Abstract
At the end of May, 17 scientists involved in an EU COST Action on Conservation Physiology of Marine Fishes met in Oristano, Sardinia, to discuss how physiology can be better used in modelling tools to aid in management of marine ecosystems. Current modelling approaches incorporate physiology to different extents, ranging from no explicit consideration to detailed physiological mechanisms, and across scales from a single fish to global fishery resources. Biologists from different sub-disciplines are collaborating to rise to the challenge of projecting future changes in distribution and productivity, assessing risks for local populations, or predicting and mitigating the spread of invasive species.
Keywords: conservation physiology, species distribution, modelling, climate effects
1. Introduction
The marine environment is changing at an unprecedented rate due to natural and anthropogenic changes (warming, acidification, fishing, eutrophication, hypoxia and pollutants [1,2]). In recent decades, climate warming has generally caused poleward shifts in distribution [3], and evidence is mounting of changes in predator–prey relationships affecting ecosystem dynamics [4]. The physiologist investigates how individual fish are affected by changing environments, whereas environmental managers, politicians and stakeholders are more concerned about how these changes will affect species, resources, ecosystems and human societies. Connecting these different perspectives requires tools that properly scale individual-level responses to population-level consequences, and which can harness physiological principles to gain a cause-and-effect understanding of environmental change on fishes [5,6]. Our strategy for advancing these tools was to facilitate collaborations between physiologists, ecologists, experimentalists and modellers.
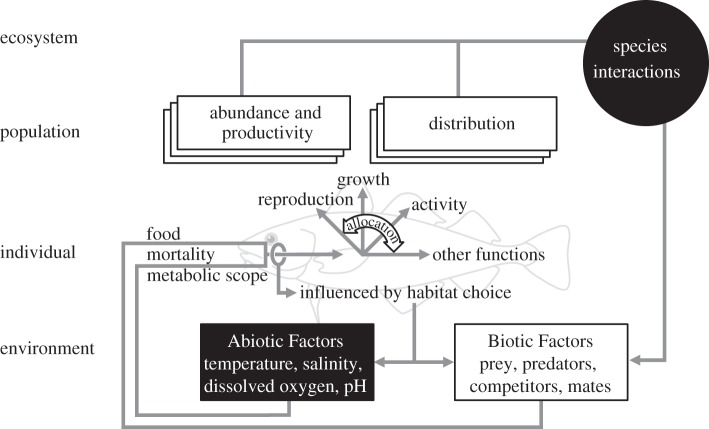
The main objective of the EU COST Action on Conservation Physiology of Marine Fishes (http://fish-conservation.nu/) is to coordinate European research efforts on the physiological mechanisms that determine distribution and abundance of marine fishes (figure 1), including invasive species, and so contribute to sustainable management of biodiversity and fishery resources. A wide range of models and topics were discussed at the meeting, spanning several levels of biological complexity (tissue, organism, population and ecosystem) and allowing broad evaluation of how fish physiology could be integrated into models. Here, we provide a brief summary of these discussions.
Figure 1.
Schematic of how metabolic scope is a key link between environmental changes, such as climate warming and effects at the level of the population, species or marine ecosystem.
2. Global bioclimate models with environmental envelopes
How global change will affect species distributions and productivity depends on both the severity of local changes and the sensitivity of local species. Cheung et al. [7] quantified thermal niches and habitat preferences of some 1000 species by overlaying observed distributions with current maps of temperature and other environmental conditions. Spatial shifts in distribution and changes in fisheries catch potential were projected by merging these niches with outputs from global climate change models, including species dispersal and changes in phytoplankton productivity. Subsequently, using a simple conceptual model of how environmental factors affect growth, maximum body size and other life-history characteristics, one may project effects of temperature, oxygen and acidity on future fish distribution and abundance, with implications for fisheries [8].
