Abstract
The deep sea is one of the largest ecosystems on Earth and is home to a highly diverse fauna, with polychaetes, molluscs and peracarid crustaceans as dominant groups. A number of studies have proposed that this fauna did not survive the anoxic events that occurred during the Mesozoic Era. Accordingly, the modern fauna is thought to be relatively young, perhaps having colonized the deep sea after the Eocene/Oligocene boundary. To test this hypothesis, we performed phylogenetic analyses of nuclear ribosomal 18S and 28S and mitochondrial cytochrome oxidase I and 16S sequences from isopod crustaceans. Using a molecular clock calibrated with multiple isopod fossils, we estimated the timing of deep-sea colonization events by isopods. Our results show that some groups have an ancient origin in the deep sea, with the earliest estimated dates spanning 232–314 Myr ago. Therefore, anoxic events at the Permian–Triassic boundary and during the Mesozoic did not cause the extinction of all the deep-sea fauna; some species may have gone extinct while others survived and proliferated. The monophyly of the ‘munnopsid radiation’ within the isopods suggests that the ancestors of this group evolved in the deep sea and did not move to shallow-water refugia during anoxic events.
Keywords: isopoda, deep sea, ancient colonization, anoxic events, molecular clock
1. Introduction
The deep sea, defined as the layer of the ocean below 200 m depth, is the largest ecosystem on Earth and contains a high species diversity [1,2]. Polychaetes, molluscs and peracarid crustaceans (amphipods, cumaceans, tanaids and isopods) are dominant groups in this environment [2]. There are divergent hypotheses concerning the timing of deep-sea colonization by these taxa. Some authors have proposed that anoxic events and increases in deep-sea floor temperatures from the end of the Palaeozoic to the early Cenozoic caused complete extinction of the deep-sea fauna (reviewed in McClain & Hardy [3]). For example, Jacobs & Lindberg [4] argued that ‘all, or virtually all, of the deep marine habitat (during anoxic events in the mid-Mesozoic and Palaeocene) must have been uninhabitable for both normal marine invertebrate faunas and vent faunas’ (p. 9400). Therefore, under the ‘extinction and recolonization’ hypothesis, the modern deep-sea fauna either arose from colonization by shallow-water fauna after the Palaeocene [4–8], or survived during anoxic events by moving to oxygenated shallow-water refuges [4].
Other authors propose that the ancestors of some deep-sea lineages colonized this environment during or prior to the Mesozoic, and survived the multiple anoxic events that occurred subsequently [3,5]. Rather than being a cause of extinction, anoxia may have contributed to speciation in these lineages via a reduction in gene flow across anoxic waters [3].
Isopod crustaceans provide an excellent opportunity for testing hypotheses concerning the evolutionary origins of deep-sea organisms. Many of the 119 families of Isopoda are found in the deep sea [9], including most of those in one of the largest suborders, Asellota. Recent studies using molecular sequence data identified at least four independent asellotan colonizations of the deep sea, with subsequent evolution and radiation of the families in situ [10,11]. On the basis of high endemic morphological diversity within Asellota, and an early phylogenetic origination of this group, it has been argued that members of this group may have colonized the deep sea prior to the Mesozoic [5]. On the other hand, other non-asellotan deep-sea taxa, which have relatively few representatives in this environment, may have colonized more recently [5].
Hypotheses concerning the timeframe of deep-sea colonization by isopods remain untested. A lack of fossil data for deep-sea isopods has hindered attempts to understand the time of their first appearance. To address this issue, we performed a phylogenetic analysis of isopods as a whole using molecular data. We then used fossil isopod taxa to calibrate molecular-clock estimates of the timeframe for deep-sea colonizations. This allowed us to address a fundamental question of deep-sea evolution: has the modern deep-sea fauna survived through anoxic events during the Mesozoic?
