Abstract
Lung collapse is considered the primary mechanism that limits nitrogen absorption and decreases the risk of decompression sickness in deep-diving marine mammals. Continuous arterial partial pressure of oxygen 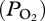 profiles in a free-diving female California sea lion (Zalophus californianus) revealed that (i) depth of lung collapse was near 225 m as evidenced by abrupt changes in
profiles in a free-diving female California sea lion (Zalophus californianus) revealed that (i) depth of lung collapse was near 225 m as evidenced by abrupt changes in 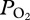 during descent and ascent, (ii) depth of lung collapse was positively related to maximum dive depth, suggesting that the sea lion increased inhaled air volume in deeper dives and (iii) lung collapse at depth preserved a pulmonary oxygen reservoir that supplemented blood oxygen during ascent so that mean end-of-dive arterial
during descent and ascent, (ii) depth of lung collapse was positively related to maximum dive depth, suggesting that the sea lion increased inhaled air volume in deeper dives and (iii) lung collapse at depth preserved a pulmonary oxygen reservoir that supplemented blood oxygen during ascent so that mean end-of-dive arterial 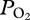 was 74 ± 17 mmHg (greater than 85% haemoglobin saturation). Such information is critical to the understanding and the modelling of both nitrogen and oxygen transport in diving marine mammals.
was 74 ± 17 mmHg (greater than 85% haemoglobin saturation). Such information is critical to the understanding and the modelling of both nitrogen and oxygen transport in diving marine mammals.
Keywords: diving physiology, lung collapse, decompression sickness, marine mammals, partial pressure of oxygen
1. Introduction
Lung collapse and subsequent lack of gas exchange have long been considered the mechanisms that limit nitrogen absorption and decrease the risk of decompression sickness in deep-diving marine mammals [1,2]. Despite the importance of this concept, documentation of lung collapse and estimation of the depth at which collapse occurs have been difficult and only obtained in a few species [3–6]. In elephant seals, Weddell seals and dolphins, estimates of the depth of lung collapse, based on blood/tissue nitrogen measurements, have ranged from 20 to 70 m [3,5,6]. In harbour seals and California sea lions, lung collapse was estimated at depths as deep as 160–170 m from pulmonary shunt determinations [4]. Depths of lung collapse in the pressure chamber studies were variable and, at least partially, dependent on inhaled air volume [3,4,7]. In free-diving elephant seals, arterial partial pressure of oxygen 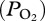 profiles revealed that
profiles revealed that 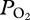 continued to increase in some dives at depths as deep as 83 m, consistent with increased inhaled air volumes and maintenance of gas exchange to greater depths in such dives [8]. Recent modelling suggests that depth of lung collapse in seals and dolphins may be much deeper than estimated in earlier field studies [9,10]. Evidence for lung collapse (cessation of gas exchange) and estimation of its depth in free-diving animals are thus critical in the modelling of blood nitrogen uptake and distribution, and in the evaluation of the potential for decompression sickness in marine mammals [9,11,12], especially considering the reports of decompression sickness in deep-diving ziphiid whales stranded after exposure to naval sonar [11,13].
continued to increase in some dives at depths as deep as 83 m, consistent with increased inhaled air volumes and maintenance of gas exchange to greater depths in such dives [8]. Recent modelling suggests that depth of lung collapse in seals and dolphins may be much deeper than estimated in earlier field studies [9,10]. Evidence for lung collapse (cessation of gas exchange) and estimation of its depth in free-diving animals are thus critical in the modelling of blood nitrogen uptake and distribution, and in the evaluation of the potential for decompression sickness in marine mammals [9,11,12], especially considering the reports of decompression sickness in deep-diving ziphiid whales stranded after exposure to naval sonar [11,13].
We investigated lung collapse using a backpack 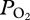 data logger in the free-diving adult female California sea lion (Zalophus californianus). Abrupt inflections in the continuous arterial
data logger in the free-diving adult female California sea lion (Zalophus californianus). Abrupt inflections in the continuous arterial 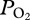 profiles during diving can provide evidence for lung collapse and be used to estimate the depth at which pulmonary gas exchange ceases (or at least significantly decreases). We hypothesized that the depth of lung collapse would be approximately 160–180 m, consistent with the previous pulmonary shunt studies in sea lions [4].
profiles during diving can provide evidence for lung collapse and be used to estimate the depth at which pulmonary gas exchange ceases (or at least significantly decreases). We hypothesized that the depth of lung collapse would be approximately 160–180 m, consistent with the previous pulmonary shunt studies in sea lions [4].