3. Resolving temporal and spatial scales
To be computationally feasible, global models rely on coarse spatial grids and sometimes annual time steps. When projecting changes within a regional sea or a single ecosystem, temporal and spatial resolution of models can (and must) be much finer. Shorter model time steps (hours to minutes) and finer spatial resolution allow mesoscale hydrographic features (2–200 km) important to biological processes to be represented (e.g. tides, fronts and eddies). These temporal and spatial scales also match better with individual-level processes where physiology can translate local environmental factors into performance metrics, such as growth and survival. These models demand more detailed physiological knowledge, such as species-specific rates of respiration, consumption and digestion [9,10]. A single physiological trait with much ecological relevance may be scope for aerobic activity (also termed metabolic scope), the ability to provide oxygen for energy-using activities, such as locomotion, digestion, tissue repair and turnover [11]. Such detailed information can be directly useful to managers, for example, as maps of quantitative physiological traits and how these vary on daily and seasonal timescales, at local geographical scales, or between different adjacent habitats [12].
These smaller-scale models need to deal with increasingly complex aspects of physiology, for example, cues for movement. For larvae, hydrodynamic, particle tracking and physiological-based foraging and growth modules are often coupled to estimate the three-dimensional trajectory of environments experienced by larvae, often revealing key processes affecting survival and year-class (recruitment) success [13]. The vertical swimming behaviour of larvae may be tailored to specific environmental preferences, food abundance or individual state such as size or satiation [14], and can greatly influence modelled outcomes. In larger organisms, horizontal movements must also be accounted for. By translating local environmental gradients into gradients of physiological performance, movement rules using only local information can be devised, and their consequences for species distributions compared with observations [15]. Differences in behavioural strategy cause different environments to be experienced among individuals, contributing to variation in growth and survival.
For models at this regional or ecosystem level, fisheries institutions routinely collect monitoring data on species distributions, abundance, age and size composition and trophic interactions. All of this information, plus fishermen's knowledge [16], can be used either directly to parametrize physiological functions or indirectly to provide estimates of unknown physiological variables [17].
4. Behavioural ecology connects environment and performance
Although regional, bio-physically coupled models have higher temporal and spatial resolution than global models, simplifications are needed to represent individual responses to the local environment. Important behaviours may occur very infrequently and within short time windows, or depend on rare events such as predation attempts [18]. The relationships between environmental variables and species responses can emerge within physiologically based behavioural models. As an example, models, including prey and predator environments may yield insights into optimal foraging ecology and risk-taking behaviour [19] in situations where changes in food availability not only affect growth but also risk-taking and therefore individual survival.
Recent developments in sensors and data storage tags promise exciting insights into highly detailed individual behaviour in wild fish [20]. Accelerometers can be calibrated to estimate metabolic rates and swimming patterns, magnetic sensors on the jaws can detect foraging episodes, pressure sensors record vertical behaviour, etc. The potential to couple temporally resolved behavioural and physiological data, also within models, is particularly appealing.
5. The adapted organism
An important question related to environmental change is: will species be able to adapt to the new environments or will they become locally extinct? At a most fundamental level, organisms adapt to environmental changes through evolutionary changes (slowly) or there can be phenotypically plastic responses (faster). A related question is: how will the strength of trophodynamic coupling change if predators and prey exhibit markedly different physiological responses to environmental change [6]? Individual growth rate is commonly used as a proxy for fitness, but growth is only one process competing for the resources available to an organism [21]. The performances that experimental physiologists quantify in controlled laboratory experiments, such as aerobic scope, are complex traits that reflect more fundamental physiological and biochemical processes that may have evolved within specific environmental and ecological contexts. Examples of questions one can ask are what causes scaling relationships [22,23], and do metabolic differences relate to diet specialization [24]?
6. A hierarchy of models
The above demonstrates that models can be arranged in a hierarchy, from global models revealing general patterns to specific projections for individuals in their habitat, and how physiological knowledge can be infused at every level to refine model predictions. Furthermore, detailed models can test implicit assumptions of more general models. Scaling from smaller to larger spatial scales may also be possible via coupling models. For example, estimates of larval survival from local, risk-based foraging models can be input to bio-physical models of drift, which in turn can be implemented as recruitment modules within global models of fish productivity. In this way, physiological-based mechanistic effects within individuals can be systematically scaled up to consequences at the population level, while being consistent about the role of behaviour. With this in mind, the value of incorporating physiology should always be assessed relative to null models without physiology. For example, a metric of the horizontal velocity a species would need to move to stay within the same thermal niche can be mapped simply as the expected rate of change in surface temperature divided by the local spatial gradient in temperature [25]. Such projections can be directly compared with those of physiologically driven bioclimate envelope models [7] to reveal the effect of incorporating species-level information on predicted changes.
