Abstract
Signal plasticity is considered an important step in the evolution of animal communication. In acoustic communication, signal transmission is often constrained by background noise. One adaptation to evade acoustic signal masking is the Lombard effect, in which an animal increases its vocal amplitude in response to an increase in background noise. This form of signal plasticity has been found in mammals, including humans, and some birds, but not frogs. However, the evolution of the Lombard effect is still unclear. Here we demonstrate for the first time the Lombard effect in a phylogentically basal bird species, the tinamou Eudromia elegans. By doing so, we take a step towards reconstructing the evolutionary history of noise-dependent vocal plasticity in birds. Similar to humans, the tinamous also raised their vocal pitch in noise, irrespective of any release from signal masking. The occurrence of the Lombard effect in a basal bird group suggests that this form of vocal plasticity was present in the common ancestor of all living birds and thus evolved at least as early as 119 Ma.
Keywords: acoustic communication, Lombard effect, phenotypic plasticity, noise, signal masking
1. Introduction
Noise is a major constraint on any form of communication. In particular, animals that use sound to communicate must deal with various biotic and abiotic noises in their habitats. Solutions to the problem of acoustic signal masking involve special adaptations in the receiver as well as in the sender [1–3]. On the sender's side, one mechanism to increase the signal-to-noise ratio in a noisy environment is the Lombard effect, an involuntary vocal phenomenon in which a calling animal increases its vocal amplitude in response to an increase in background noise [4]. This noise-dependent vocal plasticity requires a neural feedback loop between vocal production and perception [5], two systems that are often viewed in isolation. The Lombard effect is well known in human speech and it has also been reported in several other mammalian species, as well as some phylogentically derived bird groups, but is absent in frogs [4].
Signal plasticity is considered an important step in the evolution of animal communication systems [6]. Thus, elucidating the phylogenetic origins of vocal flexibility is important for understanding the diversification and versatility of animal signals in general [7]. However, our understanding of the evolution of the Lombard effect in birds is still incomplete, as only members of the more derived neognath lineage have been studied. Therefore, it is ambiguous whether the Lombard effect is a derived trait of the Neognathae or a shared trait of all birds. Here we investigated for the first time vocal plasticity in one of the most ‘ancient’ living groups of birds, tinamous, members of the Palaeognathae [8]. Specifically, we tested (i) whether the elegant crested tinamou, Eudromia elegans (figure 1a), exhibits the Lombard effect and (ii) whether a noise-dependent amplitude adjustment affects call frequency, as demonstrated in humans [4] and suggested for neognath birds [9,10].
