Abstract
Some species have sensory systems divided into subsystems with morphologically different sense organs that acquire different types of information within the same modality. Jumping spiders (family Salticidae) have eight eyes. Four eyes are directed anteriorly to view objects in front of the spider: a pair of principal eyes track targets with their movable retinae, while the immobile anterior lateral (AL) eyes have a larger field of view and lower resolution. To test whether the principal eyes, the AL eyes, or both together mediate the response to looming stimuli, we presented spiders with a video of a solid black circle that rapidly expanded (loomed) or contracted (receded). Control spiders and spiders with their principal eyes masked were significantly more likely to back away from the looming stimulus than were spiders with their AL eyes masked. Almost no individuals backed away from the receding stimulus. Our results show that the AL eyes alone mediate the loom response to objects anterior to the spider.
Keywords: salticid, spider, vision, looming, stimulus
1. Introduction
Some sensory systems are divided into subsystems with multiple morphologically different receptors that gather information within the same modality. An example is the visual system of the jumping spiders (family Salticidae), a group known for visually mediated behaviour in prey capture [1], courtship [2] and learning [3]. Their eight eyes include a pair of large, forward-facing principal eyes with an immovable cornea formed from the spider's cuticle, and a small retina at the back of a long eye tube (for a review of salticid eye structure, see [4]). The retina has high spatial acuity and colour vision and has recently been shown to function in depth perception [5], but its small size limits its field of view. However, the principal eye tubes are movable, allowing the retinae to explore different areas of the image projected by the cornea and to track moving targets while the spider is motionless [6]. In contrast, the three pairs of secondary eyes cannot move, and have fixed corneas and large fixed retinae with lower spatial acuity than that of the principal eyes [7–9]. These eyes are superb motion detectors, and recent evidence suggests that their spatial acuity is higher than previously suspected [10,11]. Of particular interest are the forward-facing anterior lateral (AL) eyes, which overlap in their field of view with the principal eyes. We are interested in how the principal and AL eyes divide up the acquisition of visual information about objects in front of the spider.
Animals from diverse taxa rapidly detect looming objects that appear to be on a collision course, and quickly act to evade them or protect their most vulnerable body parts [12]. For example, human infants jerk their heads back and block their faces with their arms [13], fiddler crabs retreat to their burrow [14] and jumping spiders back away [15]. In many species, avoidance responses can be induced not only by the approach of three-dimensional objects, but also by two-dimensional images that grow swiftly in size, such as unfurling pieces of black cloth [14] or expanding computer images [16]. This shared response to looming objects is striking given the diversity of the eye structures of the animals that exhibit it, including the camera eye of vertebrates and the compound eyes of insects and crustaceans.
Taking advantage of the physical separation and fixed corneas of the principal and AL eyes, we used eye-masking to test whether one or both eye types together mediate a response to looming objects in the jumping spider Phidippus audax. Our looming stimulus was the one commonly used in other studies [16]: an animation of a solid black circle that grew rapidly in size. To test whether spiders responded solely to movement regardless of the circle's change in size, we also presented them with an animation of a shrinking circle.
2. Material and methods
We tested 48 adult P. audax (41 females and seven males distributed across treatment groups), captured in meadows and on structures in Hampshire and Franklin counties, MA, USA. Spiders were housed in clear plastic cages (18 × 13 × 10 cm), each containing a stick, a black tube for refuge and plastic foliage for habitat enrichment [17]. Spiders were fed crickets (Acheta domesticus) weekly, maintained at 25°C on a 15 L : 9 D cycle, and provided with water in cotton-stoppered test tubes.
We masked eyes with paint, following previous studies [18,19]. We used three treatment groups: principal eyes masked (n = 16), AL eyes masked (n = 14) and a control group with paint on their dorsal posterior cephalothorax and all eyes unpainted (n = 16). Spiders were anesthetized with carbon dioxide for less than 30 s and restrained between layers of soft foam. Under a dissecting microscope, we used an artist's spotting brush to apply opaque, non-toxic acrylic paint (Chroma Art) mixed with a gel medium (Golden Gel Medium, Heavy Gel, semi-gloss) in a 1 : 1 ratio. We applied two brightly coloured paint layers that were easily visible against the black eyes: green (Permanent Green, Deep Hue) followed by orange (Cadmium, Orange Hue). Spiders, in their restraints, were returned to their cages for at least 5 min after the first paint layer and 15 min after the final layer. They were then released into their home cages for 2–5 days before testing. After testing, we removed the paint with soft forceps.
