolic response during acute CO2 exposure. In addition, it is of paramount importance to determine whether CO2 anaesthesia has persistent metabolic effects, as this may confound studies of physiological responses if unaccounted for.
In the present study, we used GC/MS-based metabolic fingerprinting to assess the effect of CO2 anaesthesia on Drosophila melanogaster females. We analysed metabolic trajectories in order to understand (i) the degree to which the metabolome is altered by an acute CO2 exposure and (ii) the pattern of homeostatic response during short- and long-term recovery.
2. Material and methods
(a). Fly culture
We conducted our experiments on a mass-bred D. melanogaster line derived from mixing two wild populations collected in October 2010 at Plancoët and Rennes (Brittany, France). The flies were maintained in the laboratory for 15 generations when tested, using a previously described rearing method [8].
(b). Treatments and CO2 anaesthesia
The treatments consisted of (i) untreated control (C), (ii) CO2 stress (S) and (iii) recovery post-CO2 stress for 1, 4 and 14 h (R1, R4 and R14). In order to check for any circadian changes in metabolic concentrations [9], a set of time-of-day untreated controls was also tested at 0, 1, 4 and 14 h (C0, C1, C4 and C14), starting at 08.00. Potential persistent metabolic changes following CO2 anaesthesia were also assessed through additional tests. For this, we compared metabolic profiles among the control (C0) and recovery periods of 24 and 48 h post-CO2 treatment (R24 and R48). Only females were used in these experiments. They were sexed visually with an aspirator. For each condition, 10 biological replicates consisting of a pool of 15 females were used for metabolic fingerprinting. For the additional tests, four replicates were tested. The CO2 treatment was conducted in a 42 ml glass vial, which was flushed with pure CO2 (N45, Air Liquide, France) from a compressed canister (or air pump for control), using a regulator with a 1.5 mm diameter glass needle tip inserted into a foam plug. The vial containing flies was sealed with parafilm and left undisturbed for 7 min with continuous CO2 flux. CO2-stressed flies were sampled at the end of CO2 exposure. For each treatment, females were directly snap-frozen in liquid nitrogen and stored at −80°C until metabolites extraction. Flies took about 6–10 min to recover from 7 min CO2 anaesthesia on food.
(c). Metabolic fingerprinting
We used a GC–MS platform to measure metabolites from the whole insect body as described by Colinet et al. [8]. Briefly, metabolites were extracted using methanol–chloroform (2 : 1) and then derivatized with methoxyamine HCl in pyridine followed by N-Methyl-N-trifluoroacetamide (MSTFA). MS detection was achieved using selective ion monitoring (SIM) mode. Quantification was based on calibration curves obtained from pure reference compounds. The system consisted of a CTC CombiPal autosampler (GERSTEL GmbH & Co. KG, Germany), a Trace GC Ultra chromatograph and a Trace DSQII quadrupole MS (Thermo Fischer Inc., USA).
(d). Statistical analyses
The levels of individual metabolites were compared with one-way ANOVAs. Dunnett's comparisons were used to compare treated values with the control ones. Bonferroni's corrections for multiple comparisons were applied. Metabolic differences among classes were also investigated using a linear discriminant analysis (LDA). Statistical significance of LDA was checked using the Monte Carlo test. Analyses were performed using the ‘ade4’ library in the statistical software R v. 2.13.0.