2. Material and methods
In August 2011 on San Nicolas Island, CA, USA, a lactating California sea lion (82.4 kg) was captured by hoop net, anaesthetized with isoflurane, and equipped with data loggers (see the electronic supplementary material, S1). A 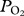 electrode was placed percutaneously in the aorta [8,14] and connected via a waterproof cable to a custom built microprocessor
electrode was placed percutaneously in the aorta [8,14] and connected via a waterproof cable to a custom built microprocessor 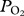 data logger mounted dorsally midline above the hips. Additionally, a time-depth recorder (TDR) and a radio transmitter were mounted above the logger. After a foraging trip to sea, the sea lion was recaptured and manually restrained while the instruments were removed. All procedures were approved by the University of California, San Diego Animals Subjects Committee and completed under National Marine Fisheries Service marine mammal permit no. 14676.
data logger mounted dorsally midline above the hips. Additionally, a time-depth recorder (TDR) and a radio transmitter were mounted above the logger. After a foraging trip to sea, the sea lion was recaptured and manually restrained while the instruments were removed. All procedures were approved by the University of California, San Diego Animals Subjects Committee and completed under National Marine Fisheries Service marine mammal permit no. 14676.
TDR data were analysed in MatLab (MathWorks, Natick, MA, USA) using a custom-written dive analysis program (IKNOS, Y. Tremblay) which calculates a zero offset correction at the surface and identifies dives on the basis of a minimum depth (5 m) and duration (20 s). 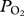 and TDR data were synchronized and a custom written MatLab code was used to obtain maximum, minimum and final
and TDR data were synchronized and a custom written MatLab code was used to obtain maximum, minimum and final 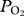 and depth at maximum
and depth at maximum 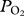 . Dives were visually inspected and estimated depths of lung collapse (first data point after the initial peak) and re-inflation (data point when
. Dives were visually inspected and estimated depths of lung collapse (first data point after the initial peak) and re-inflation (data point when 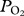 starts to increase towards the end of the dive) were determined for each serial deep dive (figure 1). As pulmonary gas exchange must occur while arterial
starts to increase towards the end of the dive) were determined for each serial deep dive (figure 1). As pulmonary gas exchange must occur while arterial 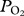 is increasing, we used the first data point after the peak to estimate a conservative depth for the large decrease in gas exchange expected with lung collapse. All means are expressed as mean ± s.d. Linear regression was used to investigate the relationship between maximum depth of dive and depth of lung collapse.
is increasing, we used the first data point after the peak to estimate a conservative depth for the large decrease in gas exchange expected with lung collapse. All means are expressed as mean ± s.d. Linear regression was used to investigate the relationship between maximum depth of dive and depth of lung collapse.
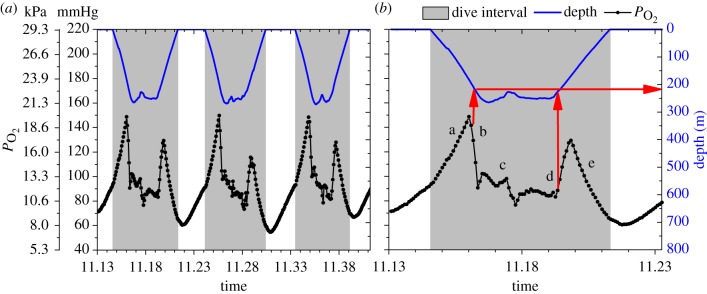
Figure 1.
