Abstract
Gestation duration and lactation duration are usually treated as independently evolving traits in primates, but the metabolic theory of ecology (MTE) suggests both durations should be determined by metabolic rate. We used phylogenetic generalized least-squares linear regression to test these different perspectives. We found that the allometries of the durations are divergent from each other and different from the scaling exponent predicted by the MTE (0.25). Gestation duration increases much more slowly (0.06 < m < 0.12), and lactation duration much more quickly (0.36 < m < 0.52) with body mass than the MTE predicts. By contrast, we found that the combined duration of gestation and lactation is consistent with the MTE's predictions (0.22 < m < 0.35). These results suggest that gestation duration and lactation duration might best be viewed as distinct but coupled adaptations. When transferring energy to their offspring, primate mothers must meet metabolically dictated physiological requirements while optimizing the timing of the switch from gestation to lactation in relation to some as-yet-unidentified body-size-related factor.
Keywords: allometry, constraint, energy transfer, metabolic theory of ecology
1. Introduction
Gestation and lactation are commonly regarded as independently evolving life-history traits in primates. Past research into the causes of variation in gestation duration has focused mainly on brain size [1–3]. Factors hypothesized to explain variation in lactation duration include maternal condition, resource abundance, diet, allomaternal care and female philopatry [4–8]. By contrast, it has recently been claimed that key aspects of mammalian life history, including the length of gestation and lactation, are governed by metabolism [9]. On this hypothesis, which derives from the metabolic theory of ecology (MTE; [10]), primate gestation and lactation evolve under a common selective regime.
Here, we report a study designed to test the independent evolution versus common cause perspectives on primate lactation and gestation. The MTE contends that metabolic rate governs the allocation of resources to growth, maintenance and reproduction, and therefore controls many physiological and ecological processes. Metabolic rate is in turn dictated by temperature and body size. According to the MTE, the mass specific rate of energy transfer to a growing individual should scale to the minus one-quarter power of body mass [11]. Thus, because durations are expected to be the inverse of rates, we tested the prediction that primate gestation and lactation durations scale with a slope of 0.25 against body mass. Following Hamilton et al. [9], we also examined the scaling exponent of the sum of gestation duration and lactation duration, a variable which Hamilton et al. call ‘development time’.
2. Material and methods
The dataset comprised loge-transformed values for female body mass (g), gestation duration (days), lactation duration (days) and development time (days) for 83 primate species. Two-thirds of extant genera and all six extant primate families are represented among the species (see the electronic supplementary material). The values for female body mass were obtained from Redding [12], while gestation durations were obtained from Redding et al. [12], Martin [13] and Jones et al. [14]. Lactation duration values were traced from compendia [14,15] to the primary literature as far as possible, and are presented in the electronic supplementary material. Following Hamilton et al. [9], we calculated development time as the sum of gestation and lactation durations.
We used the APE package [16] in the R software environment [17] to build three phylogenetic generalized least-squares linear (PGLS) regression models, using the maximum-likelihood (rather than the restricted maximum likelihood) search algorithm. Loge(female mass) was used as the independent variable in all three models. The dependent variables in the models were loge(gestation duration), loge(lactation duration) and loge(development time), respectively. Because of the confounding effects of phylogenetic autocorrelation, where species have similar trait values owing to common descent, we ran PGLS regression using a variance–covariance matrix derived from the dated consensus phylogenetic tree in Arnold et al. [18]. Because there was reason to believe that the dependent variables might show different strengths of phylogenetic autocorrelation, which can be quantified by the parameter λ [19], we fitted the parameter λ separately for each variable using the package Geiger ([20]; see table 1). The variables were indeed found to have different phylogenetic autocorrelation (table 1), justifying a full regression model with a free λ parameter. Other recent phylogenies for primates [21] yielded near-identical results (not shown).
Table 1.
