Abstract
Avian influenza viruses (AIVs) pose significant danger to human health. A key step in managing this threat is understanding the maintenance of AIVs in wild birds, their natural reservoir. Ruddy turnstones (Arenaria interpres) are an atypical bird species in this regard, annually experiencing high AIV prevalence in only one location—Delaware Bay, USA, during their spring migration. While there, they congregate on beaches, attracted by the super-abundance of horseshoe crab eggs. A relationship between ruddy turnstone and horseshoe crab (Limulus polyphemus) population sizes has been established, with a declining horseshoe crab population linked to a corresponding drop in ruddy turnstone population sizes. The effect of this interaction on AIV prevalence in ruddy turnstones has also been addressed. Here, we employ a transmission model to investigate how the interaction between these two species is likely to be altered by climate change. We explore the consequences of this modified interaction on both ruddy turnstone population size and AIV prevalence and show that, if climate change leads to a large enough mismatch in species phenology, AIV prevalence in ruddy turnstones will increase even as their population size decreases.
Keywords: avian influenza virus, Delaware Bay, climate change, transmission model
1. Introduction
Avian influenza viruses (AIVs) infect many bird species throughout the world, usually with relatively modest prevalence [1]. In some sites, however, the confluence of biotic and abiotic factors results in the proliferation of AIVs among multiple host species, leading to unusually high prevalence. Surveillance has identified Delaware Bay as such a ‘hotspot’, with virus isolation levels in shorebirds (ruddy turnstones in particular) exceeding cumulative figures across global surveillance sites by more than an order of magnitude [2]. Recent dissection of AIV epidemiology at Delaware Bay has identified the factors that generate this phenomenon [3], with food source (horseshoe crab eggs) availability during shorebird migration considered key. The interaction between these consumer (migratory shorebirds) and resource (horseshoe crabs) species is central to our understanding of both ruddy turnstone population biology and the contribution of these birds to the remarkably high AIV prevalence in Delaware Bay, a site of ‘hemispheric importance’ [2]. It is not surprising, therefore, that understanding and predicting the impact of climate change on horseshoe crab spawning times and bird migration timing [4], and their attendant consequences for AIV maintenance and spread, have been identified as both timely and important.
Horseshoe crabs have, for millions of years [5], existed in large numbers along the mid-Atlantic coast of North America [6]. Each year, adult crabs (more than 9 years old) travel to the beaches of Delaware Bay to spawn and lay their eggs [5], more than 99 per cent of which are lost through predation or exposure to the elements [7]. Declining populations of horseshoe crabs in recent years—owing to a combination of over-harvesting and habitat loss—are hypothesized [8] to account for declining numbers of ruddy turnstones [6] and other shorebirds [8].
Several studies have investigated the possible impact of climate change on bird populations [9–11]. Empirical evidence suggests that long-distance migrants with a seasonal food peak are more likely to experience population declines than ‘residents and short-distance migrants’ [11]. Ruddy turnstones are hypothesized to be impacted by climate-related changes in two distinct ways. Firstly, seasonal migration patterns may shift, with studies suggesting that migrating birds are now leaving their wintering grounds earlier, possibly in response to a changing climate [10]. Secondly, food resource (horseshoe crab eggs) availability may change, with evidence demonstrating that timing of horseshoe crab spawning is influenced by water temperature [12]. Moreover, because these changes in migration and resources are in response to environmental cues from different geographical regions, the predicted heterogeneous impact of climate change across the globe [13] means that their dynamical impacts are likely to be complex, rather than simply additive.
In this paper, we use a mathematical model to explore the consequences of an altered interaction between ruddy turnstones and horseshoe crabs in Delaware Bay as a result of climate change. Employing the most detailed parametrized model available of AIV dynamics in Delaware Bay—a model which includes three host species, seasonal forcing and multiple transmission routes [3]—we explore the consequences of two prominent potential scenarios: (I) climate change impacts migration in ruddy turnstones, altering their arrival date in Delaware Bay and (II) climate change affects horseshoe crab spawning, altering the timing of peak numbers of horseshoe crab's eggs on the Delaware Bay beaches. We show that a change in the availability of horseshoe crabs impacts the prevalence of AIV in ruddy turnstones and further demonstrate the impact of changing ruddy turnstone migration and horseshoe crab spawning on both AIV prevalence and population numbers of ruddy turnstones. We conclude that a small shift in either of these events will not qualitatively affect the dynamics of the system, but that the ecological and epidemiological consequences will be significant with greater mismatch in phenology.