We created looming and receding solid black circles in Adobe Flash for Macintosh and presented them on an iPod Touch (Apple Inc., Cupertino, CA). Animations were exported as .mov files (32 frames per second), opened in Apple iTunes, and converted to MPEG-4 for iPod compatibility. The looming circle changed from 0.6 to 5.5 cm in 1 s (speed = 4.9 cm s−1). The receding circle was identical but shrunk from large to small. To attract the spider's attention, the circles first vibrated side to side (a span of 13 pixels) for 3.8 s before looming or receding.
The testing arena (6.4 × 8.9 × 5.1 cm high) had three acetate-lined foam-core walls lightly sprayed with silicone to prevent spider escape. A remote-controlled, horizontally oriented iPod Touch served as the fourth wall. Indirect natural light came through a window; most illumination came from the iPod screen, which in pilot trials increased spiders' attention to it.
Before each trial, we checked that the spider's eyes were still covered. We introduced a spider into the arena by placing it into an opaque 20 cc syringe with the tip cut off, plugging the syringe with a plastic-wrap covered cotton ball, and inserting it through a hole in the arena wall. After 5 min, we removed the cotton plug and slowly depressed the plunger to release the spider gently into the arena. We watched trials on a monitor to avoid disturbing the spiders and videorecorded them for later scoring.
Spiders acclimated to the arena for 3–10 min. When the spider's anterior eyes were facing the screen, we used the remote to play the stimulus. If the spider turned away during the vibration and before the loom began, we restarted the stimulus when the spider turned back to the screen. Several spiders did not see the loom within 15 min, and we retested them at least 1 h later. We recorded whether the spider backed away from the stimulus (anterior eyes oriented towards the stimulus while moving the body backwards), took a defensive posture (raised anterior legs), or showed no response, and its distance from the screen when the stimulus played. Each spider was tested with both stimuli on the same day in random order.
We used logistic regression using JMP for Macintosh (SAS Institute) with two potential outcomes (backed away or did not back away) and four independent variables: eye-masking treatment, test stimulus, spider identity and the spider's distance from screen.
3. Results
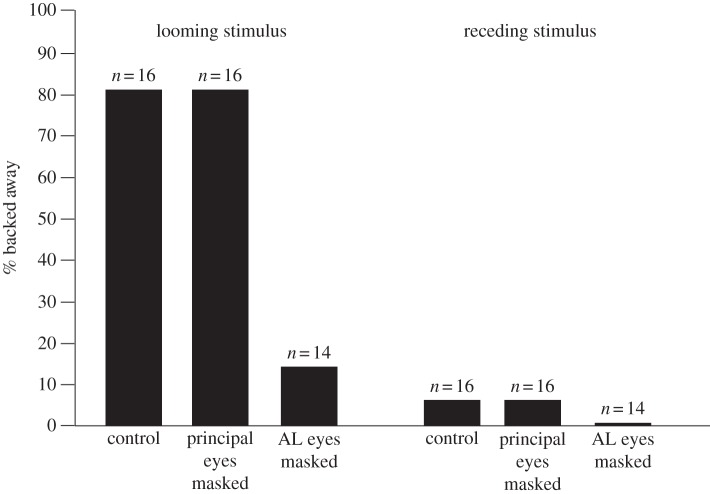
Spiders were 1.88 ± 0.12 cm (mean ± s.e.) from the screen when the stimulus played, so the expanding stimulus subtended a visual angle of approximately 18°–111°, mimicking a predator whose approach filled the visual field. Masking the AL eyes significantly reduced the looming response, whereas masking the principal eyes had no effect (figure 1; whole model test: χ2 = 56.33, d.f. = 5, p < 0.0001). Both test stimulus (effect likelihood ratio test, χ2 = 37.87, d.f. = 1, p < 0.0001) and eye-masking treatment (χ2 = 14.44, d.f. = 2, p < 0.0007) were highly significant. Eighty-one per cent of control and spiders with their principal eyes masked backed away from the looming stimulus (see electronic supplementary material), while only 14.3 per cent of AL-masked spiders backed away. The receding stimulus rarely elicited backing behaviour from any group; only two spiders (one from the group with principal eyes masked and one from the control group) backed away. Neither spider identity (χ2 = 0.48, d.f. = 1, p = 0.49) nor distance of spiders from the screen when the stimulus played (χ2 = 0.31, d.f. = 1, p = 0.57) significantly affected spider behaviour. All but two spiders raised their legs while backing away.
