Abstract
About 10 per cent of birds' eggs fail to hatch, but the incidence of failure can be much higher in endangered species. Most studies fail to distinguish between infertility (due to a lack of sperm) and embryo mortality as the cause of hatching failure, yet doing so is crucial in order to understand the underlying problem. Using newly validated techniques to visualize sperm and embryonic tissue, we assessed the fertility status of unhatched eggs of five endangered species, including both wild and captive birds. All eggs were classified as ‘infertile’ when collected, but most were actually fertile with numerous sperm on the ovum. Eggs of captive birds had fewer sperm and were more likely to be infertile than those of wild birds. Our findings raise important questions regarding the management of captive breeding programmes.
Keywords: helmeted honeyeater, hihi, captive breeding, orange-bellied parrot, Spix's macaw, yellow-shouldered Amazon parrot
1. Introduction
Hatching failure is common in birds [1], especially in endangered species [2–8]. Understanding why eggs fail to hatch is crucial for conservation strategies targeting endangered birds, particularly in captive breeding programmes. Hatching failure can result from either (i) failure of the ovum to be fertilized or (ii) failure of the fertilized ovum to develop and hatch [9–11]. Distinguishing between these two causes of hatching failure is important: infertility may result from insufficient or defective sperm [12–14], possibly reflecting poor male health/quality or copulation failure, whereas embryo mortality may more probably be influenced by maternal condition, environmental factors and inbreeding depression [15–23].
To date, few studies of hatching failure in birds have distinguished between infertility and embryo mortality [2,24]. Although some researchers have opened eggs to check for embryo development [2,4,6,25,26], ambiguity remains if there is no obvious sign of development [27]. Brekke et al. [6], for example, were unable to detect hihi (Notiomystis cincta) embryos that died in the first 3.5 days of incubation and were therefore unable to identify the cause of hatching failure in 72 per cent of unhatched eggs. In poultry, most embryo death occurs early in development (including the period between fertilization and oviposition; [22,28]). Simple macroscopic examination (as described above) will therefore over-estimate infertility. Here, we use new methods [11] to determine the fertility of, and number of sperm in, unhatched eggs from five endangered species.
2. Material and methods
Unhatched eggs were collected several days after the incubation period was complete, from helmeted honeyeaters (Lichenostomus melanops cassidix; n = 23), hihi (n = 120), orange-bellied parrots (Neophema chrysogaster; n = 146), Spix's macaws (Cyanopsitta spixii; n = 40) and yellow-shouldered Amazon parrots (Amazona barbadensis; n = 10). The hihi and yellow-shouldered Amazon parrots were wild; the orange-bellied parrots and Spix's macaws were captive. Of the helmeted honeyeater eggs, four were from wild birds and 19 were from captive birds.
The researchers who collected the eggs inspected their contents for any embryo development, and all were classified as ‘infertile’. After this initial inspection, egg contents were preserved in 5 per cent formalin and stored until detailed examination. Where possible (when the ovum was intact), the diameter of the fixed ovum (yolk) was measured to the nearest 0.1 mm using Vernier calipers.
All of the perivitelline layer (PVL) found in each sample was cleaned thoroughly and examined for the presence of sperm, following the procedure described in the study of Birkhead et al. [11] (which was validated for incubated eggs; see the electronic supplementary material). Sufficient material was obtained from all eggs to determine their fertility status, and for fertile eggs where the entire PVL was retrieved (63 hihi, 16 helmeted honeyeater (four wild and 12 captive), 37 orange-bellied parrot and 17 Spix's macaw eggs) the total number of sperm was counted. This was not possible for any yellow-shouldered Amazon parrot eggs, for which only fragments of PVL could be retrieved due to degradation.
In a comparative study, Birkhead et al. [29] found a positive relationship between ovum size and the total sperm number on the PVL in wild birds, where log10 total sperm number = 3.91 × log10 ovum diameter − 1.43 (equation applies where 0.9 ≤ log10 ovum diameter ≤ 1.7; derived from fig. 1a in Birkhead et al. [29]). From this, we calculated the expected number of sperm for each species' ova (given their size) and compared this with our sperm counts from fertile eggs.