3. Results
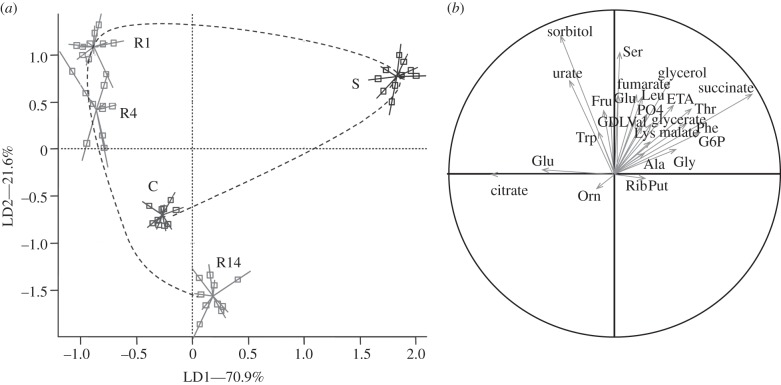
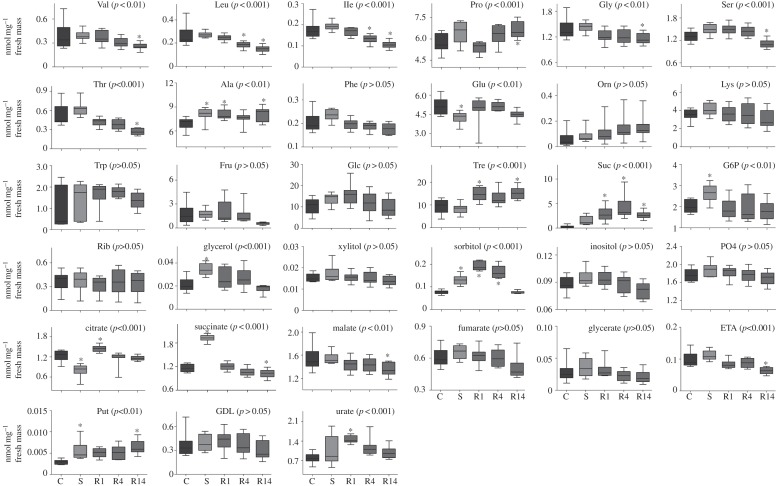
Thirty-three metabolites were detected and quantified in the samples (see the electronic supplementary material, table S1). Four metabolites showed a significant temporal variation among time-of-day controls (xylitol, proline, trehalose and sucrose; electronic supplementary material, table S2), and were thus discarded from further analyses because of their changes unrelated to CO2 treatment. The distribution of the samples in the space formed by the two first linear discriminant axes (figure 1a) showed a significant clustering effect according to the different classes (Monte Carlo test, simulated p-value < 0.001). The first discriminant axis (LD1) accounted for 70.9 per cent of the total inertia. LD1 was characterized by a clear-cut opposition between treatment S and the other treatments. The compounds that contributed the most to the structure separation were succinate and glucose-6-phosphate (G6P) that were positively correlated with LD1, whereas citrate and glutamate were negatively correlated with LD1 (figure 1b and the electronic supplementary material, table S3). LD2 accounted for 21.6 per cent of the total inertia and was characterized by a temporal pattern opposing R1–R14. The compounds contributing the most to this separation were sorbitol, serine (Ser) and urate (figure 1b and electronic supplementary material, table S3). Treatment variations of individual metabolites are illustrated in figure 2. In addition, we found no evidence of metabolic variations between control and stressed flies when a long recovery period was allowed (see the electronic supplementary material, table S4). This was further supported by LDA, which showed no clustering effect among classes (C0, R24, R48) (Monte Carlo test, simulated p-value = 0.329; figure 3).
Figure 1.
(a) Projection of samples onto the first discriminant plane of the LDA. Lines link samples to the centroid of their group (n = 10). Control flies (C), CO2-stressed flies (S) and recovering flies assessed 1, 4 and 14 h following CO2 treatment (R1, R4 and R14). The dotted line indicates the temporal succession of treatments. (b) Projection of variables on the correlation circle. Refer to electronic supplementary material, table S1 for compound abbreviations and electronic supplementary material, table S3 for correlation values.
Figure 2.
Box plots illustrating treatment and temporal variations of all individual metabolites detected in the D. melanogaster metabolome. A box consists of upper and lower hinges and a centre line corresponding to the 25th percentile, the 75th percentile and the median, respectively. Data are based on 10 replicates (n = 10). Control flies (C), CO2-stressed flies (S) and recovering flies assessed 1, 4 and 14 h following CO2 treatment (R1, R4 and R14). For each compound, statistical outcome is summarized within the figure (ANOVA1: p-value) and symbols (asterisks) indicate when a mean was significantly different from control (Dunnett's test). Bonferroni's correction for multiple comparisons was applied.
Figure 3.
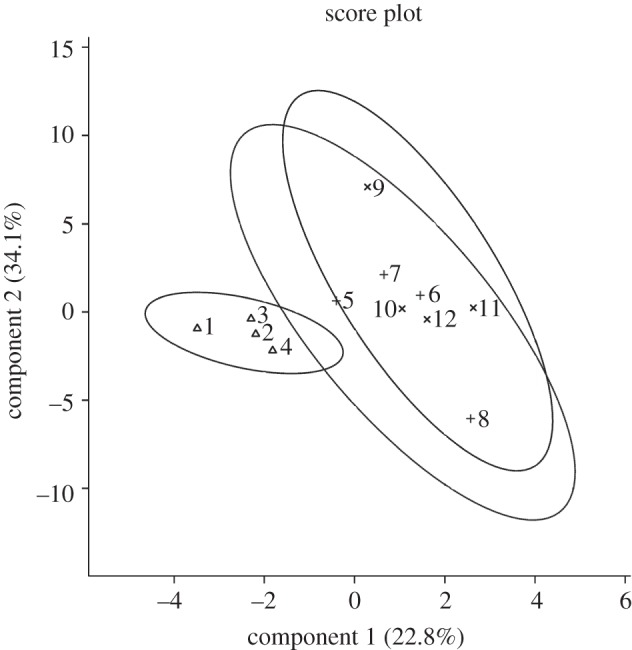
Projection of samples onto the first discriminant plane of the LDA. Control flies (C0, symbol Delta) and recovering flies assessed 24 and 48 h following CO2 treatment (R24, symbol ‘plus’ and R48, symbol ‘multiplication’; n = 4).