Figure 1.
The percentage of spiders that backed away when presented with videos of looming and receding stimuli. We tested three treatment groups: control (with paint on cephalothorax only); principal eyes masked and anterior lateral eyes masked.
4. Discussion
The AL eyes mediated the response to a looming object anterior to the spider. Masking the AL eyes significantly reduced the spiders' reaction to the looming stimulus, whereas spiders with their principal eyes masked did not differ from control spiders. Regardless of their eye-mask treatment, spiders rarely backed away from the receding stimulus; thus, it was not simply the change in size of the stimulus that caused spiders to respond.
The loom response has become a model for exploring the neural connections between sensory input and motor output [20], especially in locusts [21] and frogs [22]. In spiders, the neural basis underlying the loom response will be difficult to examine directly: their hydraulic musculature makes them poor candidates for neural recording, as puncturing the exoskeleton often causes death. However, there are themes in the findings from other species that probably apply to spiders as well. Looming sensitive neurons share the characteristics of large dendritic fields, a weak or non-existent response to optical cues related to self-initiated egocentric movement (i.e. optic flow), and responsiveness to all looming stimuli in spite of variation in texture, shape, contrast and angle of approach [12]. In locusts, the size of the image on the retina rather than the estimated time to collision generally predicts the loom response, even though the latter would appear to be more useful [12]. In salticids, although the principal eye tracks moving objects, the AL eye with its larger retina, larger visual field and motion-detecting ability [11] appears to be better suited for mediating the response to an object that looms in front of the spider. The posterior lateral (PL) eyes, similar in structure to the AL eyes, are likely to respond to looming objects approaching from the side or rear.
Although looming responses are generally induced regardless of the shape of the looming object, some species have been shown to distinguish among looming stimuli. For example, Jacky dragons (Amphibolurus muricatus) respond differently to realistic raptors than to shapes, suggesting that response specificity is determined by a hierarchical system of simple attributes combined with more complex features [16]. Whether jumping spiders are able to differentiate among looming stimuli of different shapes remains to be tested.
Acknowledgements
This work was supported by NSF IOS 0952822 to EMJ. Aram Bedrosian, Renae Brodie, Ethan Clotfelter, Anne Leonard, Sarah Partan and Denise Pope provided valuable comments.
References
- 1.Jackson R. R., Pollard S. D. 1996. Predatory behavior of jumping spiders. Annu. Rev. Entomol. 41, 287–308 10.1146/annurev.en.41.010196.001443 (doi:10.1146/annurev.en.41.010196.001443) [DOI] [PubMed] [Google Scholar]
- 2.Masta S. E., Maddison W. P. 2002. Sexual selection driving diversification in jumping spiders. Proc. Natl Acad. Sci. USA 99, 4442–4447 10.1073/pnas.072493099 (doi:10.1073/pnas.072493099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skow C. D., Jakob E. M. 2006. Jumping spiders attend to context during learned avoidance of aposematic prey. Behav. Ecol. 17, 34–40 10.1093/beheco/ari094 (doi:10.1093/beheco/ari094) [DOI] [Google Scholar]
- 4.Harland D. P., Li D., Jackson R. R. 2012. How jumping spiders see the world. In How animals see the world (eds Lazareva O. F., Shimizu T., Wasserman E. A.), pp. 133–163 Oxford, UK: Oxford University Press [Google Scholar]