Figure 1.
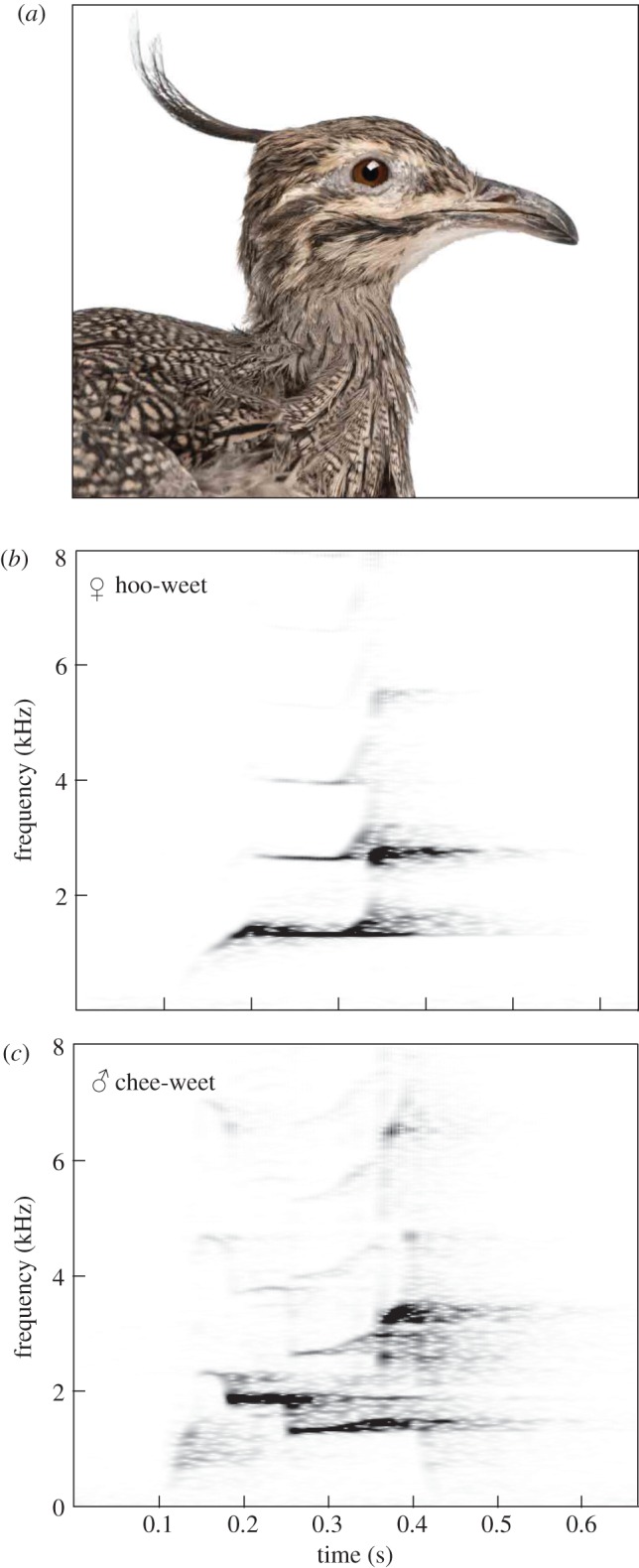
(a) Elegant crested tinamou (photo by Eric Isselée/Shutterstock.com). (b) Female hoo-weet and (c) male chee-weet call (see the electronic supplementary material, Audio S11, S12).
2. Material and methods
(a). Experimental set-up
Very little is known about the repertoire and function of elegant crested tinamou calls [11]. We monitored our captive group of three females (F1, F2 and F3) and two males (M1 and M2) for six months in a housing room (4.3 × 3.5 m and 2.2 m high, 12 L : 12 h cycle with a 10 min artificial dawn and dusk) during which time all their vocalizations were automatically recorded. For the Lombard experiments, we tested each of the tinamous singly in an aviary (1 × 1 m and 2 m high) in a sound-shielded room monitored by five video cameras. Digital sound recordings (44.1 kHz sample rate, 16-bit accuracy) were made with an omnidirectional microphone (Sennheiser ME62) suspended 1.6 m above the centre of the cage floor to a computer through an external sound card (Edirol UA-101). White noise in the frequency band from 0.01 to 10 kHz was played from a computer through an amplifier (Dynavox CS-PA1) to two loudspeakers (JBL pro III) (see the electronic supplementary material, figure S1). The speakers were mounted opposite each other at the approximate height of a tinamou's head (30 cm), 1.3 m from the centre of the cage. We broadcast the noise at two levels, varying their order systematically between birds. The playback amplitude was set at 45 dB(A) sound pressure level (SPL) for the low-noise condition and at 65 dB(A) for the high-noise condition (measured at the position of the birds' heads at the centre of the cage). Depending on the bird's exact position the received noise level varied by up to 5 dB. To elicit calling, we played a male tinamou call at 75 dB(A) at the beginning of each session using a digital playback device (Foxpro Scorpion X1-A). The noise amplitude was changed when the tested bird had called at least 12 times.
(b). Acoustic analyses and statistics
The sound analyses were carried out using the software Avisoft-SASLab Pro (v. 5.1.17) (see the electronic supplementary material). Briefly, for each call, the maximum SPL was measured (integration time 50 ms) and then the background noise value was subtracted [4]. Peak frequencies were measured in power spectra with a resolution of 0.7 Hz. Individual differences in call amplitude and frequency between the noise conditions were tested with two-sided Mann–Whitney U-tests. All statistically significant differences retained significance at p < 0.01 after Bonferroni–Holm correction. The relationship between amplitude and frequency across birds was investigated with a general linear mixed model. Sex was included in the model as a fixed factor, and individual as a random effect. We used a Wald-χ2 test to investigate the link between call amplitude and frequency.