For confirmation of fertilization, the germinal disc (GD) was stained with Hoechst 33 342, a fluorescent DNA marker, and examined under a fluorescence microscope for the presence of embryonic cells [11]. Eggs were scored as either (i) infertile (no cell nuclei in the GD) or (ii) fertile but having suffered embryo mortality (cell nuclei in the GD and sperm on the PVL).
3. Results
Sperm were found on the PVL of unhatched eggs in all five species. The proportion of unhatched eggs that were fertile was 88 per cent and 100 per cent in the wild hihi and yellow-shouldered Amazon parrot, respectively, and 45 per cent and 40 per cent in the captive Spix's macaw and orange-bellied parrot, respectively (table 1). Six of 19 (32%) captive helmeted honeyeater eggs were infertile, whereas all four wild eggs (100%) were fertile. Overall, early embryo mortality—as opposed to true infertility—was the principal cause of hatching failure. However, eggs from the captive populations had a higher rate of infertility than those from the wild populations (table 1).
Table 1.
Comparison of apparent and true levels of infertility in five endangered bird species.
| species | Order | captive (C) or wild (W) | number of eggs collected (no sign of development) | number of eggs with no cells in the GD (infertile) | number of eggs with no cells in the GD or sperm on the PVL | % infertility of non-developing eggsa |
|---|---|---|---|---|---|---|
| helmeted honeyeater (Lichenostomus melanops cassidix) | Passeriformes | W | 4 | 0 | 0 | 0 |
| C | 19 | 6 | 5 | 32 | ||
| hihi (Notiomystis cincta) | Passeriformes | W | 120 | 14 | 10 | 12 |
| orange-bellied parrot (Neophema chrysogaster) | Psittaciformes | C | 146 | 88 | 82 | 60 |
| Spix's macaw (Cyanopsitta spixii) | Psittaciformes | C | 40 | 22 | 19 | 55 |
| yellow-shouldered Amazon parrot (Amazona barbadensis) | Psittaciformes | W | 10 | 0 | 0 | 0 |
aThe number of eggs with no GD cells as a percentage of the number of non-developing eggs examined.
In the orange-bellied parrot and Spix's macaw, the number of PVL sperm was markedly lower than predicted (figure 1). The magnitude of the difference between observed and predicted sperm numbers was much smaller for the two passerine species: hihi and wild helmeted honeyeater sperm numbers were only slightly lower than predicted (figure 1), and fell within the 95 per cent prediction limits of Birkhead et al.'s [29] relationship (figure 1), suggesting that lack of sperm is not a problem for these birds. Twelve eggs from captive helmeted honeyeaters had fewer sperm than the four from the wild, falling outside of the 95 per cent prediction limits. Given the relatively small sample size, however, these data should be interpreted with caution.
Figure 1.
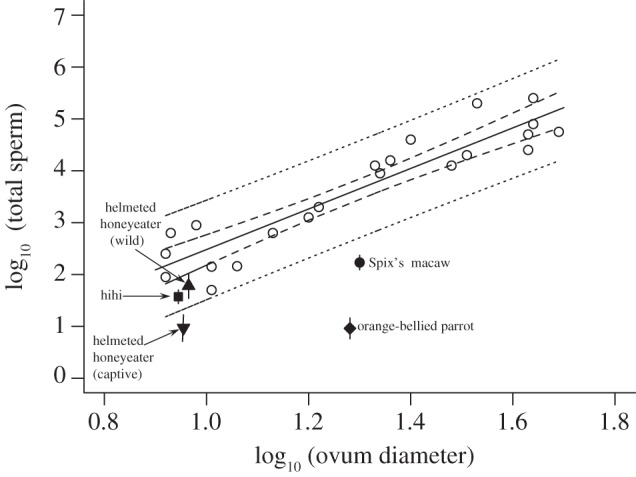
The relationship between the number of sperm found on the PVL of fertile ova and mean ovum diameter (from [29]) with 95% confidence limits (dashed lines) and prediction limits (dotted lines; generated using the ‘predict’ function in R v. 2.10.1 [30]). Open circles are data from [29] and closed shapes are data from this study. Helmeted honeyeater points are offset along the x-axis for clarity; true log10 ovum diameter = 0.95.
