Abstract
Spatial cognition is predicted to be a fundamental component of fitness in many lizard species, and yet some studies suggest that it is relatively slow and inflexible. However, such claims are based on work conducted using experimental designs or in artificial contexts that may underestimate their cognitive abilities. We used a biologically realistic experimental procedure (using simulated predatory attacks) to study spatial learning and its flexibility in the lizard Eulamprus quoyii in semi-natural outdoor enclosures under similar conditions to those experienced by lizards in the wild. To evaluate the flexibility of spatial learning, we conducted a reversal spatial-learning task in which positive and negative reinforcements of learnt spatial stimuli were switched. Nineteen (32%) male lizards learnt both tasks within 10 days (spatial task mean: 8.16 ± 0.69 (s.e.) and reversal spatial task mean: 10.74 ± 0.98 (s.e.) trials). We demonstrate that E. quoyii are capable of flexible spatial learning and suggest that future studies focus on a range of lizard species which differ in phylogeny and/or ecology, using biologically relevant cognitive tasks, in an effort to bridge the cognitive divide between ecto- and endotherms.
Keywords: spatial learning, cognition, reptile, associative learning
1. Introduction
The ability to have quick and flexible learning is predicted to arise when animals face complex and variable environmental challenges [1,2]. In consequence, testing animals using biologically relevant cognitive tasks and under conditions where they have access to the full range of stimuli available in their natural environment is particularly important for a realistic understanding of the rate and flexibility of learning [1,2]. Such studies are not easy to implement [1–3] and are therefore relatively uncommon, but they provide valuable insight into learning performance in the environments in which the cognitive traits have evolved. Experimental procedures using cognitive tests that do not adequately consider the animal's biology may thus be misleading. This has often been the case in reptiles, where many cognitive studies have been conducted in the laboratory under conditions that fail to reflect the type of ecological problems faced in the wild, or using experimental paradigms designed for mammals [4]. Not surprisingly, some of these studies have reported comparatively poor learning abilities in some reptile groups, leading to the widespread conclusion that reptile cognition is generally less sophisticated than in other vertebrate groups [4,5].
Particularly striking is the case of spatial cognition in lizards. Lizards are often faced with predatory threats that require them to quickly learn the location of suitable escape routes and refuges within their home range, and flexibly adjust the use of such refuges according to whatever contingencies may arise [6] (e.g. location of predators in their surroundings or obstacles to refuges). Although the available evidence shows that snakes and lizards are capable of learning the spatial location of food items or shelters in the laboratory [4,7–11], some studies seem to suggest that they have limited spatial cognitive abilities and require many training trials to learn simple spatial tasks [4]. Here, we used an ecologically relevant anti-predator context to study whether lizards show evidence of flexible spatial learning using two replicate groups of lizards maintained in large semi-natural outdoor enclosures.
2. Material and methods
(a). Study species
Eastern water skinks (Eulamprus quoyii) were collected from five different sites throughout the Sydney region and maintained in a captive colony at Macquarie University. We only used males because they are better spatial learners than females (unpublished data) and occupy larger home ranges, probably experiencing stronger selection/development of spatial cognitive abilities. Sixty adult male lizards were tested in two separate groups (30 males between September 1–10 (10 days) and 30 males between September 12–22 (11 days), 2011).
(b). Learning tasks
Each lizard was housed individually in a large outdoor tub measuring 3.2 m in diameter and 0.5 m high (approx. 8 m2). Each tub contained mulch substrate and two water bowls (15 × 9.5 × 7 cm) with three separate stacks of two terra-cotta roofing tiles, which were used as refuges by the lizards (see the electronic supplementary material, figure S1). Refuges were separated by approximately 1 m and were spread out along the edges of the arena. Each lizard was subjected to two consecutive learning tasks. In task 1 (spatial learning), lizards were trained to learn the location of a randomly chosen ‘safe’ refuge over four consecutive days (3 trials per day). In task 2 (reversal spatial-learning), we conducted a choice reversal by randomly choosing a new ‘safe’ refuge and repeating the protocol for up to six more consecutive days (3 trials per day). A reversal-learning task is one in which positive and negative contingencies are switched (but still present) with respect to a previous learning task so that individuals have to reverse previously created associations. Conducting a reversal allowed us to ask whether spatial cognitive ability under semi-natural conditions is flexible. We simulated predatory attacks by entering tubs and approaching and chasing lizards around their enclosure and/or by lifting incorrect refuges until lizards entered the ‘safe’ refuge (refuges were immediately replaced in their original position). Although it was not possible to scare lizards following a blind protocol, we made every attempt to reduce observer bias. First, the approach direction and order that each unsafe refuge was lifted were both randomized and different for each trial to prevent biases in flight direction. Second, each lizard was scared following a standardized protocol. Lizards were always scared from behind and gently tapped on the pelvic girdle. We followed each lizard closely from behind (within an arms length) and allowed lizards to choose their flight direction to the safe refuge. In many cases, lizards ran past the unsafe refuge in the direction of the safe refuge. Since trials were conducted under semi-natural conditions, we could not completely control for chemical cues. We implemented a control prior to the reversal task for group 2, which strongly suggests that lizards were using spatial cues to locate the ‘safe’ refuge. In addition, lizards did not tongue flick during scares, and flight responses were too quick to suggest the use of chemical tropotaxis (see the electronic supplementary material). Trials were conducted during the active period of the day (11.00–17.00 h) with at least 1 h between trials. During each trial, we recorded: (i) whether the lizard was in the safe refuge; (ii) the first refuge chosen by the lizard; (iii) the number of incorrect choices; and (iv) the latency to enter the safe refuge. For each lizard, we designated its learning trial as the last in a run of five successive correct trials in a row. Only lizards that were successful at both the spatial learning and choice reversal tasks were included in the learner group.