2. Material and methods
We investigated the interaction between ruddy turnstones and horseshoe crabs using a model designed to capture the key interactions occurring in Delaware Bay. The model includes two duck species (one resident in Delaware Bay and the other a short-distance migrant that winters in Delaware Bay) as well as ruddy turnstones, who winter in South America, pause in Delaware Bay on their northbound migration for just under a month, then breed in the Arctic. The model includes seasonal breeding and transmission for all species and has a density-dependent birth rate in ruddy turnstones to reflect their dependence on horseshoe crabs (see below). An environmental component for the virus is also included, with transmission assumed to occur both directly and via the environment [14]. The model is fully described in the electronic supplementary material.
Our focus here is the birth rate from the model for ruddy turnstones: b(t)(1 − θN(t)/E)N(t), where b(t) is the (seasonal) birth rate; θ is a shape parameter; N(t) is the (time-dependent) total ruddy turnstone population size and E is the number of horseshoe crabs. Details for calculating E are given in the electronic supplementary material. To incorporate the seasonality of horseshoe crab spawning (and hence egg availability), we calculated E from an exponential cosine function (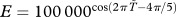 , where
, where 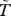 is the midpoint of the ruddy turnstones' stay in Delaware Bay), with maximum E occurring as the ruddy turnstones are usually in Delaware Bay, and declining steeply away from that point. To address scenario I, we model a temporal shift in the ruddy turnstones' arrival in Delaware Bay. A single shift corresponds to a difference of three weeks and we consider two arrival times of either side of the current one. For scenario II, we shift the timing of peak horseshoe crab spawning by a month at a time and consider the two months prior to current spawning timing.
is the midpoint of the ruddy turnstones' stay in Delaware Bay), with maximum E occurring as the ruddy turnstones are usually in Delaware Bay, and declining steeply away from that point. To address scenario I, we model a temporal shift in the ruddy turnstones' arrival in Delaware Bay. A single shift corresponds to a difference of three weeks and we consider two arrival times of either side of the current one. For scenario II, we shift the timing of peak horseshoe crab spawning by a month at a time and consider the two months prior to current spawning timing.
3. Results
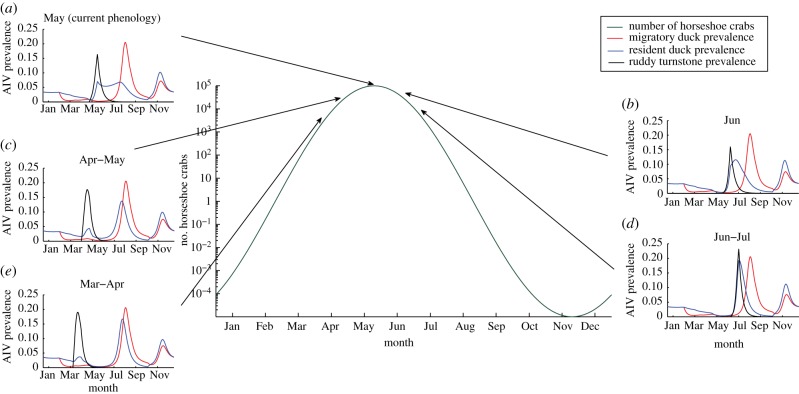
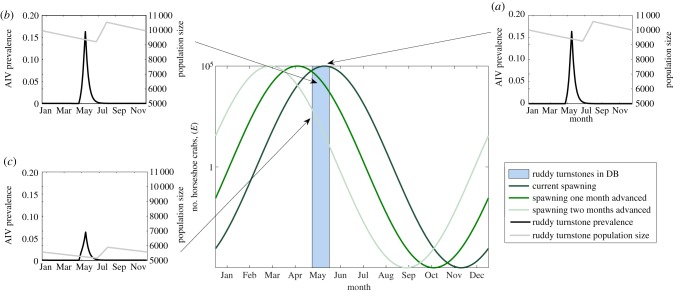
We tested the two potential scenarios outlined in §1. Figure 1 presents the results from scenario I (i.e. changing the timing of ruddy turnstone migration) using our deterministic model. We calculated the number of horseshoe crabs available to the birds when they are in Delaware Bay from the exponential cosine curve given in §2 (for sensitivity to this function see the electronic supplementary material). The main panel of figure 1 shows the function for E, and the offset panels (figure 1a–e) show the prevalence curves in all three hosts resulting from a change in migration timing (changed by three weeks each time).