Phylogenetically corrected allometric slopes of gestation and lactation durations, and of their sum (development time).
| trait | slope | intercept | 95% CI | λ in fitted model | MLE(λ) (G)a |
|---|---|---|---|---|---|
| gestation | 0.09 | 4.44 | 0.06–0.12 | 1.00 | 1 (0) |
| lactation | 0.42 | 2.01 | 0.36–0.52 | 0.46 | 0.92 (11.22) |
| development time | 0.28 | 3.80 | 0.22–0.35 | 0.60 | 0.93 (9.87) |
aG = 2×(log[maximum likelihood (ML) at λ = 1]−log[ML at λ = MLE(λ)]).
3. Results
Table 1 and figure 1 summarize the three PGLS regression models. The slope of loge(gestation duration) versus loge(body mass) is significantly shallower than the MTE expectation of 0.25 (maximum likelihood estimate (MLE) = 0.08, confidence interval (CI) = 0.06–0.12), while the slope of loge(lactation duration) on loge(body mass) is significantly steeper than the MTE expectation of 0.25 (MLE = 0.42, CI = 0.36–0.52). The slope of loge(development time) on loge(body mass) is indistinguishable from the MTE expectation of 0.25 (MLE = 0.28, CI = 0.22–0.35). Similar results were obtained with ordinary least squares regressions (not shown).
Figure 1.
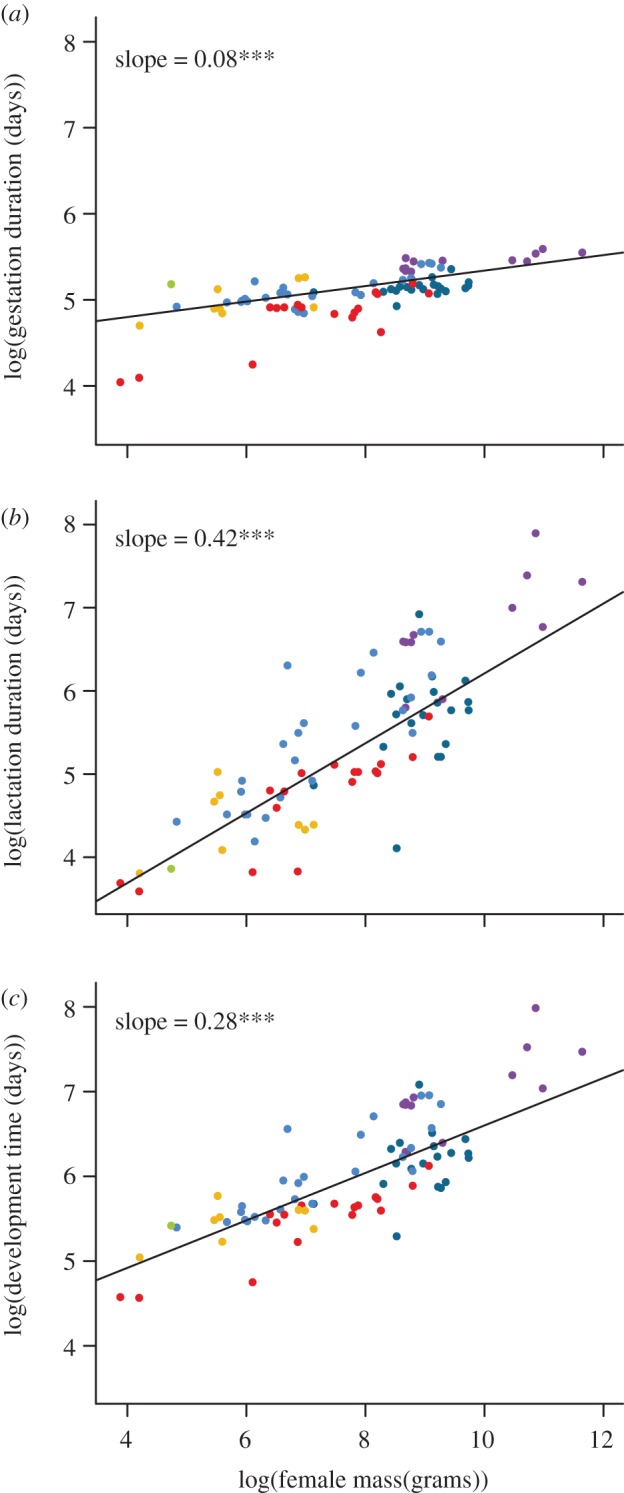
Plots of (a) gestation duration, (b) lactation duration, and (c) development time (gestation duration + lactation duration) versus species-specific female body mass across 83 species of primate. Dots are species means, colour-coded by major clade: red, Lemuriformes; gold, Lorisiformes; green, Tarsiiformes; sky blue, Cercopithecoidea; teal, Platyrrhini; purple, Hominoidea. The lines are the phylogenetically corrected best-fit lines using phylogenetic general least squares. ***p < 0.001.