Our learning criteria and curves considered lizards found within the ‘safe’ refuge at the start of the trial as having made a correct choice (see electronic supplementary material, for details). We believe that this is a biologically sensible interpretation because learners are predicted to take refuge in the ‘safe’ refuge. However, we acknowledge that this scoring method might inflate apparent learning (see electronic supplementary material). To ensure that this was not the case, we replicated our original statistical analysis of learning curves, but considering lizards already found within the safe refuge to have made ‘no choice’. Results of this analysis are similar (see electronic supplementary material) and did not affect our final conclusion. Lizards already in the safe refuge were considered to have zero latency to enter the safe refuge and these values were subsequently removed prior to analysis.
(c). Data analysis
We used generalized linear mixed models (GLMM) to test for significant decreases in mean number of incorrect choices (ICC) and mean latency to enter the safe refuge (LSR) across learning blocks (see electronic supplementary material, for details).
3. Results
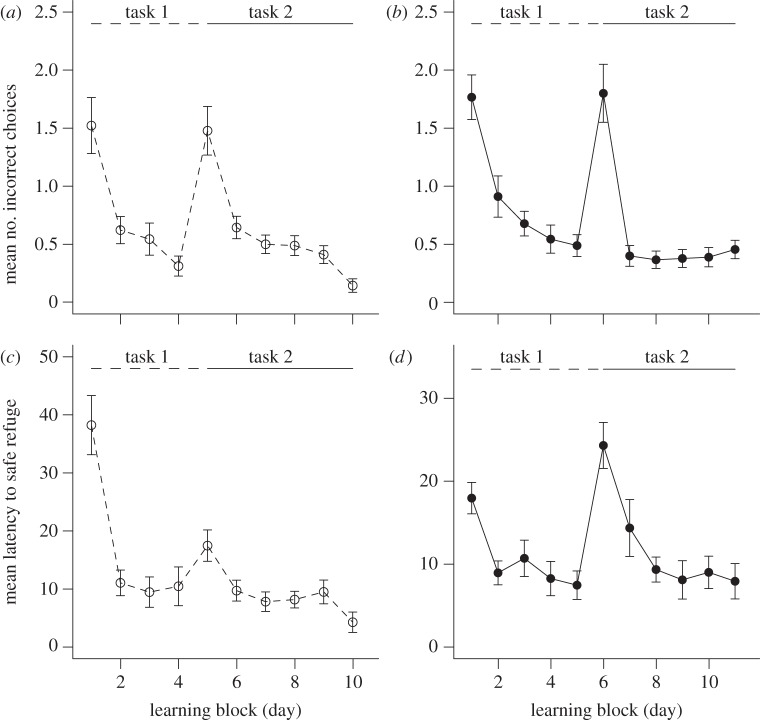
Body size and mass did not differ significantly between the groups (Wilcoxon test; SVL: W = 144, p = 0.52 and mass: W = 143.5, p = 0.59). Lizards in group 1 exhibited a significant decrease in both ICC and LSR from day 1 to day 2 in task 1 (ICC: β = −0.98 ± 0.42, p = 0.02 and LSR: β = −1.36 ± 0.45, p = 0.002; figure 1a,c); however, in task 2 (choice reversal) a significant decrease in ICC was observed by day 3 (β = −1.03 ± 0.43, p = 0.017; figure 1a) whereas LSR did not decrease significantly until day 10 (β = −1.66 ± 0.51, p = 0.001; figure 1c). In group 2, ICC and LSR decreased significantly by day 2 in task 1 (ICC: β = −0.78 ± 0.33, p = 0.02 and LSR: β = −0.76 ± 0.32, p = 0.02; figure 1b,d) and in task 2 (ICC: β = −1.52 ± 0.36, p < 0.001 and LSR: β = −1.13 ± 0.34, p = 0.001; figure 1b,d). The proportion of lizards outside the unsafe refuges in tasks 1 and 2 for both groups decreased across trials (figure 2a,b).