4. Discussion
The most important metabolic changes detected during CO2 stress were the accumulation of succinate and G6P, whereas citrate was downregulated with respect to control flies. During CO2 anaesthesia, the heartbeat stops, and oxygen delivery is markedly impaired generating anoxic conditions. Oxidative phosphorylation and ATP production are thus compromised [2,7]. Moreover, pure CO2 directly inhibits several metabolic enzymes such as succinate dehydrogenase (SDH) [10] and NADPH-producing enzymes [11]. The accumulation of succinate thus likely relates to the inhibition of SDH. In the almond moth Ephestia cautella, hypoxia and CO2 block the entrance of glycolysis products into tricarboxylic acid cycle (TCA) cycle, which results in reduction of citrate concentration [7]. Likewise, we found that citrate concentration decreased in parallel with the succinate increase during the CO2 stress. A drastic reduction in ATP level with CO2 treatment has been reported, suggesting that the phosphorylation system is affected in E. cautella [7]. The increase in G6P observed during CO2 anaesthesia may suggest that cells are forced to depend on glycolysis for ATP production. It is not clear whether these metabolic alterations resulted from the direct effect of CO2 or from anoxia or a combination thereof. Whatever the underlying causative mechanisms, it clearly appears that a consequence of CO2 anaesthesia is an alteration of energetic metabolism.
In this study, all flies quickly recovered from acute CO2 exposure. This is true from a mobility standpoint; however, we found that metabolic profiles remained different from controls for several hours (up to 14 h). During the recovery period, concentrations of several metabolites were altered relative to controls. The metabolites contributing the most to the temporal pattern during the recovery were sorbitol, serine and urate. These metabolites had a high concentration in 1 h-recovering flies followed by a temporal reduction during the recovery. Upon reperfusion, the cells experience sudden oxygen influxes, which create a burst in reactive oxygen species (ROS) production [12,13]. Urate is an important scavenger of oxygen radicals [14], and the importance of urate in oxygen defence has been unambiguously demonstrated in D. melanogaster [15]. The occurrence of this biomarker of free radical damage indicates that oxidative damage probably occurred during the recovery post-CO2 exposure. Likewise, polyols (such as sorbitol) are effective ROS scavengers in insects [16]. Sorbitol is also considered to be an anaerobic end product in insects [17]. The causative roles of these metabolic variations are not known, but these modulations clearly highlight that important metabolic changes take place during recovery from CO2 anaesthesia.
This study brings evidence for detrimental effects of CO2 anaesthesia on physiological traits. Metabolic disturbance caused by CO2 was still manifested after 14 h of recovery. However, we found no evidence of system-wide metabolic changes when a long recovery period was allowed (more than 24 h). Thus, CO2 anaesthesia should be used with caution in physiological studies, especially if only short-term recovery is allowed. We suggest that CO2 treatment is an acceptable anaesthetic method provided that a long recovery period (more than 24 h) is allowed. On the basis of the present metabolic profiling data, we corroborate the notion that at least 24 h recovery is necessary to avoid side effects of CO2 anaesthesia [4]. Further comparisons with other types of anaesthetics such as chilling, nitrogen or ether would help us to elucidate whether the metabolic disturbances seen in this study are shared across several forms of anaesthesia or specific to CO2.
Acknowledgements
This study was supported by Fonds de la Recherche Scientifique FRS–FNRS. This paper is no. BRC244 of the Biodiversity Research Centre (ELIB). We are grateful to Vanessa Larvor for technical help.