Arterial 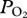 (black line, 5 s sampling interval) and depth profiles (blue line, 1 s sampling interval) from serial deep dives of a California sea lion. (a) Characteristic
(black line, 5 s sampling interval) and depth profiles (blue line, 1 s sampling interval) from serial deep dives of a California sea lion. (a) Characteristic 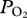 profiles of serial deep dives. (b) Typical abrupt changes in
profiles of serial deep dives. (b) Typical abrupt changes in 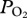 occur in this dive at approximately 200 m depth during descent and ascent (indicated with red arrows), consistent with significant cessation of gas exchange at depth. Profiles were characterized by a, an initial compression hyperoxia; b, an abrupt decline at approximately 200 m depth; c, a gradual decline in
occur in this dive at approximately 200 m depth during descent and ascent (indicated with red arrows), consistent with significant cessation of gas exchange at depth. Profiles were characterized by a, an initial compression hyperoxia; b, an abrupt decline at approximately 200 m depth; c, a gradual decline in 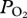 in the bottom segment of the dive; d, a rapid increase in
in the bottom segment of the dive; d, a rapid increase in 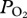 during early ascent; e, a decline in
during early ascent; e, a decline in 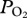 during ascent.
during ascent.
3. Results
We report new evidence for lung collapse obtained from arterial 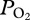 profiles in a free-diving adult female California sea lion during serial deep dives of 6.0 ± 0.5 min to depths of 306 ± 35 m (n = 48) (figure 1).
profiles in a free-diving adult female California sea lion during serial deep dives of 6.0 ± 0.5 min to depths of 306 ± 35 m (n = 48) (figure 1). 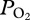 profiles were characterized by (a) an initial compression hyperoxia to a mean
profiles were characterized by (a) an initial compression hyperoxia to a mean 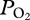 of 187 ± 46 mmHg (24.9 ± 6.1 kPa), (b) an abrupt decline at 225 ± 25 m depth, (c) a gradual decline in
of 187 ± 46 mmHg (24.9 ± 6.1 kPa), (b) an abrupt decline at 225 ± 25 m depth, (c) a gradual decline in 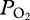 in the bottom segment of the dive, (d) a rapid increase in
in the bottom segment of the dive, (d) a rapid increase in 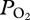 during early ascent at 247 ± 26 m, and (e) a decline in
during early ascent at 247 ± 26 m, and (e) a decline in 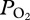 during ascent to a final
during ascent to a final 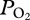 of 74 ± 17 mmHg (9.8 ± 2.3 kPa, greater than 85% haemoglobin saturation [15]; figure 1). The sudden large changes in
of 74 ± 17 mmHg (9.8 ± 2.3 kPa, greater than 85% haemoglobin saturation [15]; figure 1). The sudden large changes in 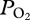 at about 225 m depth are consistent with dramatic reduction of gas exchange owing to alveolar collapse and/or pulmonary shunt. Additionally, there was a positive relationship between maximum dive depth and depth of lung collapse (F1,46 = 146.97, p < 0.001; figure 2).
at about 225 m depth are consistent with dramatic reduction of gas exchange owing to alveolar collapse and/or pulmonary shunt. Additionally, there was a positive relationship between maximum dive depth and depth of lung collapse (F1,46 = 146.97, p < 0.001; figure 2).
Figure 2.
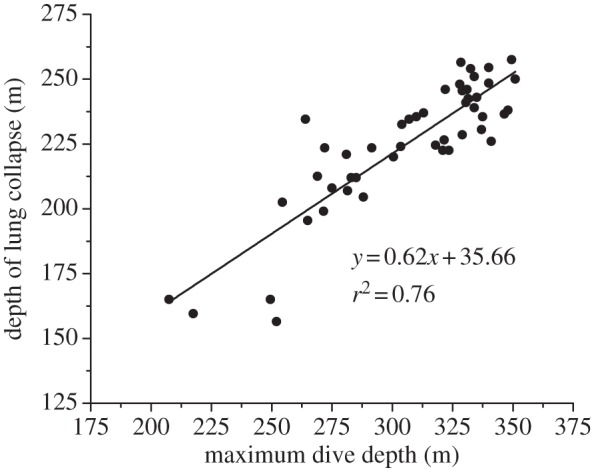
Relationship between depth of lung collapse (m) and maximum depth (m) of the dive. The positive relationship indicates that lung collapse occurs at greater depths in deeper dives, suggesting sea lions increase inhaled air volume in deeper dives (F1,46 = 146.97, p < 0.001).
