Abstract
Gliding morphologies occur in diverse vertebrate lineages in Southeast Asian rainforests, including three gecko genera, plus frogs, snakes, agamid lizards and squirrels. It has been hypothesized that repeated evolution of gliding is related to the dominance of Asian rainforest tree floras by dipterocarps. For dipterocarps to have influenced the evolution of gliding in Southeast Asian vertebrates, gliding lineages must have Eocene or later origins. However, divergence times are not known for most lineages. To investigate the temporal pattern of Asian gliding vertebrate evolution, we performed phylogenetic and molecular clock analyses. New sequence data for geckos incorporate exemplars of each gliding genus (Cosymbotus, Luperosaurus and Ptychozoon), whereas analyses of other vertebrate lineages use existing sequence data. Stem ages of most gliding vertebrates, including all geckos, cluster in the time period when dipterocarps came to dominate Asian tropical forests. These results demonstrate that a gliding/dipterocarp correlation is temporally viable, and caution against the assumption of early origins for apomorphic taxa.
Keywords: volant, parachuting, Sundaland, phylogeny, Reptilia, Mammalia
1. Introduction
Gliding vertebrates are more prevalent in Southeast Asian than other rainforests, and include squirrels (Pteromyini), colugos (Dermoptera), Chrysopelea snakes, Draco lizards, geckos and Rhacophorus frogs [1–3]. Among geckos, gliding has been described or confirmed for species in three genera: Hemidactylus (two species formerly in the genus Cosymbotus, here retained for clarity), Luperosaurus and Ptychozoon [4–8]. These genera are arboreal, obligate (Luperosaurus, Ptychozoon) or facultative (Cosymbotus) rainforest dwellers restricted to Southeast Asia. They share several morphological features that are rudimentary or absent in related geckos and seemingly facilitate gliding, including dorsoventral body flattening, extensive interdigital webbing, elaborate skin flaps and flattened tails with lateral projections (figure 1) [4]. Similarly, some combination of skin flaps and/or dorsoventral flattening has evolved in other Asian gliding vertebrate lineages.
Figure 1.
(a,c,e) Gliding geckos and (b,d,f) non-gliding relatives. Note digital webbing and lateral flaps in gliders. (a) Hemidactylus (Cosymbotus) craspedotus; (b) Hemidactylus garnotii; (c) Luperosaurus cumingii; (d) Lepidodactylus vanuatuensis; (e) Ptychozoon lionotum and (f) Gekko vittatus.
Several hypotheses have been proposed to account for this parallel evolution of gliding. Three have received the most attention. One hypothesis suggests Asian rainforests differ from other rainforests structurally, with fewer lianas interlacing the canopy, favouring gliding locomotion as a means of among-tree locomotion [9]. Subsequent studies have not supported this distinction [10,11]. Two more widely accepted hypotheses correlate the evolution of gliding with the dominance of the Asian large-tree flora by Dipterocarpaceae, a tropical tree family that is exceptionally diverse in Asia [2,3,11]. Dipterocarps are taller than other canopy trees, potentially favouring evolution of gliders by allowing for longer glides. Alternately, the unpalatable leaves and cyclic mast-fruiting of dipterocarps may increase food patchiness (directly for herbivores, indirectly for insectivores such as geckos), favouring evolution of gliding as an energy-efficient means of locomotion among foraging locations.
Dipterocarps have a Gondwanan origin, and probably colonized Asia via the Indian plate that collided with the remainder of Asia approximately 50 million years ago (Ma) [12–14]. In Southeast Asia, the first evidence of dipterocarps soon followed, from the Middle/Late Eocene (49–34 Ma) of Myanmar. Dipterocarps became increasingly common through the Oligocene and Miocene, and dominant parts of the Southeast Asian tree flora by 20 Ma [15,16]. If the evolution of gliding-associated traits is correlated with the development of dipterocarp forests, we expect a date of origin for gliding lineages during the period 50–20 Ma, as this floral shift occurred.