3. Results
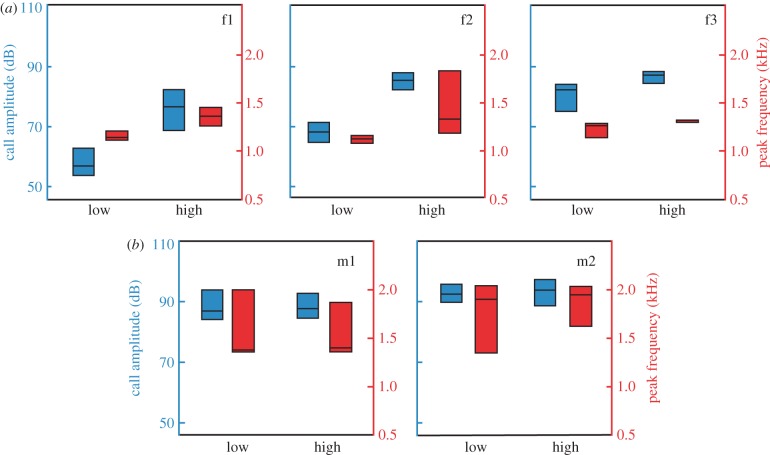
We identified at least 12 different call types in our recordings (see the electronic supplementary material, figure S3). One of the most common vocalizations were hoo-weet and chee-weet calls (figure 1b,c), which were also the only calls that were repeatably elicited by our playback. Females responded to the playbacks with hoo-weets, whereas males responded with chee-weets, which were considerably higher in amplitude than the female calls (figure 2). All females increased the amplitude of their hoo-weet calls in response to the increase in the noise (Mann–Whitney U-tests: F1: U = 46, n1 = 12, n2 = 77, p < 0.001; F2: U = 0, n1 = 17, n2 = 42, p < 0.001; F3: U = 43, n1 = 21, n2 = 17, p < 0.001; figure 2a). The increase in amplitude was associated with a rise in peak frequency (F1: U = 72.5, n1 = 12, n2 = 77, p < 0.001; F2: U = 87, n1 = 17, n2 = 42, p < 0.001; F3: U = 65.5, n1 = 21, n2 = 17, p = 0.001; figure 2a). On average, call amplitudes increased by 14 ± 8 dB (mean ± s.d.) in noise, and frequencies rose by 161 ± 92 Hz. In contrast, the males neither changed the amplitude of their chee-weet calls with increased background noise (M1: U = 371, n1 = 25, n2 = 32, p = 0.641; M2: U = 230, n1 = 21, n2 = 27, p = 0.266; figure 2b) nor their call frequency (M1: U = 399, n1 = 25, n2 = 32, p = 0.987; M2: U = 267.0, n1 = 21, n2 = 27, p = 0.732; figure 2b). However, we found a strong link between call amplitude and peak frequency across all males and females, irrespective of the level of background noise (Wald test: 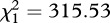 , p < 0.001).
, p < 0.001).
Figure 2.
Noise-dependent changes in call amplitude and peak frequency. Medians and interquartile ranges are given for (a) females and (b) males. Low: 45 dB(A) noise, high: 65 dB(A) noise (re. 20 µPa).
4. Discussion
We present the first evidence for the Lombard effect in a palaeognath bird. Moreover, we also found that tinamous use a large repertoire of call types that vary in structure and usage. As such, our results demonstrate that a basal bird exhibits a degree of vocal complexity and plasticity that had only been described in mammals and more derived birds. Interestingly, only the female tinamous increased their call amplitude in response to increased in background noise. The absence of a similar response in the tested males may be due to the considerably higher call amplitude of males. Males may have called closer to their physical upper limit and may therefore have had no capacity to increase their call amplitude, at least for the chee-weet call. A sex difference in the Lombard effect was also reported in a songbird, the Bengalese finch Lonchura striata [12]. Although both male and female finches exhibited the Lombard effect, the effect was weaker in females, probably because, like male tinamous, they called at higher amplitudes.
Our study also demonstrated a coupling of vocal amplitude and pitch in tinamou calls, which has previously been suggested for vocalizations of more derived birds (reviewed in [13]). Most probably this association is the result of a physical coupling during vocal production [14]. This biophysical link may have led to a frequency increase when the tinamous raised their call amplitude in elevated noise. It is important to note that the increase in call pitch did not yield an increase in the signal-to-noise ratio, as the vocalizations were masked by the broad spectrum background noise. Thus, we conclude that the noise-dependent increase in peak frequency in the tested birds is a passive response that occurs irrespective of any release from signal masking. This finding in a bird resembles the Lombard effect in humans, as speakers also involuntarily raise their vocal pitch in noise even when it would not improve signal detection [15]. Birds exposed to intense anthropogenic noise often vocalize at higher frequencies, which has been interpreted as an adaptation to mitigate masking from low frequency noise [16,17]. This frequency shift can be achieved either by using different call types [18] or by modifying the same call [17,19] similar to the Lombard effect. Higher song frequencies can be beneficial in terms of receiver responses in noise [20–22], but whether the increases in pitch are indeed the outcome of selection processes is debated [13,23]. Our data show that a noise-related increase in vocal frequency can occur irrespective of any release from signal masking, supporting the notion that the observed changes in urban bird vocalizations may be a by-product of the Lombard effect that creates a fortuitous masking release in low-frequency noise [24].