4. Discussion
The double peaked arterial 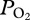 profile of the sea lion provides some of the first empirical evidence for alveolar collapse and re-expansion in a free-diving mammal. The abrupt decline in
profile of the sea lion provides some of the first empirical evidence for alveolar collapse and re-expansion in a free-diving mammal. The abrupt decline in 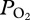 during descent and the rapid rise during ascent argue for rapid, significant changes in gas exchange at these depths, consistent with theoretical models [9,10]. Furthermore, the similarity of the two peak
during descent and the rapid rise during ascent argue for rapid, significant changes in gas exchange at these depths, consistent with theoretical models [9,10]. Furthermore, the similarity of the two peak 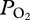 values at the same depth range suggests that the decline in
values at the same depth range suggests that the decline in 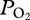 during descent is not secondary to a decrease in the O2 fraction of the lung.
during descent is not secondary to a decrease in the O2 fraction of the lung.
These data represent evidence for lung collapse in the California sea lion at a slightly deeper depth range than predicted by prior pressure chamber studies [4,7]. Differences in the exact depth of collapse in dives and chamber studies may be secondary to differences in the inhaled air volume under different conditions [3,4,7], to differences in heart rate and circulation time from the lungs to the location of the electrode in the aorta [16], or to limitations of the response time of the 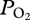 electrode [17]. Approximately 75 per cent of the variation in depth of lung collapse in this study could be attributed to maximum depth of the dive, with lung collapse occurring at greater depths in deeper dives (figure 2). As diving lung volume is positively related to depth of lung collapse [4,7], the relationship between ultimate dive depth and depth of lung collapse in a free-diving sea lion supports the concept that the sea lion inhaled larger air volumes before deeper dives (figure 2). This is similar to findings in Adélie, king and emperor penguins, in which, inhaled air volume was also positively related to dive depth [18,19]. These data suggest that sea lions, like penguins, plan their dives. On the other hand, Antarctic fur seals are thought to dive with a constant lung volume [20].
electrode [17]. Approximately 75 per cent of the variation in depth of lung collapse in this study could be attributed to maximum depth of the dive, with lung collapse occurring at greater depths in deeper dives (figure 2). As diving lung volume is positively related to depth of lung collapse [4,7], the relationship between ultimate dive depth and depth of lung collapse in a free-diving sea lion supports the concept that the sea lion inhaled larger air volumes before deeper dives (figure 2). This is similar to findings in Adélie, king and emperor penguins, in which, inhaled air volume was also positively related to dive depth [18,19]. These data suggest that sea lions, like penguins, plan their dives. On the other hand, Antarctic fur seals are thought to dive with a constant lung volume [20].
Additionally, we present the first evidence for resumption of pulmonary gas exchange upon ascent. This supplementation of blood oxygen resulted in a mean end-of-dive 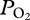 of 74 mmHg, corresponding to greater than 85 per cent haemoglobin saturation [15]. Therefore, another important function of lung collapse in the sea lion is the preservation in the upper airways of an oxygen reservoir that is then used during ascent when gas re-expands into the alveoli of the lung (figure 1). Thus, lung collapse during serial deep dives also serves to prevent severe hypoxaemia, decreasing the risk of shallow water blackout during ascent. In contrast, during the relatively shallow (less than 160 m) dives of Antarctic fur seals, it has been proposed that continued gas exchange may result in extreme lung oxygen depletion and severe hypoxaemia, necessitating the use of ascent exhalations to delay re-expansion of alveoli, and prevent reverse blood-to-lung oxygen transfer and subsequent worsening of arterial hypoxaemia [20]. Our data suggest lung collapse at depth during serial deep dives mitigates the risk of such hypoxaemia during these long duration dives of California sea lions.
of 74 mmHg, corresponding to greater than 85 per cent haemoglobin saturation [15]. Therefore, another important function of lung collapse in the sea lion is the preservation in the upper airways of an oxygen reservoir that is then used during ascent when gas re-expands into the alveoli of the lung (figure 1). Thus, lung collapse during serial deep dives also serves to prevent severe hypoxaemia, decreasing the risk of shallow water blackout during ascent. In contrast, during the relatively shallow (less than 160 m) dives of Antarctic fur seals, it has been proposed that continued gas exchange may result in extreme lung oxygen depletion and severe hypoxaemia, necessitating the use of ascent exhalations to delay re-expansion of alveoli, and prevent reverse blood-to-lung oxygen transfer and subsequent worsening of arterial hypoxaemia [20]. Our data suggest lung collapse at depth during serial deep dives mitigates the risk of such hypoxaemia during these long duration dives of California sea lions.