This timeframe seemingly precludes it from acting as a causative agent in some lineages. For example, Luperosaurus is sister to Lepidodactylus [17]. Gecko genera with similar species richness to Lepidodactylus (e.g. Gehyra) are significantly older than Mid-Cenozoic in age, and the divergence between Gekko and Lepidodactylus has been estimated to have occurred near the K/T boundary, 65 Ma [18,19], hinting at earlier ages of origin for Luperosaurus as well. We performed phylogenetic and timing analyses on a dataset, including Asian flap-bearing geckos, as well as most other Southeast Asian and Pacific gekkonid genera, to both investigate evolutionary relationships among the arboreal Asian gecko genera and evaluate the tenability of a correlation between dipterocarp forest development and the evolution of gliding morphologies. Comparative analyses were performed using existing sequence data for each of the other gliding Asian vertebrate lineages.
2. Material and methods
Our gekkonid nucleotide sequence dataset includes two Cosymbotus, two Luperosaurus, two Ptychozoon (three specimens), 20 Gekko (23 specimens), 10 Lepidodactylus (16 specimens) and two Pseudogekko. We also included five Hemidactylus, 18 gekkotan and two non-gekkotan outgroups (see the electronic supplementary material, table S1). The dataset includes the mitochondrial gene ND2 and flanking tRNAs, plus portions of nuclear genes RAG1 and PDC (2766 bp total; new sequences have GenBank accession numbers: JX515611–JX515652). Phylogenetic analyses were performed under likelihood, parsimony and Bayesian criteria. For comparative analyses of other Asian gliding vertebrates, we assembled the most comprehensive datasets possible from available GenBank sequences (see the electronic supplementary material, tables S2–S5): two genes (2059 bp) and 34 taxa (14 gliding) for Pteromyini; two genes (1169 bp) and 18 taxa (1 gliding) for Chrysopelea; six genes (2915 bp) and 39 taxa (15 gliding) for Rhacophorus; three genes (1813 bp) and 52 taxa (32 gliding) for Draco. All alignment matrices are deposited in the Dryad repository (http://dx.doi.org/10.5061/dryad.jh3sb). For Dermoptera, we use the results of a published 19-gene, 14 000 bp analysis [20]. Divergence times for the gekkonid and comparative analyses were calculated using Bayesian relaxed-clock analyses and multiple fossil calibrations; post burn-in posterior samples of node heights from these analyses were used to calculate posterior probabilities of divergences occurring from 50 to 20 Ma. Details of specimens used and methods are provided in the electronic supplementary material.
3. Results
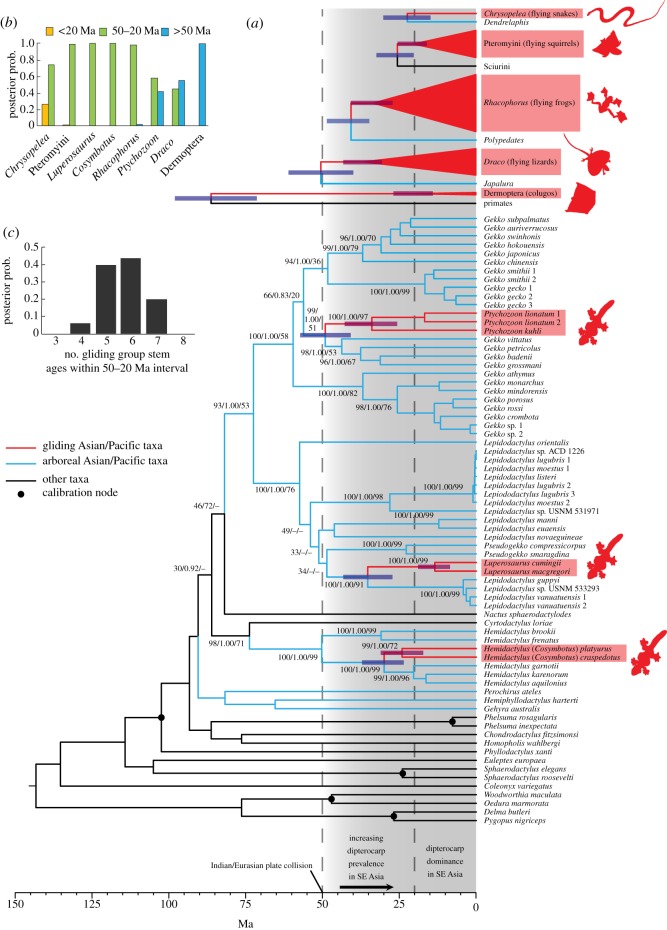
Phylogenetic analyses of the combined gekkonid dataset recover several strongly supported groups (figure 2a). Analyses support an arboreal Gekko group comprising the genera Gekko, Lepidodactylus, Pseudogekko, Luperosaurus and Ptychozoon. Monophyly of each constituent genus of the Gekko group is not supported—as in recently published analyses, sampled Ptychozoon species are embedded within Gekko [17], but we also recover Luperosaurus and Pseudogekko as embedded in Lepidodactylus. There is significant phylogenetic structure in the Gekko/Ptychozoon subgroup; Ptychozoon is most closely related to species in the Gekko vittatus and G. petricolus groups. In the Lepidodactylus/Luperosaurus/Pseudogekko subgroup, patterns of relationships are not as well established. There is strong support for the grouping as a whole, and for Luperosaurus being most closely related to the Melanesian species Lepidodactylus guppyi and L. vanuatuensis. Relationships of Cosymbotus to other Hemidactylus, as well as relationships among other sampled gekkotan taxa, conform to the results of previous studies [21] and are not discussed further. Evolutionary relationships in the Pteromyini, Chrysopelea, Draco and Rhacophorus analyses also conform to results of previous studies (see the electronic supplementary material, figures S1–S4).