7. Meeting outcomes
Our discussions indicated that (i) modellers should acquaint themselves with the details of other types of models (including null models) to understand how specific (complex) models might be compared or coupled to more general (simpler) models to test and refine tools, (ii) physiologists should consider the scale at which their knowledge can best be applied, such as accepting more approximations in the general models, (iii) an important advancement will be projecting how physiological changes in predators and their prey will affect the functioning of food webs, and (iv) cross-disciplinary discussions that may be painful at first (owing to differences in vocabulary and jargon) will ultimately be rewarding and, in our case, provided an essential first step towards building better models for conservation physiology of marine fishes.
Acknowledgments
CNR–IAMC (National Research Council, Institute for Coastal Marine Environment), Oristano, Sardinia, is thanked for great hospitality. Funding was provided by EU COST action FA1004. For more information, see http://fish-conservation.nu.
References
- 1.IPCC. 2007. Climate change 2007: the physical science basis. Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Doney S. C. 2010. The growing human footprint on coastal and open-ocean biogeochemistry. Science 328, 1512–1516 10.1126/science.1185198 (doi:10.1126/science.1185198) [DOI] [PubMed] [Google Scholar]
- 3.Perry A. L., Low P. J., Ellis J. R., Reynolds J. D. 2005. Climate change and distribution shifts in marine fishes. Science 308, 1912–1915 10.1126/science.1111322 (doi:10.1126/science.1111322) [DOI] [PubMed] [Google Scholar]
- 4.Möllmann C., Müller-Karulis B., Kornilovs G., St John M. A. 2008. Effects of climate and overfishing on zooplankton dynamics and ecosystem structure: regime shifts, trophic cascade, and feedback loops in a simple ecosystem. ICES J. Mar. Sci. 65, 302–310 10.1093/icesjms/fsm197 (doi:10.1093/icesjms/fsm197) [DOI] [Google Scholar]
- 5.Rijnsdorp A. D., Peck M. A., Engelhard G. H., Möllmann C., Pinnegar J. K. 2009. Resolving the effect of climate change on fish populations. ICES J. Mar. Sci. 66, 1570–1583 10.1093/icesjms/fsp056 (doi:10.1093/icesjms/fsp056) [DOI] [Google Scholar]
- 6.Pörtner H. O., Peck M. A. 2010. Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J. Fish Biol. 77, 1745–1779 10.1111/j.1095-8649.2010.02783.x (doi:10.1111/j.1095-8649.2010.02783.x) [DOI] [PubMed] [Google Scholar]
- 7.Cheung W .W. L., Lam V. W. Y., Sarmiento J. L., Kearney K., Watson R., Zeller D., Pauly D. 2010. Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Glob. Change Biol. 16, 24–35 10.1111/j.1365-2486.2009.01995.x (doi:10.1111/j.1365-2486.2009.01995.x) [DOI] [Google Scholar]
- 8.Cheung W. W. L., Dunne J., Sarmiento J. L., Pauly D. 2011. Integrating ecophysiology and plankton dynamics into projected maximum fisheries catch potential under climate change in the Northeast Atlantic. ICES J. Mar. Sci. 68, 1008–1018 10.1093/icesjms/fsr012 (doi:10.1093/icesjms/fsr012) [DOI] [Google Scholar]
- 9.Herbert N. A., Steffensen J. F. 2005. The response of Atlantic cod, Gadus morhua, to progressive hypoxia: fish swimming speed and physiological stress. Mar. Biol. 147, 1403–1412 10.1007/s00227-005-0003-8 (doi:10.1007/s00227-005-0003-8) [DOI] [Google Scholar]
- 10.Claireaux G., Webber D. M., Lagardère J. P., Kerr S. R. 2000. Influence of water temperature and oxygenation on the aerobic metabolic scope of Atlantic cod (Gadus morhua). J. Sea Res. 44, 257–265 10.1016/S1385-1101(00)00053-8 (doi:10.1016/S1385-1101(00)00053-8) [DOI] [Google Scholar]