- 5.Nagata T., et al. 2012. Depth perception from image defocus in a jumping spider. Science 335, 469–471 10.1126/Science.1211667 (doi:10.1126/Science.1211667) [DOI] [PubMed] [Google Scholar]
- 6.Land M. F. 1969. Movements of retinae of jumping spiders (Salticidae—Dendryphantinae) in response to visual stimuli. J. Exp. Biol. 51, 471–493 [DOI] [PubMed] [Google Scholar]
- 7.Land M. F. 1969. Structure of the retinae of the principal eyes of jumping spiders (Salticidae: Dendryphantinae) in relation to visual optics. J. Exp. Biol. 51, 443–470 [DOI] [PubMed] [Google Scholar]
- 8.Blest A. D., O'Carroll D. C., Carter M. 1990. Comparative ultrastructure of layer-I receptor mosaics in principal eyes of jumping spiders—the evolution of regular arrays of light guides. Cell Tissue Res. 262, 445–460 10.1007/BF00305241 (doi:10.1007/BF00305241) [DOI] [Google Scholar]
- 9.Williams D., McIntyre P. 1980. The principal eyes of a jumping spider have a telephoto component. Nature 288, 578–580 10.1038/288578a0 (doi:10.1038/288578a0) [DOI] [Google Scholar]
- 10.Land M. F., Nilsson D.-E. 2002. Animal Eyes. Oxford, UK: Oxford University Press [Google Scholar]
- 11.Zurek D. B., Nelson X. J. 2012. Hyperacute motion detection by the lateral eyes of jumping spiders. Vision Res. 66C, 26–30 10.1016/j.visres.2012.06.011 (doi:10.1016/j.visres.2012.06.011) [DOI] [PubMed] [Google Scholar]
- 12.Fotowat H., Gabbiani F. 2011. Collision detection as a model for sensory-motor integration. Annu. Rev. Neurosci. 34, 1–19 10.1146/annurev-neuro-061010-113632 (doi:10.1146/annurev-neuro-061010-113632) [DOI] [PubMed] [Google Scholar]
- 13.Ball W., Tronick E. 1971. Infant responses to impending collision: optical and real. Science 171, 818–820 10.1126/science.171.3973.818 (doi:10.1126/science.171.3973.818) [DOI] [PubMed] [Google Scholar]
- 14.Hemmi J. M. 2005. Predator avoidance in fiddler crabs: 2. The visual cues. Anim. Behav. 69, 615–625 10.1016/j.anbehav.2004.06.019 (doi:10.1016/j.anbehav.2004.06.019) [DOI] [Google Scholar]
- 15.Stankowich T. 2009. When predators become prey: flight decisions in jumping spiders. Behav. Ecol. 20, 318–327 10.1093/beheco/arp004 (doi:10.1093/beheco/arp004) [DOI] [Google Scholar]
- 16.Carlile P. A., Peters R. A., Evans C. S. 2006. Detection of a looming stimulus by the Jacky dragon: selective sensitivity to characteristics of an aerial predator. Anim. Behav. 72, 553–562 10.1016/j.anbehav.2005.10.027 (doi:10.1016/j.anbehav.2005.10.027) [DOI] [Google Scholar]
- 17.Carducci J. P., Jakob E. M. 2000. Rearing environment affects behaviour of jumping spiders. Anim. Behav. 59, 39–46 10.1006/anbe.1999.1282 (doi:10.1006/anbe.1999.1282) [DOI] [PubMed] [Google Scholar]
- 18.Rovner J. S. 1993. Visually mediated responses in the lycosid spider Rabidosa rabida: the roles of different pairs of eyes. Mem. Queensl. Mus. 33, 635–638 [Google Scholar]
- 19.Forster L. M. 1979. Visual mechanisms of hunting behavior in Trite planiceps, a jumping spider (Araneae, Salticidae). N Z. J. Zool. 6, 79–93 10.1080/03014223.1979.10428351 (doi:10.1080/03014223.1979.10428351) [DOI] [Google Scholar]
- 20.Card G. M. 2012. Escape behaviors in insects. Curr. Opin. Neurobiol. 22, 180–186 10.1016/j.conb.2011.12.009 (doi:10.1016/j.conb.2011.12.009) [DOI] [PubMed] [Google Scholar]
- 21.Gray J. R., Blincow E., Robertson R. M. 2010. A pair of motion-sensitive neurons in the locust encode approaches of a looming object. J. Comp. Physiol. A 196, 927–938 10.1007/s00359-010-0576-7 (doi:10.1007/s00359-010-0576-7) [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa H., Hongjian K. 2010. Collision-sensitive neurons in the optic tectum of the bullfrog, Rana catesbeiana. J. Neurophysiol. 104, 2487–2499 10.1152/jn.01055.2009 (doi:10.1152/jn.01055.2009) [DOI] [PubMed] [Google Scholar]