Our findings suggest that the Lombard effect may be a shared trait of extant birds, and may therefore have evolved more than 119 Ma [25]. Presuming it is also an ancestral trait in mammals, one can put forward at least two alternative phylogenetic hypotheses: (i) the Lombard effect evolved independently in the most recent common ancestor to mammals and again in ancestral birds or (ii) it is a trait shared through common descent. If the latter is true, then the common ancestor of birds and mammals, i.e. an early amniote, must have exhibited the Lombard effect. The neuronal circuits essential for the Lombard effect in mammals are located in the brainstem [5], the phylogenetically oldest part of the vertebrate brain. Moreover, given that many amphibians vocalize but do not seem to show a Lombard response [26], it is conceivable that this form of vocal plasticity might have evolved in an early amniote. To test these two hypotheses, the closest living relative of birds, the crocodilians and other members of the Sauropsidae need to be studied.
Acknowledgements
We thank Wolfgang Kunz for bird care, and Niels Rattenborg and two anonymous reviewers for comments. Financial support was provided by the Max Planck Society and the DFG (award BR 2309/6-1).
References
- 1.Klump G. M. 1996. Bird communication in the noisy world. In Ecology and evolution of acoustic communication in birds (eds Kroodsma D. E., Miller E. H.), pp. 321–338 Ithaca, NY: Comstock Publishing Associates [Google Scholar]
- 2.Brumm H., Slabbekoorn H. 2005. Acoustic communication in noise. Adv. Study Behav. 35, 151–209 10.1016/S0065-3454(05)35004-2 (doi:10.1016/S0065-3454(05)35004-2) [DOI] [Google Scholar]
- 3.Bee M. A. 2012. Sound source perception in anuran amphibians. Curr. Opin. Neurobiol. 22, 301–310 10.1016/j.conb.2011.12.014 (doi:10.1016/j.conb.2011.12.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brumm H., Zollinger S. A. 2011. The evolution of the Lombard effect: 100 years of psychoacoustic research. Behaviour 148, 1173–1198 10.1163/000579511X605759 (doi:10.1163/000579511X605759) [DOI] [Google Scholar]
- 5.Hage S. R., Jürgens U., Ehret G. 2006. Audio-vocal interaction in the pontine brainstem during self-initiated vocalization in the squirrel monkey. Eur. J. Neurosci. 23, 3297–3308 10.1111/j.1460-9568.2006.04835.x (doi:10.1111/j.1460-9568.2006.04835.x) [DOI] [PubMed] [Google Scholar]
- 6.Ord T. J., Stamps J. A., Losos J. B. 2010. Adaptation and plasticity of animal communication in fluctuating environments. Evolution 64, 3134–3148 10.1111/j.1558-5646.2010.01056.x (doi:10.1111/j.1558-5646.2010.01056.x) [DOI] [PubMed] [Google Scholar]
- 7.Kimbrough D. O., Griebel U. 2008. Evolution of communicative flexibility: complexity, creativity, and adaptability in human and animal communication. Boston, MA: MIT Press [Google Scholar]
- 8.Hackett S. J., et al. 2008. A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763–1768 10.1126/science.1157704 (doi:10.1126/science.1157704) [DOI] [PubMed] [Google Scholar]
- 9.Leonard M. L., Horn A. G. 2005. Ambient noise and the design of begging signals. Proc. R. Soc. B 272, 651–656 10.1098/rspb.2004.3021 (doi:10.1098/rspb.2004.3021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osmanski M. S., Dooling R. J. 2009. The effect of altered auditory feedback on control of vocal production in budgerigars (Melopsittacus undulatus). J. Acoust. Soc. Am. 126, 911–919 10.1121/1.3158928 (doi:10.1121/1.3158928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohl W. H. 1970. A study of the crested tinamou of Argentina. US Department of the Interior, Special Scientific Report—Wildlife No. 131, pp. 1–110. [Google Scholar]