- 11.Fry F. E. J. 1971. The effect of environmental factors on the physiology of fish. In Fish physiology, vol. 6 (eds Hoar W. S., Randall D. J.), pp. 1–98 London, UK: Academic Press [Google Scholar]
- 12.Cucco A., Sinerchia M., Lefrançois C., Magni P., Ghezzo M., Umgiesser G., Perilli A., Domenici P. 2012. A metabolic scope based model of fish response to environmental changes. Ecol. Mod. 237–238, 132–141 10.1016/j.ecolmodel.2012.04.019 (doi:10.1016/j.ecolmodel.2012.04.019) [DOI] [Google Scholar]
- 13.Peck M. A., Hufnagl M. 2012. Can IBMs explain why most larvae die in the sea? Model scenarios and sensitivity analyses reveal research needs. J. Mar. Sys. 93, 77–93 10.1016/j.jmarsys.2011.08.005 (doi:10.1016/j.jmarsys.2011.08.005) [DOI] [Google Scholar]
- 14.Fiksen Ø., Jørgensen C., Kristiansen T., Vikebø F., Huse G. 2007. Linking behavioural ecology and oceanography: larval behaviour determines growth, mortality and dispersal. Mar. Ecol. Progr. Ser. 347, 195–205 10.3354/meps06978 (doi:10.3354/meps06978) [DOI] [Google Scholar]
- 15.Teal L.R., van Hal R., van Kooten T., Ruardij P., Rijnsdorp A. In press Bio-energetics underpins the spatial response of North Sea plaice (Pleuronectes platessa L.) and sole (Solea solea L.) to environmental change. Glob. Change Biol. [Google Scholar]
- 16.Azzurro E., Moschella P., Maynou F. 2011. Tracking signals of change in Mediterranean fish diversity based on local ecological knowledge. PLoS ONE 6, e24885. 10.1371/journal.pone.0024885 (doi:10.1371/journal.pone.0024885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kooijman S. A. L. M. 2000. Dynamic energy and mass budgets in biological systems. Cambridge, UK: Cambridge University Press [Google Scholar]
- 18.Domenici P., Claireaux G., McKenzie D. J. 2007. Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms: an introduction. Phil. Trans. R. Soc. B 362, 1929–1936 10.1098/rstb.2007.2078 (doi:10.1098/rstb.2007.2078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiksen Ø., Jørgensen C. 2011. Model of optimal behaviour in fish larvae predicts that food availability determines survival, but not growth. Mar. Ecol. Progr. Ser. 432, 207–219 10.3354/meps09148 (doi:10.3354/meps09148) [DOI] [Google Scholar]
- 20.Metcalfe J. D., Le Quesne W. J. F., Cheung W. W. L., Righton D. A. 2012. Conservation physiology for applied management of marine fish: an overview with perspectives on the role and value of telemetry. Phil. Trans. R. Soc. B 367, 1746–1756 10.1098/rstb.2012.0017 (doi:10.1098/rstb.2012.0017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enberg K., Jørgensen C., Dunlop E. S., Varpe Ø., Boukal D. S., Baulier L., Eliassen S., Heino M. 2012. Fishing-induced evolution of growth: concepts, mechanisms and the empirical evidence. Mar. Ecol. 33, 1–25 10.1111/j.1439-0485.2011.00460.x (doi:10.1111/j.1439-0485.2011.00460.x) [DOI] [Google Scholar]
- 22.Darveau C.-A., Suarez R. K., Andrews R. D., Hochachka P. W. 2002. Allometric cascade as a unifying principle of body mass effects on metabolism. Nature 417, 166–170 10.1038/417166a (doi:10.1038/417166a) [DOI] [PubMed] [Google Scholar]
- 23.Killen S. S., Atkinson D., Glazier D. S. 2010. The intraspecific scaling of metabolic rate with body mass in fishes depends on lifestyle and temperature. Ecol. Lett. 13, 184–193 10.1111/j.1461-0248.2009.01415.x (doi:10.1111/j.1461-0248.2009.01415.x) [DOI] [PubMed] [Google Scholar]
- 24.McNab B. K. 1986. The influence of food habits on the energetics of eutherian mammals. Ecol. Monogr. 56, 1–19 10.2307/2937268 (doi:10.2307/2937268) [DOI] [Google Scholar]
- 25.Burrows M. T., et al. 2011. The pace of shifting climate in marine and terrestrial ecosystems. Science 334, 652–655 10.1126/science.1210288 (doi:10.1126/science.1210288) [DOI] [PubMed] [Google Scholar]