Figure 1.
Exploring the consequences of potential scenario I. The assumed function for the number of horseshoe crabs, E, against time (main plot). The offset figures (a–e) show prevalence throughout the year for each host species (for the final year of a 500 year simulation), with arrows showing when the ruddy turnstones were present in Delaware Bay.
As ruddy turnstones deviate from their current arrival time in Delaware Bay, AIV prevalence increases (figure 1d,e). Arrival earlier in the year (figure 1e) coincides with a higher AIV prevalence in the resident ducks (and hence higher virion concentration in the environmental reservoir). This, combined with two reasonable assumptions in the model—density-dependent direct transmission and seasonal breeding—leads to a greater number of ruddy turnstones present in Delaware Bay if they migrate a few weeks earlier. The reduction in horseshoe crab eggs, E, is not enough to significantly decrease the ruddy turnstone population size, so transmission is higher compared with later arrival. The latter increase in prevalence (figure 1d) occurs as their timing overlaps with the main peak in prevalence in resident ducks, sparking larger peak prevalences in both species than is otherwise seen. Current evidence from migrating bird species suggests their migration dates are advancing [10]. Applied to our results, this suggests AIV prevalence in ruddy turnstones may increase, all else being equal.
The results from scenario II (i.e. changing the timing of horseshoe crab spawning) are presented in figure 2. The offset figures show the AIV prevalence in ruddy turnstones and their population size in three different scenarios—the current situation and two earlier peaks in horseshoe crab spawning. As in figure 1, we find that for a small alteration in timing, prevalence (and population size) is not greatly affected, but for the earliest peak in horseshoe crab spawning both ruddy turnstone population size and AIV prevalence have declined hugely (figure 2c; for equivalent results for scenario I, see the electronic supplementary material).
Figure 2.
Three functions for peak horseshoe crab spawning, shifted through time (main plot). The offset figures show AIV prevalence and population size in ruddy turnstones for each of these functions (for the final year of a 500 year simulation). The blue bar shows when the tuddy turnstones are in Delaware Bay.
4. Discussion
We considered the consequences of climate change on both AIV prevalence and population sizes in ruddy turnstones. We compared two contrasting potential scenarios—that climate change modifies either ruddy turnstone migration timing or the horseshoe crab spawning season. Qualitatively, our results indicate that, as the mismatch between supply and demand for horseshoe crab eggs increases, so will the ruddy turnstone population decline. An unexpected finding, however, was that a change in the arrival time of ruddy turnstones in Delaware Bay is predicted to generate greater variability in outcomes. From stochastic (Markov process) realizations of the model, we observe a notable increase in AIV extinction frequency (table 1), which clearly dampens the potential for cross-species transmission. However, in instances where AIV persists, prevalence and, as a result, spillover potential to domestic birds, is substantially enhanced (electronic supplementary material, figure S3).
Table 1.
Results of stochastic simulations (150 simulations for each time period). Parameters for stochastic model as in electronic supplementary material, table S1. Extinction probability is calculated as the number of simulations for which AIV does not persist in any of the three host species in the model after 500 years of simulation.
| timing of ruddy turnstone stopover | AIV extinction probability |
|---|---|
| March–April (midpoint 0.2595 yr) | 0.35 |
| April–May (midpoint 0.3225 yr) | 0.12 |
| May (midpoint 0.3855 yr) | 0.05 |
| June (midpoint 0.4485 yr) | 0.25 |
| June–July (midpoint 0.5155 yr) | 0.41 |
This research has exposed the potential reduction in population size faced by North American ruddy turnstones. In addition to current anthropogenic factors, such as harvesting of horseshoe crabs [8], which directly affect resource supply, ruddy turnstones are vulnerable to the effects of climate-driven shifts in phenological synchrony of horseshoe crab spawning and ruddy turnstone spring migration. These events, in turn, bear on AIV transmission in Delaware Bay, though the overall impact is predicted to be nuanced. Our analyses indicate a relatively small change in AIV prevalence among migratory ducks, but substantial effect on the phase and amplitude of AIV peak prevalence in resident ducks. Crucially, this affects Delaware Bay's role as a potential AIV gateway site: a change in ruddy turnstone migration biology may well to lead to an increase in peak prevalences among two of the host species in the system, and hence a possible increase in emergence threat.