Figure 2.
(a) Timetrees of gliding geckos and other vertebrates. The gecko timetree is based on the combined dataset, with support values (ML bootstrap/Bayesian PP/MP bootstrap) and 95% credibility intervals of age indicated for key nodes. For other vertebrates, schematics of stem and crown ages of gliding taxa are depicted, with 95% credibility intervals of age; corresponding full timetrees are in the electronic supplementary material. (b) Posterior probability distributions for gliding vertebrate stem ages falling inside the 50–20 Ma temporal interval. (c) Posterior probabilities for the number of gliding vertebrate stem ages (of eight total) falling within the 50–20 Ma temporal interval.
Molecular clock analyses support Cainozoic stem ages for all Asian gliding vertebrates save Dermoptera (figure 2). Among geckos, Ptychozoon is the earliest diverging gliding genus, diverging from Gekko 49 (57–41) Ma. Of the remaining non-gekkotan gliders, Draco diverged from Japalura during a similar timeframe 50 (61–40 Ma). Credibility intervals of stem ages for all other Asian gliding vertebrates do not predate 50 Ma. Posterior probabilities of stem ages (figure 2b) show that only the Dermoptera stem age falls significantly outside the 50–20 Ma interval, and it is most probable that six of eight stem ages fall within the 50–20 Ma interval (figure 2c).
4. Discussion
The results of our timing analyses broadly overlap the period in which dipterocarps dispersed into Southeast Asia and came to dominate the large-tree flora. Median stem ages for six of eight gliding groups fall within this period. The two exceptions, Dermoptera and Draco, both have potential caveats. The long time period (more than 65 myr) between the dermopteran/primate split and divergence between living dermopterans suggests that in this group stem age is not a reliable time estimate for the evolution of gliding morphology. Meanwhile, Draco belongs to an ancestrally Indian group [22], and thus probably evolved in the presence of dipterocarp forest. Among other gliding groups, differences in stem ages may partly result from distinct biogeographic histories. For example, among geckos, Ptychozoon, the oldest genus, is deeply nested within Gekko, whose centre of diversity is Southeast Asia. By contrast, Lepidodactylus (in which Luperosaurus is nested) has its centre of diversity in Melanesia, and most Hemidactylus (in which Cosymbotus is nested) occur in relatively arid habitats stretching from East Africa to India. In both cases, the evolution of gliding morphologies seems to have quickly followed dispersal into Southeast Asian rainforests. It is possible that Cosymbotus and Luperosaurus evolved gliding morphologies in areas of Southeast Asia not occupied by Ptychozoon, occupying an empty niche, but our sampling lacks the necessary comprehensiveness required to estimate such fine-scale biogeographic patterns.
While our results are consistent with a temporal overlap between the evolution of gliding Southeast Asian vertebrates and development of dipterocarp forests, they cannot differentiate between either of the dipterocarp-related hypotheses (tree height or food patchiness). Likewise, we do not discount alternative evolutionary pressures, such as to promote crypsis, as having had no effect in the evolution of body flaps and webbing in Asian gliding vertebrates [4]. Instead, we suspect that a complex interplay among evolutionary pressures, morphological structures and behaviours has contributed to the evolution of gliding morphologies in Southeast Asian geckos and other vertebrates, and based on temporal patterns evident from our analyses, the development of dipterocarp forests cannot be discounted as having played a role.