2. Material and methods
We obtained nucleotide sequences of two nuclear (18S and 28S rRNA) and two mitochondrial (cytochrome oxidase I, COI and 16S rRNA) genes, comprising a combination of novel and published data (see the electronic supplementary material) for a list of taxa and accession numbers. We selected these genes because they were best represented among deep-sea isopods. 18S sequences were available for all of the organisms included in our study, whereas other genes were available only for a subset of these (see the electronic supplementary material).
Nucleotide sequences were aligned by either eye or using a combination of Muscle v. 3.8.31 and Gblocks v. 0.91b (to remove ambiguously aligned regions) with default settings. Both of these alignment methods produced almost identical results; analyses based on Muscle/Gblocks are presented. Alignments and trees are available at TreeBase.org.
To estimate the phylogeny and divergence times, we analysed the concatenated sequence alignment using maximum-likelihood (ML) and Bayesian methods. In both cases, the dataset was partitioned into four subsets: 18S, 16S, 28S and first + second codon sites of COI. We excluded the third codon sites of COI because of saturation. To examine the effects of composition heterogeneity, which can mislead phylogenetic inference and produce biases in estimates of branch lengths [12], we conducted a posterior predictive analysis in PhyloBayes [13]. On the basis of results of this test, we excluded eight ingroup taxa from subsequent analyses (see the electronic supplementary material). Using cross-validation analysis in PhyloBayes, we found that the CAT (so named because it classifies sites into categories)–general time-reversible (GTR) model provided a better fit to the dataset than the CAT or GTR models. The CAT–GTR model assumes a mixture of GTR matrices differing in their equilibrium base frequencies. For comparison, we also analysed the data using an ML phylogenetic approach (see the electronic supplementary material).
Estimates of divergence times were obtained using the autocorrelated lognormal-relaxed clock in PhyloBayes. For comparison, we also conducted a dating analysis using the uncorrelated lognormal-relaxed clock in the software BEAST [14] (see the electronic supplementary material). The two sets of date estimates were qualitatively similar and supported the same conclusions; the estimates from BEAST are presented only in the electronic supplementary material. The list of fossils used for calibration of the molecular clock is provided in the electronic supplementary material.
3. Results and discussion
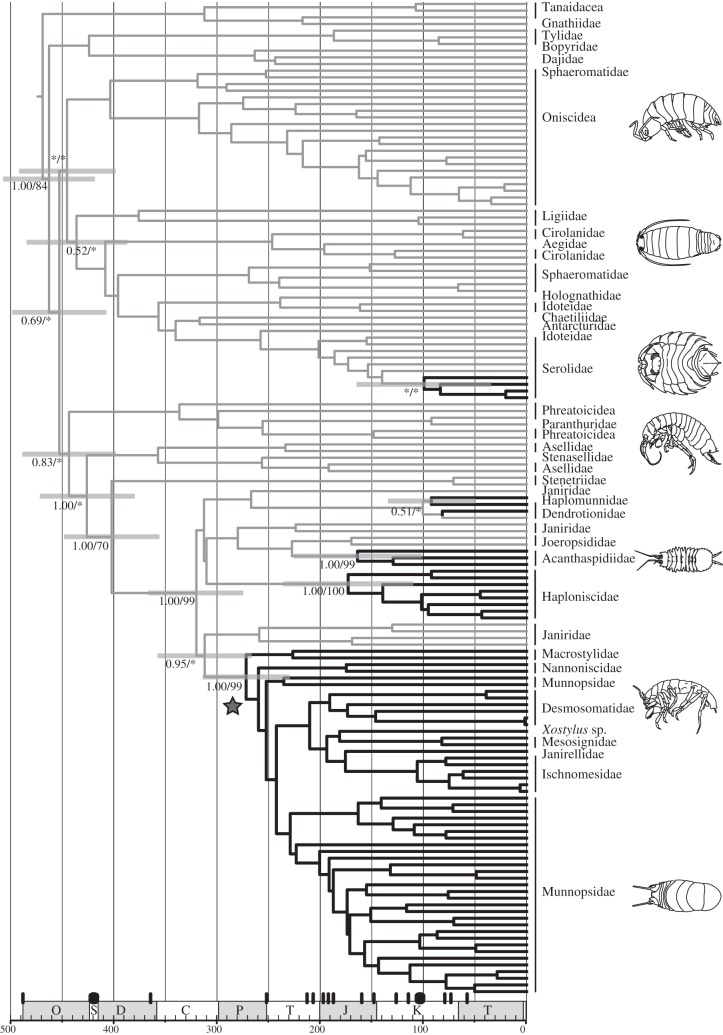
Using a combined nuclear and mitochondrial sequence dataset, we have confirmed that isopods have colonized the deep sea on multiple occasions from shallow water [10,11,15] (figure 1). Strong support (99% likelihood bootstrap support, BS; 1.0 posterior probability, PP) was found for a large asellotan clade of deep-sea isopods known as the munnopsid radiation. Our molecular-clock analysis showed that the ancestors of this clade colonized the deep sea during the Early Permian (272 Ma, 95% credibility interval: 232–314 Myr) and diversified in the deep sea. This lineage thus survived throughout the Mesozoic and Cenozoic, when the major anoxic events are thought to have