4. Discussion
Our results challenge both the independent evolution and MTE perspectives on primate gestation duration and lactation duration. The allometric slope for development time is consistent with this duration being constrained by metabolic rate. The contrasting and divergent slopes for gestation duration and lactation duration suggest that the timing of the switch from gestation to lactation is governed by a factor or set of factors other than metabolic rate. The observation that gestation duration and lactation duration scale with a slope of 0.25 when combined, but not when analysed separately, suggests that decreases in gestation duration have to be offset by increases in lactation duration, and vice versa. These results suggest that primate gestation duration and lactation duration act as coupled traits evolving under the influence of metabolic rate and at least one other factor, rather than as traits evolving entirely independently or completely under the influence of metabolic rate.
On the face of it, our results conflict with those of Hamilton et al. [9], who found that gestation duration, lactation duration and development time all scale with exponents that are not statistically different from 0.25 in placental mammals, marsupials and monotremes, and consequently concluded that all three intervals are governed by metabolic rate. However, this conflict may be more apparent than real. Hamilton et al.'s [9] analyses were at either the subclass or cohort level while we focused on a single order. So, it is possible that the two sets of results are in fact compatible. Primates may be unique among mammals in how their gestation and lactation durations scale with body mass, perhaps owing to their relatively large, metabolically costly brains. Alternatively, development time may be governed by metabolic rate in all mammalian orders, but orders divide development time into gestation and lactation in different ways, and do so such that orders with scaling exponents above 0.25 are balanced by orders with scaling exponents below 0.25.
The first of these hypotheses is more parsimonious than the second. However, the second hypothesis deserves scrutiny. There are clade and grade effects on relative gestation and lactation durations in mammals, such that species from larger-bodied orders (e.g. Cetartiodactyla) produce more developed (more precocial) neonates than species from smaller-bodied orders (e.g. Rodentia) [22]: this is broadly (and intriguingly) in opposition to the scaling we show here within primates, but is consistent with the idea that mammalian orders differ in the relative timing of the switch from gestation to lactation. Additionally, the relative weight at birth in mammals varies many-fold more than relative weight at weaning [22]. This is consistent with the idea that the timing of the switch from gestation to lactation is order specific, while the combined duration of gestation and lactation follows a common cross-order allometry. Ultimately, determining which of the two hypotheses is correct will require the allometries of gestation duration, lactation duration and development time to be examined in other mammalian orders.
In addition to repeating our analyses across other mammalian orders, future work should identify what influences the timing of the switch from gestation to lactation within Order Primates. So far, we have identified four possibilities. First, gestation duration is restricted by the allometrically increasing mechanical costs associated with carrying a foetus (e.g. reduced feeding efficiency, increased predation risk) [23]. This hypothesis predicts that relative gestation durations should be shorter in species that forage more actively and/or experience higher predation pressure. Second, gestation duration is restricted by allometrically increasing biomechanical constraints. These constraints may be associated with passage of the foetus through the birth canal [24] or with locomotor efficiency [25]. This hypothesis predicts that, controlling for body size, primates with a high frequency of cephalopelvic disproportion and high rates of maternal death during birth should have shorter gestation durations and longer lactation durations than primates with lower frequency of cephalopelvic disproportion and lower rates of maternal death during birth. Third, gestation is shortened and lactation lengthened in larger primates as a response to selection for increased flexibility of early cognitive development [24]. Early birth relative to body mass may result in enhanced neural plasticity and facilitate the behavioural flexibility required for being long-lived. Here, we need to test whether primates with shorter gestation times produce young that are relatively more altricial, and, if so, whether the level of altriciality is associated with increased flexibility of cognitive development. Fourth, relative energy transfer efficiency for gestation and lactation may change with body size in primates [26,27], perhaps mediated by placental morphology [28]. Unlike the previous three, this hypothesis is not consistent with the MTE, and does not predict any particular allometric slope for the combined duration. However, it predicts that lactation in larger-bodied primates is relatively more efficient than lactation in smaller-bodied primates. This list of hypotheses is obviously neither exhaustive nor fully mutually exclusive. However, it may begin to refocus efforts to explain when a primate mother shifts from provisioning via gestation to provisioning via lactation—that is, to explain when she gives birth.