Figure 1.
(a,b) Mean (±s.e.) number of incorrect choices and (c,d) the mean (±s.e.) latency until entering the safe refuge across trials for group 1 (a,c; open circle and dashed line) and group 2 (b,d) filled circle and solid line) for learners and non-learners combined. Refer to the GLMM results for statistical significance.
Figure 2.
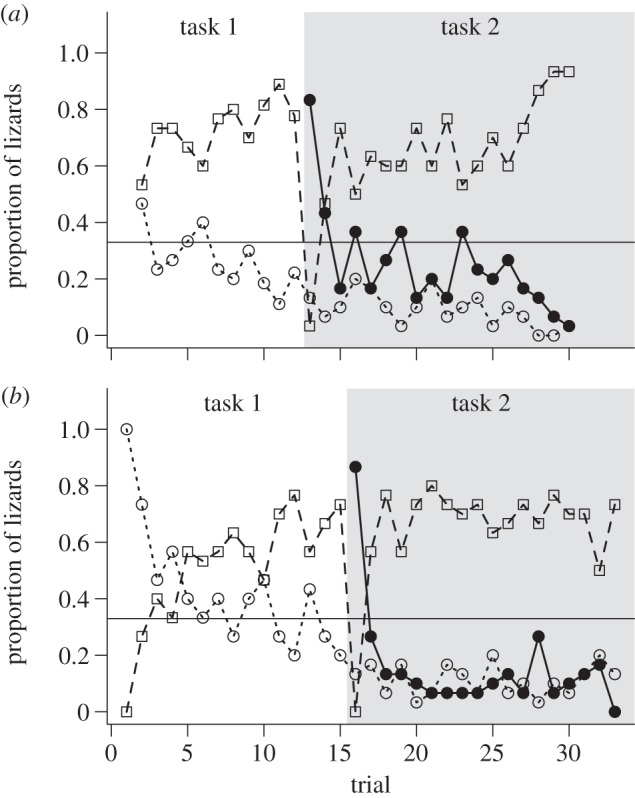
(a) Group 1; (b) group 2. Proportion of lizards in the correct safe refuge (open square and dashed line), in the incorrect refuge (open circle and dotted line) and in the former safe refuge (filled circle and solid line) in successive trials for learners and non-learners combined. In task 2, the sum of the lizards near the former safe refuge and lizards near the incorrect refuge equals the lizards near the incorrect refuge in task 1. Each refuge is predicted to be chosen by chance 33% of the time and this is represented by the horizontal solid line.
Nineteen lizards (32%) were categorized as learners (group 1, n = 11; group 2, n = 8; see table S1 in electronic supplementary material). In task 1, lizards learnt the position of the safe refuge within 5–14 trials (mean = 8.16 ± 0.69; n = 19). In task 2, lizards learned the position of the new safe refuge after 6–17 trials (mean = 10.74 ± 0.98; n = 19). The difference in median learning trial between tasks was marginally non-significant (V = 30, p = 0.052).
4. Discussion
The long-standing idea that squamates (lizards and snakes) have poor cognitive abilities has been spurred in part by the use of inadequate or ecologically irrelevant cognitive tasks [4,5], and is being challenged by recent studies [11–14]. Here, we provide evidence of flexible spatial learning in lizards tested under a biologically meaningful anti-predatory context in semi-natural conditions. In both tasks, lizards were performing above chance in the span of only 3–4 days (3 trials per day), and at least 19 out of 60 lizards learned to avoid unsafe refuges in both the spatial and the reversal task. It is important to note that our data do not provide insight into the mechanism(s) that may be responsible for spatial learning. Nonetheless, our findings contrast with previously available studies reporting that lizards require dozens of trials before learning a relatively simple spatial task if they learn at all [4,8]. This may be due to the larger spatial scale of our experiment, or to the fact that lizards had access to the whole range of spatial cues that they would have access to in their natural habitat (e.g. landmarks, distal cues, geometric cues), whereas laboratory experiments usually focus on a restricted range of cues.