- 12.Kobayasi K. I., Okanoya K. 2003. Sex differences in amplitude regulation of distance calls in Bengalese finches, Lunchula striata var. domestica. Anim. Biol. 53, 173–182 10.1163/157075603769700368 (doi:10.1163/157075603769700368) [DOI] [Google Scholar]
- 13.Nemeth E., Zollinger S. A., Brumm H. 2012. Effect sizes and the integrative understanding of urban bird song. Am. Nat. 180, 146–152 10.1086/665994 (doi:10.1086/665994) [DOI] [Google Scholar]
- 14.Goller F., Cooper B. G. 2008. Peripheral mechanisms of sensorimotor integration during singing. In Neuroscience of birdsong (eds Marler P., Zeigler H. P.), pp. 99–114 Cambridge, UK: Cambridge University Press [Google Scholar]
- 15.Lu Y., Cooke M. 2009. Speech production modifications produced in the presence of low-pass and high-pass filtered noise. J. Acoust. Soc. Am. 126, 1495–1499 10.1121/1.3179668 (doi:10.1121/1.3179668) [DOI] [PubMed] [Google Scholar]
- 16.Slabbekoorn H., Peet M. 2003. Birds sing at a higher pitch in urban noise. Nature 424, 267. 10.1038/424267a (doi:10.1038/424267a) [DOI] [PubMed] [Google Scholar]
- 17.Potvin D. A., Parris K. M., Mulder R. A. 2011. Geographically pervasive effects of urban noise on frequency and syllable rate of songs and calls in silvereyes (Zosterops lateralis). Proc. R. Soc. B 278, 2464–2469 10.1098/rspb.2010.2296 (doi:10.1098/rspb.2010.2296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halfwerk W., Slabbekoorn H. 2009. A behavioural mechanism explaining noise-dependent frequency use in urban birdsong. Anim. Behav. 78, 1301–1307 10.1016/j.anbehav.2009.09.015 (doi:10.1016/j.anbehav.2009.09.015) [DOI] [Google Scholar]
- 19.Bermúdez-Cuamatzin E., Ríos-Chelén A. A., Gil D., Garcia C. M. 2011. Experimental evidence for real-time song frequency shift in response to urban noise in a passerine bird. Biol. Lett. 7, 36–38 10.1098/rsbl.2010.0437 (doi:10.1098/rsbl.2010.0437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mockford E. J., Marshall R. C. 2009. Effects of urban noise on song and response behaviour in great tits. Proc. R. Soc. B 276, 2979–2985 10.1098/rspb.2009.0586 (doi:10.1098/rspb.2009.0586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halfwerk W., Bot S., Buikx J., van der Velde M., Komdeur J., ten Cate C., Slabbekoorn H. 2011. Low-frequency songs lose their potency in noisy urban conditions. Proc. Natl Acad. Sci. USA 108, 14 549–14 554 10.1073/pnas.1109091108 (doi:10.1073/pnas.1109091108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pohl N. U., Leadbeater E., Slabbekoorn H., Klump G. M., Langemann U. 2012. Great tits in urban noise benefit from high frequencies in song detection and discrimination. Anim. Behav. 83, 711–721 10.1016/j.anbehav.2011.12.019 (doi:10.1016/j.anbehav.2011.12.019) [DOI] [Google Scholar]
- 23.Eens M., Rivera-Gutierrez H. F., Pinxten R. 2012. Are low-frequency songs sexually selected, and do they lose their potency in male–female interactions under noisy conditions? Proc. Natl Acad. Sci. USA 109, E208. 10.1073/pnas.1119570109 (doi:10.1073/pnas.1119570109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemeth E., Brumm H. 2010. Birds and anthropogenic noise: are urban songs adaptive? Am. Nat. 176, 465–475 10.1086/656275 (doi:10.1086/656275) [DOI] [PubMed] [Google Scholar]
- 25.van Tuinen M., Hedges S. B. 2001. Calibration of avian molecular clocks. Mol. Biol. Evol. 18, 206–213 10.1093/oxfordjournals.molbev.a003794 (doi:10.1093/oxfordjournals.molbev.a003794) [DOI] [PubMed] [Google Scholar]
- 26.Love E. K., Bee M. A. 2010. An experimental test of noise-dependent voice amplitude regulation in Cope's grey treefrog, Hyla chrysoscelis. Anim. Behav. 80, 509–515 10.1016/j.anbehav.2010.05.031 (doi:10.1016/j.anbehav.2010.05.031) [DOI] [PMC free article] [PubMed] [Google Scholar]