Our study has additional implications beyond testing the tenability of the dipterocarp/gliding correlation. Most notably, our phylogenetic results highlight the frequency with which morphologically aberrant taxa are embedded within radiations of more conservative species. Just within geckos, many similar findings have been made in recent years (e.g. Palmatogecko in Pachydactylus [23]). These results should serve as a caution against assigning distinct taxonomic status or assuming ancient origins for species exhibiting morphological specializations or novelties.
Acknowledgements
We thank Rafe Brown (Kansas University), Lee Grismer (La Sierra University), Ivan Ineich (Muséum National d'Histoire Naturelle, Paris), Fred Kraus (Bishop Museum), Kelvin Lim (Zoological Reference Collection, Singapore), Jim McGuire and Carol Spencer (Museum of Vertebrate Zoology), Jens Vindum (California Academy of Sciences) and George Zug and Addison Wynn (United States National Museum) for providing samples used in this study and/or some photos used in figure 1. Funding was provided by NSF grants (nos DEB 0515909 and DEB 0844523).
References
- 1.Corlett R. T. 2007. What's so special about Asian tropical forests? Curr. Sci. 93, 1551–1557 [Google Scholar]
- 2.Corlett R. T., Primack R. B. 2011. Tropical rain forests: an ecological and biogeographical comparison, 2nd edn, pp. 336 New York, NY: Wiley-Blackwell [Google Scholar]
- 3.Singh S. P., Sharma C. M. 2009. Tropical ecology: an overview. Trop. Ecol. 50, 7–21 [Google Scholar]
- 4.Russell A. P. 1979. Origin of parachuting locomotion in gekkonid lizards (Reptilia, Gekkonidae). Zool. J. Linn. Soc. Lond. 65, 233–249 10.1111/j.1096-3642.1979.tb01093.x (doi:10.1111/j.1096-3642.1979.tb01093.x) [DOI] [Google Scholar]
- 5.Russell A. P., Dijkstra L. D., Powell G. L. 2001. Structural characteristics of the patagium of Ptychozoon kuhli (Reptilia: Gekkonidae) in relation to parachuting locomotion. J. Morphol. 247, 252–263 (doi:10.1002/1097-4687(200103)247:3<252::aid-jmor1015>3.0.co;2-z) [DOI] [PubMed] [Google Scholar]
- 6.Jusufi A., Goldman D. I., Revzen S., Full R. J. 2008. Active tails enhance arboreal acrobatics in geckos. Proc. Natl Acad. Sci. USA 105, 4215–4219 10.1073/pnas.0711944105 (doi:10.1073/pnas.0711944105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudley R., Byrnes G., Yanoviak S. P., Borrell B., Brown R. M., McGuire J. A. 2007. Gliding and the functional origins of flight: biomechanical novelty or necessity. Annu. Rev. Ecol. Evol. Syst. 38, 179–201 10.1146/annurev.ecolsys.37.091305.110014 (doi:10.1146/annurev.ecolsys.37.091305.110014) [DOI] [Google Scholar]
- 8.Young B. A., Lee C. E., Daley K. M. 2002. On a flap and a foot: aerial locomotion in the ‘flying’ gecko, Ptychozoon kuhli. J. Herpetol. 36, 412–418 10.1670/0022-1511(2002)036[0412:oafaaf]2.0.co;2 (doi:10.1670/0022-1511(2002)036[0412:oafaaf]2.0.co;2) [DOI] [Google Scholar]
- 9.Emmons L. H., Gentry A. H. 1983. Tropical forest structure and the distribution of gliding and prehensile-tailed vertebrates. Am. Nat. 121, 513–524 10.1086/284079 (doi:10.1086/284079) [DOI] [Google Scholar]
- 10.Appanah S., Gentry A. H., LaFrankie J. V. 1992. Liana diversity and species richness of Malaysian rain forests. J. Trop. For. Sci. 6, 116–123 [Google Scholar]