occurred and extinguished the deep-sea fauna. This result is incongruent with the ‘extinction and recolonization’ hypothesis, which holds that all of the deep-sea fauna became extinct during periods of anoxia and were later replaced [6–8]. Under this hypothesis, deep-sea organisms should not be older than 90 Myr, considering that subsequent anoxic events have been less severe [4]; or 57.8 Myr, the date of the Eocene/Oligocene boundary and last major anoxic event (see Wilson [5]). Another explanation for the origin of the ancient deep-sea fauna is that the lineages persisted during anoxia by taking refuge in shallow waters. This does not appear to apply to the munnopsid radiation, because its members are almost exclusively found in the deep sea [5,10].
Figure 1.
Bayesian phylogenetic reconstruction of isopods, showing the time of colonizations by ancestral deep-sea taxa. The tree is based on analysis of 149 species, using DNA sequences of nuclear 18S and 28S and mitochondrial 16S and COI. Support values for deep-sea clades (thick lines) and stem nodes are given as ML bootstrap support (BS) percentages (1000 replicates) and Bayesian posterior probabilities (PP) (trees with support values for all nodes are provided in the electronic supplementary material). The tree was rooted using outgroups from the pericarid order Tanaidacea. Asterisks indicate <50% BS or <0.5 PP. Branch lengths are proportional to time and node bars denote 95% credibility intervals of the estimated node ages of interest. Black bars on the geological timescale show the major anoxic events [3]. The star indicates the clade known as the ‘munnopsid radiation’. Character states on ancestral branches are based on the assumption that the common ancestor (root) inhabited shallow water. We made the conservative assumption that transitions to the deep sea occurred later rather than earlier (analogous to the DELTRAN parsimony criterion, which favours parallelisms over reversals).
We found strong support for parallel colonization of the deep sea by ancestors of the other asellotan families Acanthaspidiidae and Haploniscidae (99–100% BS, 1.0 PP). These colonizations were also inferred to be ancient, occurring at 164 Ma (95% CI: 101–223 Myr) and 173 Ma (95% CI: 110–234 Myr), respectively. Although these colonizations are estimated to have occurred more recently than that of the munnopsid colonization, they are nonetheless prior to key anoxia events of the late Mesozoic and early Cenozoic.
The survival of fauna through major anoxic events might have been possible if the Mesozoic deep sea contained oxygenated refuges that allowed fauna to subsist [5,6]. Such refugia, separated by anoxic areas, may have promoted speciation in the deep sea [3]. It is also possible that anoxia, in the areas where it occurred, caused the extinction of some groups and perhaps selected those able to survive at low oxygen concentration, which would then have recolonized the environment when oxygen restrictions disappeared [8,16]. Conditions of low oxygen and food supply at the end of the Permian resulted in low-diversity communities composed of small organisms such as gastropods [8,17]. Similarly, the asellotan families that evolved in the deep sea and survived the anoxic periods comprise small-bodied isopods when compared with primarily shallow-water families [5].