4. Discussion
Between 40 and 100 per cent of apparently ‘infertile’ eggs produced by five endangered bird species were actually fertile and had numerous sperm associated with the PVL. This suggests that, particularly in wild populations, a lack of sperm reaching the ova is not the only major cause of hatching failure. The captive orange-bellied parrots and Spix's macaws were two exceptions. Both species had a relatively high level of infertility owing to a lack of sperm.
A number of factors may explain the marked difference in fertility levels between the orange-bellied parrots and Spix's macaws and the other species. First, Psittaciformes (parrots) may inseminate relatively low numbers of sperm compared with other taxa. Although total sperm counts were not possible for the yellow-shouldered Amazon parrot, all eggs were fertile, suggesting that lack of sperm is not a problem for this species. However, little is known about the number of sperm inseminated and reaching the egg in parrots and this requires further study [31,32].
Second, high levels of inbreeding within a population may result in genetic incompatibility between mates, leading to fertilization failure. All five populations considered here are known or assumed to be inbred: the hihi, for example, have been through a series of population bottlenecks [6] and the Spix's macaws originate almost entirely from a single pair (R. Watson 2010, personal communication). If inbreeding causes infertility in orange-bellied parrots and Spix's macaws, it is reasonable to expect the same in the other three species. However, infertility was rare in the other species; most hatching failure (88–100%) resulted from early embryo death. Since inbreeding causes the exposure of recessive lethals in the homozygous state [1], and major genes are expressed early in development, the high incidence of early embryo death is perhaps a more expected outcome of inbreeding depression [23].
Finally, all orange-bellied parrot and Spix's macaw samples were from captive birds. Captivity can reduce reproductive success [33,34], and it is possible that the lack of sperm in many orange-bellied parrot and Spix's macaw eggs was due to either (i) copulation failure or (ii) low sperm production by males (e.g. due to stress [35] or inbreeding). Laparoscopies have shown, for example, that some Spix's macaw males have under-developed testes (R. Watson 2010, personal communication). Problems like these may be less likely in the wild. Moreover, since these captive birds were maintained as pairs there was no opportunity for females to engage in extra-pair copulations, which might compensate for insufficient sperm from pair males [13].
To identify and understand the specific reproductive problems faced by endangered birds, future studies should first discriminate between infertility and embryo death as the cause of hatching failure. Combining this approach with behavioural observations will allow discrimination between factors such as male infertility, mate compatibility and environmental conditions as potential causes of failure. Knowledge of this underlying biology will increase the efficacy of conservation strategies, especially captive breeding programmes.
Acknowledgements
We thank M. Shelton at Healesville Sanctuary, R. Watson, S. Hammer, C. Hebel and J. Soares at Al Wabra Wildlife Preservation, S. Williams, R. Evans, P. Brekke and J. Ewen for collecting unhatched eggs, P. McMullin and Cranberry Foods for providing turkey eggs (see the electronic supplementary material), and B. Montgomerie for comments on the manuscript. N.H. was funded by an NERC PhD studentship and T.R.B. by the Leverhulme Trust.
References
- 1.Koenig W. D. 1982. Ecological and social factors affecting hatchability of eggs. Auk 99, 526–536 [Google Scholar]