Acknowledgements
This work was supported by the James S. McDonnell Foundation and the National Science Foundation (DEB-0917853). P.R. was also supported by the RAPIDD program of the Science and Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health.
References
- 1.Olsen B., Munster V. J., Wallensten A., Waldenström J., Osterhaus A. D. M. E., Fouchier R. A. M. 2006. Global patterns of influenza A virus in wild birds. Science 312, 384–388 10.1126/science.1122438 (doi:10.1126/science.1122438) [DOI] [PubMed] [Google Scholar]
- 2.Krauss S., Stallknecht D. E., Negovetich N. J., Niles L. J., Webby R. J., Webster R. G. 2010. Coincident ruddy turnstone migration and horseshoe crab spawning creates an ecological ‘hot spot’ for influenza viruses. Proc. R. Soc. B 277, 3373–3379 10.1098/rspb.2010.1090 (doi:10.1098/rspb.2010.1090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown V. L., Drake J. M., Stallknecht D. E., Brown J. D., Pedersen K., Rohani P. In review. The determinants of a wildlife disease hotspot: host diversity, migration, seasonal breeding and environmental transmission. J. R. Soc. Interface. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith D. R., Jackson N. L., Nordstrom K. F., Weber R. G. 2011. Beach characteristics mitigate effects of onshore wind on horseshoe crab spawning: implications for matching with shorebird migration in Delaware Bay Anim. Conservat. 14, 575–584 10.1111/j.1469-1795.2011.00481.x (doi:10.1111/j.1469-1795.2011.00481.x) [DOI] [Google Scholar]
- 5.Loveland R. E. 2001. The Life History of Horseshoe Crabs. In Limulus in the spotlight: a species 350 years in the making and in peril? (ed. Tanacredi J. T.), pp. 93–102 Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- 6.Eagle J. 2001. Issues and approaches in regulation of the horseshoe crab fishery. In Limulus in the spotlight: a species 350 years in the making and in peril? (ed. Tanacredi J. T.), pp. 85–92 Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- 7.Carmichael R. H., Rutecki D., Valiela I. 2003. Abundance and population structure of the Atlantic horseshoe crab Limulus polyphemus in Pleasant Bay and Cape Cod. Mar. Ecol.: Prog. Ser. 246, 225–239 [Google Scholar]
- 8.Niles L. J., et al. 2009. Effects of horseshoe crab harvest in Delaware Bay on Red Knots: are harvest restrictions working? Bioscience 59, 153–164 10.I525/bio.2009.59.2.8 (doi:10.I525/bio.2009.59.2.8) [DOI] [Google Scholar]
- 9.Tøttrup A. P., Rainio K., Coppack T., Lehikoinen E., Rahbek C., Thorup K. 2010. Local temperature fine-tunes the timing of spring migration in birds. Integr. Comp. Biol. 50, 293–304 10.1093/icb/icq028 (doi:10.1093/icb/icq028) [DOI] [PubMed] [Google Scholar]
- 10.Jonzén N., et al. 2006. Rapid advance of spring arrival dates in long-distance migratory birds. Science 312, 1959–1961 10.1126/science.1126119 (doi:10.1126/science.1126119) [DOI] [PubMed] [Google Scholar]
- 11.Both C., Van Turnhout C. A. M., Bijlsma R. G., Siepel H., Van Strien A. J., Foppen R. P. B. 2010. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc. R. Soc. B 277, 1259–1266 10.1098/rspb.2009.1525 (doi:10.1098/rspb.2009.1525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith D. R., Michels S. F. 2006. Seeing the elephant. Fisheries 31, 485–491 10.1577/1548-8446(2006)31[485:STE]2.0.CO;2 (doi:10.1577/1548-8446(2006)31[485:STE]2.0.CO;2) [DOI] [Google Scholar]
- 13.Field C., et al. (eds) 2012. IPCC, 2012: managing the risks of extreme events and disasters to advance climate change adaptation. A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 14.Rohani P., Breban R., Stallknecht D. E., Drake J. M. 2009. Environmental transmission of low pathogenicity avian influenza viruses and its implications for pathogen invasion. Proc. Natl Acad. Sci. USA 106, 10 365–10 369 10.1073/pnas.0809026106 (doi:10.1073/pnas.0809026106) [DOI] [PMC free article] [PubMed] [Google Scholar]