In contrast with the ancient groups, other lineages appear to have colonized the deep sea more recently. Members of the non-asellotan family Serolidae, the genera Ceratoserolis and Cuspidocerolis (99 Ma 95% CI: 44–172 Myr), colonized the deep sea well after the origins of the serolid shallow-water lineages 186 Ma (95% CI: 133–279 Myr), although nodal support for the grouping Cuspidiserolis and Ceratoserolis was low (<50% BS; <0.5 PP). Those genera have reduced eyes, which is a typical adaptation to deep waters. Although blind species of deep-sea serolids exist, these were not available for our study.
A molecular dating study of deep-sea echinoids found that generalist omnivore taxa have migrated to the deep sea in relatively low numbers over the last 200 Myr, with only small numbers of taxa having survived major anoxic events [18]. On the other hand, the study found several independent colonizations of the deep sea by detritivore echinoids between 75 and 55 Ma, after the last major global anoxic event at 93 Ma [18]. The authors concluded that anoxic events have played only a subsidiary role in determining diversity of deep-sea echinoids. These results are similar to ours in that deep-sea colonizations by isopods have occurred on multiple occasions over the last 210 Myr. However, the major radiation of deep-sea isopods (munnopsids) followed an ancient colonization, whereas that of echinoids (detritivores) occurred relatively recently. Our study, combined with those on echinoids and other taxa (e.g. hydrothermal vent taxa; reviewed in McClain & Hardy [3]), argues against hypotheses of a uniformly ‘young’ or ‘old’ deep-sea fauna.
Our study is, to our knowledge, the first to use a molecular-clock approach to demonstrate that the deep-sea isopod fauna has persisted since the end of the Palaeozoic Era. Our results are in conflict with the widely held notion that anoxic events and temperature fluctuations caused the extinction of most major deep-sea groups, and that recolonization from shallow water occurred after the Cretaceous Period. Multiple groups seem to show alternative patterns of deep-sea colonization, even within isopods. Date estimates for deep-sea colonizations in other groups will help us to clarify the factors that influence deep-sea biodiversity.
Acknowledgements
This research was supported by a University of Sydney International Scholarship to L.L. We thank Torben Riehl, Tim Lee and Shane Ahyong for valuable discussion, and two anonymous referees and Patrick Stewart for comments on the manuscript.
References
- 1.Nybakken W., Bertness M. D. 2004. Marine biology: an ecological approach, 6th edn San Francisco, CA: Benjamin Cummings [Google Scholar]
- 2.Gage J. D., Tyler P. A. 1991. Deep-sea biology: a natural history of organisms at the deep-sea floor. Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.McClain C. R., Hardy S. M. 2010. The dynamics of biogeographic ranges in the deep sea. Proc. R. Soc. B 277, 3533–3546 10.1098/rspb.2010.1057 (doi:10.1098/rspb.2010.1057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs D. K., Lindberg D. R. 1998. Oxygen and evolutionary patterns in the sea: onshore/offshore trends and recent recruitment of deep-sea faunas. Proc. Natl Acad. Sci. USA 95, 9396–9401 10.1073/pnas.95.16.9396 (doi:10.1073/pnas.95.16.9396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson G. D. F. 1999. Some of the deep-sea fauna is ancient. Crustaceana 72, 1020–1030 [Google Scholar]