Acknowledgements
We thank M. Elliot especially, and also B. Crespi, N. Dulvy, J. Joy, R. Huey, N. Longrich, L. McKerracher, two anonymous reviewers, the Associate Editor and members of SFU's Fab*-Lab and Human Evolution Studies Program for very valuable input. We are supported by SFU, NSERC, SSHRC, CFI, BCKDF, the CRC Program and the Bass Visiting Scholars Program at Yale University.
References
- 1.Pagel M. D., Harvey P. H. 1988. How mammals produce large-brained offspring. Evolution 42, 948–957 10.2307/2408910 (doi:10.2307/2408910) [DOI] [PubMed] [Google Scholar]
- 2.Martin R. D. 1996. Scaling of the mammalian brain: the maternal energy hypothesis. News Physiol. Sci. 11, 149–156 [Google Scholar]
- 3.Barton R. A., Capellini I. 2011. Maternal investment, life histories, and the costs of brain growth in mammals. Proc. Natl Acad. Sci. USA 108, 6169–6174 10.1073/pnas.1019140108 (doi:10.1073/pnas.1019140108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee P. C., Maljuf P., Gordon I. J. 1991. Growth, weaning and maternal investment from a comparative perspective. J. Zool. 225, 99–114 10.1111/j.1469-7998.1991.tb03804.x (doi:10.1111/j.1469-7998.1991.tb03804.x) [DOI] [Google Scholar]
- 5.Lee P. C. 1996. The meanings of weaning: growth, lactation, and life history. Evol. Anthropol. 5, 87–98 (doi:10.1002/(SICI)1520-6505(1996)5:3<87::AID-EVAN4>3.0.CO;2-T) [DOI] [Google Scholar]
- 6.Dall S. R., Boyd I. L. 2004. Evolution of mammals: lactation helps mothers to cope with unreliable food supplies. Proc. R. Soc. Lond. B 271, 2049–2057 10.1098/rspb.2004.2830 (doi:10.1098/rspb.2004.2830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross C. 1998. Primate life histories . Evol. Anthropol. 6, 54–63 (doi:10.1002/(SICI)1520-6505(1998)6:2<54::AID-EVAN3>3.0.CO;2-W) [DOI] [Google Scholar]
- 8.Lee P. C., Kappeler P. M. 2003. Socioecological correlates of phenotypic plasticity of primate life histories. In Primate life history and socioecology (eds Kappeler P. M., Pereira M. E.), pp. 41–65 Chicago, IL: University of Chicago Press [Google Scholar]
- 9.Hamilton M. J., Davidson A. D., Sibly R. M., Brown J. H. 2011. Universal scaling of production rates across mammalian lineages. Proc. R. Soc. B 278, 560–566 10.1098/rspb.2010.1056 (doi:10.1098/rspb.2010.1056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B. 2004. Towards a metabolic theory of ecology. Ecology 85, 1771–1789 10.1890/03-9000 (doi:10.1890/03-9000) [DOI] [Google Scholar]
- 11.West G. B., Brown J. H., Enquist B. J. 1997. A general model for the origin of allometric scaling laws in biology. Science 276, 122–126 10.1126/science.276.5309.122 (doi:10.1126/science.276.5309.122) [DOI] [PubMed] [Google Scholar]
- 12.Redding D. W., Wolff C. V. D., Mooers A. O. 2010. Evolutionary distinctiveness, threat status, and ecological oddity in primates. Conserv. Biol. 24, 1052–1058 10.1111/j.1523-1739.2010.01532.x (doi:10.1111/j.1523-1739.2010.01532.x) [DOI] [PubMed] [Google Scholar]
- 13.Martin R. D. 2007. The evolution of human reproduction: a primatological perspective. Yearb. Phys. Anthropol. 50, 59–84 10.1002/ajpa.20734 (doi:10.1002/ajpa.20734) [DOI] [PubMed] [Google Scholar]