- 2.Jamieson I. G., Ryan C. J. 2000. Increased egg infertility associated with translocating inbred takahe (Porphyrio hochstetteri) to island refuges in New Zealand. Biol. Conserv. 94, 107–114 10.1016/S0006-3207(99)00158-5 (doi:10.1016/S0006-3207(99)00158-5) [DOI] [Google Scholar]
- 3.Briskie J. V., Mackintosh M. 2004. Hatching failure increases with severity of population bottlenecks in birds. Proc. Natl Acad. Sci. USA 101, 558–561 10.1073/pnas.0305103101 (doi:10.1073/pnas.0305103101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooson S., Jamieson I. G. 2004. Variation in breeding success among reintroduced island populations of South Island saddlebacks Philesturnus carunculatus carunculatus. Ibis 146, 417–426 10.1111/j.1474-919X.2004.00275.x (doi:10.1111/j.1474-919X.2004.00275.x) [DOI] [Google Scholar]
- 5.Swinnerton K. J., Groombridge J. J., Jones C. G., Burn R. W., Mungroo Y. 2004. Inbreeding depression and founder diversity among captive and free-living population of the endangered pink pigeon Columba mayeri. Anim. Conserv. 7, 353–364 10.1017/S1367943004001556 (doi:10.1017/S1367943004001556) [DOI] [Google Scholar]
- 6.Brekke P., Bennett P. M., Wang J., Pettorelli N., Ewen J. G. 2010. Sensitive males: inbreeding depression in an endangered bird. Proc. R. Soc. B 277, 3677–3684 10.1098/rspb.2010.1144 (doi:10.1098/rspb.2010.1144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heber S., Briskie J. V. 2010. Population bottlenecks and increased hatching failure in endangered birds. Conserv. Biol. 24, 1674–1678 10.1111/j.1523-1739.2010.01553.x (doi:10.1111/j.1523-1739.2010.01553.x) [DOI] [PubMed] [Google Scholar]
- 8.Ortego J., Cordero P. J., Aparicio J. M., Calabuig G. 2010. Parental genetic characteristics and hatching success in a recovering population of lesser kestrels. J. Ornithol. 151, 155–162 10.1007/s10336-009-0438-7 (doi:10.1007/s10336-009-0438-7) [DOI] [Google Scholar]
- 9.Romanoff A. L. 1960. The avian embryo. New York, NY: Macmillan [Google Scholar]
- 10.Wishart G. J. 1995. New approaches to evaluating male and female fertility. In Proc. 1st Int. Symp. on the Artificial Insemination of Poultry (eds Bakst M. R., Wishart G. J.), pp. 207–223 Savoy, IL: Poultry Science Association [Google Scholar]
- 11.Birkhead T. R., Hall J., Schut E., Hemmings N. 2008. Unhatched eggs: methods for discriminating between infertility and early embryo mortality. Ibis 150, 508–517 10.1111/j.1474-919X.2008.00813.x (doi:10.1111/j.1474-919X.2008.00813.x) [DOI] [Google Scholar]
- 12.Brillard J. P. 1990. Control of fertility in birds. Paris, France: INRA [Google Scholar]
- 13.Wetton J. H., Parkin D. T. 1991. An association between fertility and cuckoldry in the house sparrow, Passer domesticus. Proc. R. Soc. Lond. B 245, 227–233 10.1098/rspb.1991.0114 (doi:10.1098/rspb.1991.0114) [DOI] [Google Scholar]
- 14.Lifjeld J. T., Laskemoen T., Fossøy F., Johnsen A., Kleven O. 2007. Functional infertility among territorial males in two passerine species, the willow warbler Phylloscopus trochilus and the bluethroat Luscinia svecica . J. Avian Biol. 38, 267–272 10.1111/j.2007.0908-8857.04126.x (doi:10.1111/j.2007.0908-8857.04126.x) [DOI] [Google Scholar]
- 15.Monaghan P., Nager R. G. 1997. Why don't birds lay more eggs? Trends Ecol. Evol. 12, 270–274 10.1016/S0169-5347(97)01094-X (doi:10.1016/S0169-5347(97)01094-X) [DOI] [PubMed] [Google Scholar]
- 16.Webb D. R. 1987. Thermal tolerance of avian embryos: a review. Condor 89, 874–898 10.2307/1368537 (doi:10.2307/1368537) [DOI] [Google Scholar]