In summary, besides providing evidence for lung collapse and reinforcing its role in limiting nitrogen absorption at depth, our findings demonstrate another important consequence of lung collapse, namely, the preservation of oxygen for use during ascent. In addition, it appears that sea lions inhale larger air volumes for deeper dives. Lastly, the vascular access and documentation of lung collapse reported here make the well-studied, trainable California sea lion an excellent candidate for investigation of blood nitrogen uptake during free dives and evaluation of the numerical modelling of nitrogen transport.
Acknowledgements
This research was funded by the Office of Naval Research (award no. N000141010514). We thank the US Navy (specifically G. Smith and J. Ugoretz), J. Meir, M. Fowler, P. Robinson and D. Costa for logistical support and advice, B. DaValle and S. Nelson (US Navy) for anaesthesia assistance and P. Thorson, M. Tift, R. Walsh, C. Williams and G. L. Kooyman for assistance in the field.
References
- 1.Scholander P. F. 1940. Experimental investigations on the respiratory function in diving mammals and birds. Hvalrådets Skrifter 22, 1–131 [Google Scholar]
- 2.Kooyman G. L., Hammond D. D., Schroeder J. P. 1970. Bronchograms and tracheograms of seals under pressure. Science 169, 82–84 10.1126/science.169.3940.82 (doi:10.1126/science.169.3940.82) [DOI] [PubMed] [Google Scholar]
- 3.Kooyman G. L., Schroeder J. P., Denison D. M., Hammond D. D., Wright J. J., Bergman W. P. 1972. Blood nitrogen tensions of seals during simulated deep dives. Am. J. Physiol. 223, 1016–1020 [DOI] [PubMed] [Google Scholar]
- 4.Kooyman G. L., Sinnett E. E. 1982. Pulmonary shunts in harbor seals and sea lions during simulated dives to depth. Physiol. Zool. 55, 105–111 10.2307/30158447 (doi:10.2307/30158447) [DOI] [Google Scholar]
- 5.Ridgway S. H., Howard R. 1979. Dolphin lung collapse and intramuscular circulation during free diving: evidence from nitrogen washout. Science 206, 1182–1183 10.1126/science.505001 (doi:10.1126/science.505001) [DOI] [PubMed] [Google Scholar]
- 6.Falke K. J., Hill R. D., Qvist J., Schneider R. C., Guppy M., Liggins G. C., Hochachka P. W., Elliott R. E., Zapol W. M. 1985. Seal lungs collapse during free diving: evidence from arterial nitrogen tensions. Science 229, 556–558 10.1126/science.4023700 (doi:10.1126/science.4023700) [DOI] [PubMed] [Google Scholar]
- 7.Moore M. J., Hammar T., Arruda J., Cramer S., Dennison S., Montie E., Fahlman A. 2011. Hyperbaric computed tomographic measurement of lung compression in seals and dolphins. J. Exp. Biol. 214, 2390–2397 10.1242/jeb.055020 (doi:10.1242/jeb.055020) [DOI] [PubMed] [Google Scholar]
- 8.Meir J. U., Champagne C. D., Costa D. P., Williams C. L., Ponganis P. J. 2009. Extreme hypoxemic tolerance and blood oxygen depletion in diving elephant seals. Am. J. Physiol. 297, R927–939 10.1152/ajpregu.00247.2009 (doi:10.1152/ajpregu.00247.2009) [DOI] [PubMed] [Google Scholar]
- 9.Bostrom B. L., Fahlman A., Jones D. R. 2008. Tracheal compression delays alveolar collapse during deep diving in marine mammals. Respir. Physiol. Neurobiol. 161, 298–305 10.1016/j.resp.2008.03.003 (doi:10.1016/j.resp.2008.03.003) [DOI] [PubMed] [Google Scholar]