- 14.Jones K. E., et al. 2009. PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90, 2648. 10.1890/08-1494.1 (doi:10.1890/08-1494.1) [DOI] [Google Scholar]
- 15.Tienhoven A., Tienhoven A., Hayssen V. 1993. Asdell's patterns of mammalian reproduction: a compendium of species-specific data, 3rd edn Ithaca, NY: Cornell University Press [Google Scholar]
- 16.Paradis E., Claude J., Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 10.1093/bioinformatics/btg412 (doi:10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 17.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (www.r-project.org) [Google Scholar]
- 18.Arnold C., Matthews L. J., Nunn C. L. 2010. The10k trees website: a new online resource for primate phylogeny. Evol. Anthropol. 19, 114–118 10.1002/evan.20251 (doi:10.1002/evan.20251) [DOI] [Google Scholar]
- 19.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884 10.1038/44766 (doi:10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 20.Harmon L. J., Weir J. T., Brock C. D., Glor R. E., Challenger W. 2008. Geiger: investigating evolutionary radiations. Bioinformatics 24, 129–131 10.1093/bioinformatics/btm538 (doi:10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- 21.Fritz S. A., Bininda-Emonds O. R. P., Purvis A. 2009. Geographical variation in predictors of mammalian extinction risk: big is bad, but only in the tropics. Ecol. Lett. 12, 538–549 10.1111/j.1461-0248.2009.01307.x (doi:10.1111/j.1461-0248.2009.01307.x) [DOI] [PubMed] [Google Scholar]
- 22.Derrickson E. M. 1992. Comparative reproductive strategies of altricial and precocial Eutherian mammals. Func. Ecol. 6, 57–65 10.2307/2389771 (doi:10.2307/2389771) [DOI] [Google Scholar]
- 23.Biewener A. A. 1989. Scaling body support in mammals: limb posture and muscle mechanics. Science 245, 45–48 10.1126/science.2740914 (doi:10.1126/science.2740914) [DOI] [PubMed] [Google Scholar]
- 24.van Schaik C. P., Barrickman N., Bastian M. L., Elissa B. K., Noordwijk M. A. 2006. Primate life histories and the role of brains. In Evolution of human life history (eds Hawkes K., Paine R.), pp. 127–154 Santa Fe, NM: School of American Research [Google Scholar]
- 25.Leutenegger W. 1974. Functional aspects of pelvic morphology in simian primates. J. Hum. Evol. 3, 207–222 10.1016/0047-2484(74)90179-1 (doi:10.1016/0047-2484(74)90179-1) [DOI] [Google Scholar]
- 26.Kunkele J. 2000. Energetics of gestation relative to lactation in a precocial rodent, the guinea pig (Cavia porcellus). J. Zool. 250, 533–539 10.1111/j.1469-7998.2000.tb00794.x (doi:10.1111/j.1469-7998.2000.tb00794.x) [DOI] [Google Scholar]
- 27.Dufour D. L., Sauther M. L. 2002. Comparative and evolutionary dimensions of the energetics of human pregnancy and lactation. Am. J. Hum. Biol. 14, 584–602 10.1002/ajhb.10071 (doi:10.1002/ajhb.10071) [DOI] [PubMed] [Google Scholar]
- 28.Capellini I., Venditti C., Barton R. A. 2011. Placentation and maternal investment in mammals. Am. Nat. 177, 86–98 10.1086/657435 (doi:10.1086/657435) [DOI] [PubMed] [Google Scholar]