- 17.Fry D. M. 1995. Reproductive effects in birds exposed to pesticides and industrial chemicals. Environ. Health Persp. 103, S165–S171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook M. I., Beissinger S. R., Toranzos G. A., Rodriguez R. A., Arendt W. J. 2003. Trans-shell infection by pathogenic micro-organisms reduces the shelf life of non-incubated bird's eggs: a constraint on the onset of incubation? Proc. R. Soc. Lond. B 270, 2233–2240 10.1098/rspb.2003.2508 (doi:10.1098/rspb.2003.2508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beissinger S. R., Cook M. I., Arendt W. J. 2005. The shelf life of bird eggs: testing egg viability using a tropical climate gradient. Ecology 86, 2164–2175 10.1890/04-1624 (doi:10.1890/04-1624) [DOI] [Google Scholar]
- 20.Sittmann K., Abplanalp H., Fraser R. A. 1966. Inbreeding depression in Japanese quail. Genetics 54, 371–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempenaers B., Congdon B., Boag P., Robertson R. J. 1999. Extrapair paternity and egg hatchability in tree swallows: evidence for the genetic compatibility hypothesis? Behav. Ecol. 10, 304–311 10.1093/beheco/10.3.304 (doi:10.1093/beheco/10.3.304) [DOI] [Google Scholar]
- 22.Christensen V. L. 2001. Factors associated with early embryo mortality. World. Poultry Sci. J. 57, 359–372 10.1079/WPS20010025 (doi:10.1079/WPS20010025) [DOI] [Google Scholar]
- 23.Hemmings N. L., Slate J., Birkhead T. R. 2012. Inbreeding causes early death in a passerine bird. Nat. Commun. 3, 863. 10.1038/ncomms1870 (doi:10.1038/ncomms1870) [DOI] [PubMed] [Google Scholar]
- 24.Morrow E. H., Arnqvist G., Pitcher T. E. 2002. The evolution of infertility: does hatching rate in birds coevolve with female polyandry? J. Evol. Biol. 15, 702–709 10.1046/j.1420-9101.2002.00445.x (doi:10.1046/j.1420-9101.2002.00445.x) [DOI] [Google Scholar]
- 25.Potti J., Merino S. 1996. Causes of hatching failure in the pied flycatcher. Condor 98, 328–336 10.2307/1369151 (doi:10.2307/1369151) [DOI] [Google Scholar]
- 26.Cordero P. J., Aparicio J. M., Veiga J. P. 2004. Parental genetic characteristics and hatching success in the spotless starling, Sturnus unicolor. Anim. Behav. 67, 637–642 10.1016/j.anbehav.2003.06.005 (doi:10.1016/j.anbehav.2003.06.005) [DOI] [Google Scholar]
- 27.Kosin I. L. 1944. Macro- and microscopic methods of detecting fertility in unincubated hen's eggs. Poult. Sci. 23, 266–269 10.3382/ps.0230266 (doi:10.3382/ps.0230266) [DOI] [Google Scholar]
- 28.Romanoff A. L., Romanoff A. J. 1972. Pathogenesis of the avian embryo. New York, NY: Wiley [Google Scholar]
- 29.Birkhead T. R., Sheldon B. C., Fletcher F. 1994. A comparative study of sperm–egg interactions in birds. J. Reprod. Fertil. 101, 353–361 10.1530/jrf.0.1010353 (doi:10.1530/jrf.0.1010353) [DOI] [PubMed] [Google Scholar]
- 30.R Development Core Team 2009. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org). [Google Scholar]
- 31.Samour J. H., Moore H. D. M., Bailey I. T., Watson P. F. 1987. Annual testicular cycle in budgerigars (Melopsittacus undulatus). J. Zool. 212, 465–473 10.1111/j.1469-7998.1987.tb02917.x (doi:10.1111/j.1469-7998.1987.tb02917.x) [DOI] [Google Scholar]
- 32.Anderson S. J., Bird D. M., Hagen M. D. 2002. Semen characteristics of the quaker parakeet (Myiopsitta monachus). Zoo Biol. 21, 507–512 10.1002/zoo.10060 (doi:10.1002/zoo.10060) [DOI] [Google Scholar]
- 33.Saint Jalme M., Combreau O., Seddon P. J., Paillat P., Gaucher P., van Heezik Y. 1996. Restoration of Chlamydotis undulata macqueenii (Houbara bustard) populations in Saudi Arabia: a progress report. Restor. Ecol. 4, 81–87 10.1111/j.1526-100X.1996.tb00110.x (doi:10.1111/j.1526-100X.1996.tb00110.x) [DOI] [Google Scholar]
- 34.Ricklefs R. E., Scheuerlein A., Cohen A. 2003. Age-related patterns of fertility in captive populations of birds and mammals. Exp. Gerontol. 38, 741–745 10.1016/S0531-5565(03)00101-3 (doi:10.1016/S0531-5565(03)00101-3) [DOI] [PubMed] [Google Scholar]
- 35.Mazaro R., Lamano-Carvalho T. L. 2006. Prolonged deleterious effects of neonatal handling on reproductive parameters of pubertal males. Reprod. Fertil. Develop. 18, 497–500 10.1071/RD04076 (doi:10.1071/RD04076) [DOI] [PubMed] [Google Scholar]