- 10.Fahlman A., Hooker S. K., Olszowka A., Bostrom B. L., Jones D. R. 2009. Estimating the effect of lung collapse and pulmonary shunt on gas exchange during breath-hold diving: the Scholander and Kooyman legacy. Respir. Physiol. Neurobiol. 165, 28–39 10.1016/j.resp.2008.09.013 (doi:10.1016/j.resp.2008.09.013) [DOI] [PubMed] [Google Scholar]
- 11.Hooker S. K., Baird R. W., Fahlman A. 2009. Could beaked whales get the bends? Effect of diving behaviour and physiology on modelled gas exchange for three species: Ziphius cavirostris, Mesoplodon densirostris and Hyperoodon ampullatus. Respir. Physiol. Neurobiol. 167, 235–246 10.1016/j.resp.2009.04.023 (doi:10.1016/j.resp.2009.04.023) [DOI] [PubMed] [Google Scholar]
- 12.Zimmer W. M. X., Tyack P. L. 2007. Repetitive shallow dives pose decompression risk in deep-diving beaked whales. Mar. Mammal. Sci. 23, 888–925 10.1111/j.1748-7692.2007.00152.x (doi:10.1111/j.1748-7692.2007.00152.x) [DOI] [Google Scholar]
- 13.Jepson P. D., et al. 2003. Gas-bubble lesions in stranded cetaceans. Nature 425, 575–576 10.1038/425575a (doi:10.1038/425575a) [DOI] [PubMed] [Google Scholar]
- 14.Ponganis P. J., Stockard T. K., Meir J. U., Williams C. L., Ponganis K. V., van Dam R. P., Howard R. 2007. Returning on empty: extreme blood O2 depletion underlies dive capacity of emperor penguins. J. Exp. Biol. 210, 4279–4285 10.1242/jeb.011221 (doi:10.1242/jeb.011221) [DOI] [PubMed] [Google Scholar]
- 15.Horvath S. M., Chiodi H., Ridgway S. H., Azar S. 1968. Respiratory and electrophoretic characteristics of hemoglobin of porpoises and sea lion. Comp. Biochem. Physiol. 24, 1027–1033 10.1016/0010-406X(68)90815-3 (doi:10.1016/0010-406X(68)90815-3) [DOI] [PubMed] [Google Scholar]
- 16.Ponganis P. J., Kooyman G. L., Winter L. M., Starke L. N. 1997. Heart rate and plasma lactate responses during submerged swimming and trained diving in California sea lions, Zalophus californianus. J. Comp. Physiol. B. 167, 9–16 10.1007/s003600050042 (doi:10.1007/s003600050042) [DOI] [PubMed] [Google Scholar]
- 17.Stockard T. K., Heil J., Meir J. U., Sato K., Ponganis K. V., Ponganis P. J. 2005. Air sac PO2 and oxygen depletion during dives of emperor penguins. J. Exp. Biol. 208, 2973–2980 (doi:10.1242/jeb.01687) [DOI] [PubMed] [Google Scholar]
- 18.Sato K., Shiomi K., Marshall G., Kooyman G. L., Ponganis P. J. 2011. Stroke rates and diving air volumes of emperor penguins: implications for dive performance. J. Exp. Biol. 214, 2854–2863 10.1242/jeb.055723 (doi:10.1242/jeb.055723) [DOI] [PubMed] [Google Scholar]
- 19.Sato K., Naito Y., Kato A., Niizuma Y., Watanuki Y., Charrassin J. B., Bost C. A., Handrich Y., Le Maho Y. 2002. Buoyancy and maximal diving depth in penguins: do they control inhaling air volume? J. Exp. Biol. 205, 1189–1197 [DOI] [PubMed] [Google Scholar]
- 20.Hooker S. K., Miller P. J. O., Johnson M. P., Cox O. P., Boyd I. L. 2005. Ascent exhalations of Antarctic fur seals: A behavioural adaptation for breath-hold diving? Proc. R. Soc. B 292, 355–363 10.1098/rspb.2004.2964 (doi:10.1098/rspb.2004.2964) [DOI] [PMC free article] [PubMed] [Google Scholar]