- 11.Dudley R., DeVries P. 1990. Tropical rain forest structure and the geographical distribution of gliding vertebrates. Biotropica 22, 432–434 10.2307/2388564 (doi:10.2307/2388564) [DOI] [Google Scholar]
- 12.Rowley D. B. 1996. Age of initiation of collision between India and Asia: a review of stratigraphic data. Earth Planet. Sci. Lett. 145, 1–13 10.1016/s0012-821x(96)00201-4 (doi:10.1016/s0012-821x(96)00201-4) [DOI] [Google Scholar]
- 13.Rust J., et al. 2010. Biogeographic and evolutionary implications of a diverse paleobiota in amber from the early Eocene of India. Proc. Natl Acad. Sci. USA 107, 18 360–18 365 10.1073/pnas.1007407107 (doi:10.1073/pnas.1007407107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashton P. S. 1988. Dipterocarp biology as a window to the understanding of tropical forest structure. Annu. Rev. Ecol. Syst. 19, 347–370 10.1146/annurev.ecolsys.19.1.347 (doi:10.1146/annurev.ecolsys.19.1.347) [DOI] [Google Scholar]
- 15.Morley R. J. 2000. Origin and evolution of tropical rain forests, pp. 362 Chichester, UK: Wiley [Google Scholar]
- 16.Wong K. M. 2011. A biogeographic history of Southeast Asian rainforests. In Managing the future of Southeast Asias valuable tropical rainforests (eds Wickneswari R., Cannon C.), pp. 21–55 Dordrecht, The Netherlands: Springer [Google Scholar]
- 17.Brown R. M., Siler C. D., Das I., Min Y. 2012. Testing the phylogenetic affinities of Southeast Asia's rarest geckos: flap-legged geckos (Luperosaurus), flying geckos (Ptychozoon) and their relationship to the pan-Asian genus Gekko. Mol. Phylogenet. Evol. 63, 915–921 10.1016/j.ympev.2012.02.019 (doi:10.1016/j.ympev.2012.02.019) [DOI] [PubMed] [Google Scholar]
- 18.Gamble T., Bauer A. M., Greenbaum E., Jackman T. R. 2008. Evidence for Gondwanan vicariance in an ancient clade of gecko lizards. J. Biogeogr. 35, 88–104 10.1111/j.1365-2699.2007.01770.x (doi:10.1111/j.1365-2699.2007.01770.x) [DOI] [Google Scholar]
- 19.Heinicke M. P., Greenbaum E., Jackman T. R., Bauer A. M. 2011. Phylogeny of a trans-Wallacean radiation (Squamata, Gekkonidae, Gehyra) supports a single early colonization of Australia. Zool. Scr. 40, 584–602 10.1111/j.1463-6409.2011.00495.x (doi:10.1111/j.1463-6409.2011.00495.x) [DOI] [Google Scholar]
- 20.Janecka J. E., Miller W., Pringle T. H., Wiens F., Zitzmann A., Helgen K. M., Springer M. S., Murphy W. J. 2007. Molecular and genomic data identify the closest living relative of primates. Science 318, 792–794 10.1126/science.1147555 (doi:10.1126/science.1147555) [DOI] [PubMed] [Google Scholar]
- 21.Bauer A. M., Jackman T. R., Greenbaum E., Giri V. B., de Silva A. 2010. South Asia supports a major endemic radiation of Hemidactylus geckos. Mol. Phylogenet. Evol. 57, 343–352 10.1016/j.ympev.2010.06.014 (doi:10.1016/j.ympev.2010.06.014) [DOI] [PubMed] [Google Scholar]
- 22.Macey J. R., Schulte J. A., Larson A., Ananjeva N. B., Wang Y. Z., Pethiyagoda R., Rastegar-Pouyani N., Papenfuss T. J. 2000. Evaluating trans-Tethys migration: an example using acrodont lizard phylogenetics. Syst. Biol. 49, 233–256 10.1093/sysbio/49.2.233 (doi:10.1093/sysbio/49.2.233) [DOI] [PubMed] [Google Scholar]
- 23.Bauer A. M., Lamb T. 2005. Phylogenetic relationships of southern African geckos in the Pachydactylus group (Squamata: Gekkonidae). Afr. J. Herpetol. 54, 105–129 10.1080/21564574.2005.9635525 (doi:10.1080/21564574.2005.9635525) [DOI] [Google Scholar]