References
- 1.Ashburner M., Golic K. G., Hawley R. S. 2005. Drosophila. A laboratory handbook. New York, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- 2.Nicolas G., Sillans D. 1989. Immediate and latent effects of carbon dioxide on insects. Annu. Rev. Entomol. 34, 97–116 10.1146/annurev.en.34.010189.000525 (doi:10.1146/annurev.en.34.010189.000525) [DOI] [Google Scholar]
- 3.Nilson T. L., Sinclair B. J., Robert S. P. 2006. The effects of carbon dioxide anaesthesia and anoxia on rapid cold-hardening and chill coma recovery in Drosophila melanogaster. J. Insect Physiol. 52, 1027–1033 10.1016/j.jinsphys.2006.07.001 (doi:10.1016/j.jinsphys.2006.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champion de Crespigny F. E., Wedell N. 2008. The impact of anaesthetic technique on survival and fertility in Drosophila. Physiol. Entomol. 33, 310–315 10.1111/j.1365-3032.2008.00632.x (doi:10.1111/j.1365-3032.2008.00632.x) [DOI] [Google Scholar]
- 5.Milton C. C., Partridge L. 2008. Brief carbon dioxide exposure blocks heat hardening but not cold acclimation in Drosophila melanogaster. J. Insect Physiol. 54, 32–40 10.1016/j.jinsphys.2007.08.001 (doi:10.1016/j.jinsphys.2007.08.001) [DOI] [PubMed] [Google Scholar]
- 6.Badre N. H., Martin M. E., Cooper R. L. 2005. The physiological and behavioral effects of carbon dioxide on Drosophila melanogaster larvae. Comp. Biochem. Physiol. A 140, 363–376 10.1016/j.cbpb.2005.01.019 (doi:10.1016/j.cbpb.2005.01.019) [DOI] [PubMed] [Google Scholar]
- 7.Friedlander A., Navarro S. 1979. The effect of controlled atmospheres on carbohydrate metabolism in the tissue of Ephestia cautella (walker) pupae. Insect Biochem. 9, 79–83 10.1016/0020-1790(79)90029-5 (doi:10.1016/0020-1790(79)90029-5) [DOI] [Google Scholar]
- 8.Colinet H., Larvor V., Laparie M., Renault D. 2012. Exploring the plastic response to acclimation through metabolomics. Funct. Ecol. 26, 711–722 10.1111/j.1365-2435.2012.01985.x (doi:10.1111/j.1365-2435.2012.01985.x) [DOI] [Google Scholar]
- 9.Seay D. J., Thummel C. S. 2011. The circadian clock, light, and cryptochrome regulate feeding and metabolism in Drosophila. J. Biol. Rhythms 26, 497–506 10.1177/0748730411420080 (doi:10.1177/0748730411420080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards J. 1968. Carbon dioxide anaesthesia and succinic dehydrogenase in the corn earworm, Heliothis zea. J. Insect Physiol. 14, 1045–1048 10.1016/0022-1910(68)90041-3 (doi:10.1016/0022-1910(68)90041-3) [DOI] [Google Scholar]
- 11.Friedlander A., Navarro S., Silhacek D. L. 1984. The effect of carbon dioxide on NADPH production in Ephestia cautella (Wlk.) pupae. Comp. Biochem. Physiol. B 77, 839–842 10.1016/0305-0491(84)90321-3 (doi:10.1016/0305-0491(84)90321-3) [DOI] [Google Scholar]
- 12.Coquin L., Feala J. D., McCuloch A. D., Paternostro G. 2008. Metabolomic and flux-balance analysis of age-related decline of hypoxia tolerance in Drosophila muscle tissue. Mol. Syst. Biol. 4, 233. 10.1038/msb.2008.71 (doi:10.1038/msb.2008.71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lighton J. R. B., Schilman P. E. 2007. Oxygen reperfusion damage in an insect. PLoS ONE 2, e1267. 10.1371/journal.pone.0001267 (doi:10.1371/journal.pone.0001267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glantzounis G. K., Tsimoyiannis E. C., Kappas A. M., Galaris D. A. 2005. Uric acid and oxidative stress. Curr. Pharm. Design 11, 4145–4151 10.2174/138161205774913255 (doi:10.2174/138161205774913255) [DOI] [PubMed] [Google Scholar]
- 15.Hilliker A. J., Duyf B., Evans D., Phillips J. P. 1992. Urate-null rosy mutants of Drosophila melanogaster are hypersensitive to oxygen stress. Proc. Natl Acad. Sci. USA 89, 4343–4347 10.1073/pnas.89.10.4343 (doi:10.1073/pnas.89.10.4343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felton G. W., Summers C. B. 1995. Antioxidant systems in insects . Arch. Insect Biochem. Physiol. 29, 187–197 10.1002/arch.940290208 (doi:10.1002/arch.940290208) [DOI] [PubMed] [Google Scholar]
- 17.Storey K. B., Wilkie D. R., Cairns A., Thomas H., Cowan D. A., Baust J., Pullin A. S., Russell N. J., Jaenicke R. 1990. Biochemical adaptation for cold hardiness in insects. Phil. Trans. R. Soc. Lond. B 326, 635–654 10.1098/rstb.1990.0036 (doi:10.1098/rstb.1990.0036) [DOI] [Google Scholar]