- 6.Horne D. J. 1999. Ocean circulation modes of the Phanerozoic: implications for the antiquity of deep-sea benthonic invertebrates. Crustaceana 72, 999–1018 10.1163/156854099503906 (doi:10.1163/156854099503906) [DOI] [Google Scholar]
- 7.Stock J. H. 1986. Deep sea origin of cave faunas: an unlikely supposition. Stygologia 2, 105–111 [Google Scholar]
- 8.Benton M. J., Twitchett R. J. 2003. How to kill (almost) all life: the end-Permian extinction event. Trends Ecol. Evol. 18, 358–365 10.1016/S0169-5347(03)00093-4 (doi:10.1016/S0169-5347(03)00093-4) [DOI] [Google Scholar]
- 9.Schotte M., Boyko C. B., Bruce N. L., Poore G. C. B., Taiti S., Wilson G. D. F. 2008. World list of marine freshwater and terrestrial isopod crustaceans. Washington, DC: National Museum of Natural History Smithsonian Institution. See http://invertebrates.si.edu/isopod/. [Google Scholar]
- 10.Raupach M. J., Mayer C., Malyutina M., Wagele J.-W. 2009. Multiple origins of deep-sea Asellota (Crustacea: Isopoda) from shallow waters revealed by molecular data. Proc. R. Soc. B 276, 799–808 10.1098/rspb.2008.1063 (doi:10.1098/rspb.2008.1063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raupach M. J., Held C., Wagele J. W. 2004. Multiple colonization of the deep sea by the Asellota (Crustacea: Peracarida: Isopoda). Deep-Sea Res. Part II-Top. Stud. Oceanogr. 51, 1787–1795 10.1016/j.dsr2.2004.06.035 (doi:10.1016/j.dsr2.2004.06.035) [DOI] [Google Scholar]
- 12.Jermiin L. S., Ho S. Y. W., Ababneh F., Robinson J., Larkum A. W. D. 2004. The biasing effect of compositional heterogeneity on phylogenetic estimates may be underestimated. Syst. Biol. 53, 638–643 10.1080/10635150490468648 (doi:10.1080/10635150490468648) [DOI] [PubMed] [Google Scholar]
- 13.Lartillot N., Lepage T., Blanquart S. 2009. PhyloBayes 3: a Bayesian software package for phylogenetic reconstruction and molecular dating. Bioinformatics 25, 2286–2288 10.1093/bioinformatics/btp368 (doi:10.1093/bioinformatics/btp368) [DOI] [PubMed] [Google Scholar]
- 14.Drummond A. J., Ho S. Y. W., Phillips M. J., Rambaut A. 2006. Relaxed phylogenetics and dating with confidence. PLoS Biol. 4, e88. 10.1371/journal.pbio.0040088 (doi:10.1371/journal.pbio.0040088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson G. D. 1980. New insights into the colonization of the deep sea: systematics and zoogeography of the Munnidae and the Pleurogoniidae comb. nov. (Isopoda; Janiroidea). J. Nat. Hist. 14, 215–236 10.1080/00222938000770201 (doi:10.1080/00222938000770201) [DOI] [Google Scholar]
- 16.Raup D. M. 1979. Size of the Permo-Triassic bottleneck and its evolutionary implications. Science 206, 217–218 10.1126/science.206.4415.217 (doi:10.1126/science.206.4415.217) [DOI] [PubMed] [Google Scholar]
- 17.Payne J. L. 2005. Evolutionary dynamics of gastropod size across the end-Permian extinction and through the Triassic recovery interval. Paleobiology 31, 269–290 10.1666/0094-8373(2005)031[0269:EDOGSA]2.0.CO;2 (doi:10.1666/0094-8373(2005)031[0269:EDOGSA]2.0.CO;2) [DOI] [Google Scholar]
- 18.Smith A. B., Stockley B. 2005. The geological history of deep-sea colonization by echinoids: roles of surface productivity and deep-water ventilation. Proc. R. Soc. B 272, 865–869 10.1098/rspb.2004.2996 (doi:10.1098/rspb.2004.2996) [DOI] [PMC free article] [PubMed] [Google Scholar]