Our data fit well with theoretical expectations given that, in the wild, most lizards need to process complex spatial information that is crucial to fitness [11]. For example, some lizard species exhibit wide-ranging mate searching while others defend territories, both of which require knowledge of the spatial location of mates and resources. Comparative cognition studies of lizards from a range of clades and with diverse mating systems and ecology will enable us to test the generality of these findings and allow us to better understand how lizard spatial cognition stacks up with the traditional bird and mammal model systems.
Acknowledgements
All research was carried out under the approval of the AEC committee of Macquarie University (2011–018).
References
- 1.Dukas R. 1998. Cognitive ecology: prospects. In Cognitive ecology: the evolutionary ecology of information processing and decision making (ed. Dukas R.), pp. 405–408 Chicago, IL: University of Chicago Press [Google Scholar]
- 2.Healy S., Rowe C. 2010. Information processing: the ecology and evolution of cognitive abilities. In Evolutionary behavioral ecology (eds Westneat D., Fox C. W.), pp. 162–174 Oxford, UK: Oxford University Press [Google Scholar]
- 3.Healy S. D., Hurly T. A. 2003. Cognitive ecology: foraging in hummingbirds as a models system. Adv. Study Behav. 32, 325–359 10.1016/S0065-3454(03)01007-6 (doi:10.1016/S0065-3454(03)01007-6) [DOI] [Google Scholar]
- 4.Burghardt G. M. 1977. Learning processes in reptiles. In Biology of the reptilia (eds Gans C., Tinkle T. W.), pp. 555–681 New York, NY: Academic Press [Google Scholar]
- 5.Wilkinson A., Huber L. 2012. Cold-blooded cognition: reptilian cognitive abilities. In Oxford handbook of comparative evolutionary psychology (eds Vonk J., Shackleford T. K.), pp. 1–8 Oxford, UK: Oxford University Press [Google Scholar]
- 6.Cooper W. F., Jr, Wilson D. S. 2007. Beyond optimal escape theory: microhabitats as well as predation risk affect escape and refuge use by the phrynosomatid lizard Sceloporus virgatus. Behaviour 144, 1235–1254 10.1163/156853907781890940 (doi:10.1163/156853907781890940) [DOI] [Google Scholar]
- 7.Holtzman D. A., Harris T. W., Aranguren G., Bostock E. 1999. Spatial learning of an escape task by young corn snakes, Elaphe guttata guttata. Anim. Behav. 57, 51–60 10.1006/anbe.1998.0971 (doi:10.1006/anbe.1998.0971) [DOI] [PubMed] [Google Scholar]
- 8.Day L. B., Crews D., Wilczynski W. 1999. Spatial and reversal learning in congeneric lizards with different foraging strategies. Anim. Behav. 57, 393–407 10.1006/anbe.1998.1007 (doi:10.1006/anbe.1998.1007) [DOI] [PubMed] [Google Scholar]
- 9.Day L. B., Ismail N., Wilczynski W. 2003. Use of position and feature cues in discrimination learning by the whiptail lizard (Cnemidophorus inornatus). J. Comp. Psychol. 117, 440–448 10.1037/0735-7036.117.4.440 (doi:10.1037/0735-7036.117.4.440) [DOI] [PubMed] [Google Scholar]
- 10.Punco F., Madragon S. 2002. Spatial learning in Australian skinks of the genus Ctenotus (Scindidae). Amphib-Reptilia 23, 233–238 [Google Scholar]
- 11.Carazo P., Font E., Desfilis E. 2008. Beyond ‘nasty neighbours’ and ‘dear enemies’? Individual recognition by scent marks in a lizard (Podarcis hispanica). Anim. Behav. 76, 1953–1963 10.1016/j.anbehav.2008.08.018 (doi:10.1016/j.anbehav.2008.08.018) [DOI] [Google Scholar]
- 12.Davis K. M., Burghardt G. M. 2011. Turtles (Pseudemys nelsoni) learn about visual cues indicating food from experienced turtles. J. Comp. Psychol. 125, 267–273 [DOI] [PubMed] [Google Scholar]
- 13.Leal M., Powell B. J. 2012. Behavioural flexibility and problem-solving in a tropical lizard. Biol. Lett. 8, 28–30 10.1098/rsbl.2011.0480 (doi:10.1098/rsbl.2011.0480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manrod J. D., Hartdegen R., Burghardt G. M. 2008. Rapid solving of a problem apparatus by juvenile black-throated monitor lizards (Varanus albigularis albigularis). Anim. Cogn. 11, 267–273 10.1007/s10071-007-0109-0 (doi:10.1007/s10071-007-0109-0) [DOI] [PubMed] [Google Scholar]