Abstract
The antibiotic myxopyronin (Myx) functions by inhibiting bacterial RNA polymerase (RNAP). The binding site on RNAP for Myx—the RNAP “switch region SW1/SW2 subregion”—is different from the binding site on RNAP for the RNAP inhibitor currently used in broad-spectrum antibacterial therapy, rifampin (Rif). Here, we report the frequency, spectrum, and fitness costs of Myx resistance in Staphylococcus aureus. The resistance rate for Myx is 4 × 10−8 to 7 × 10−8 per generation, which is equal within error to the resistance rate for Rif (3 × 10−8 to 10 × 10−8 per generation). Substitutions conferring Myx resistance were obtained in the RNAP β subunit [six substitutions: V1080(1275)I, V1080(1275)L, E1084(1279)K, D1101(1296)E, S1127(1322)L, and S1127(1322)P] and the RNAP β′ subunit [five substitutions: K334(345)N, T925(917)K, T925(917)R, G1172(1354)C, and G1172(1354)D] (residues numbered as in Staphylococcus aureus RNAP and, in parentheses, as in Escherichia coli RNAP). Sites of substitutions conferring Myx resistance map to the RNAP switch region SW1/SW2 subregion and do not overlap the binding site on RNAP for Rif, and, correspondingly, Myx-resistant mutants exhibit no cross-resistance to Rif. All substitutions conferring Myx resistance exhibit significant fitness costs (4 to 15% per generation). In contrast, at least three substitutions conferring Rif resistance exhibit no fitness costs (≤0% per generation). The observation that all Myx-resistant mutants have significant fitness costs whereas at least three Rif-resistant mutants have no fitness costs, together with the previously established inverse correlation between fitness cost and clinical prevalence, suggests that Myx resistance is likely to have lower clinical prevalence than Rif resistance.
INTRODUCTION
Myxopyronin (Myx) is an α-pyrone antibiotic produced by Myxococcus fulvus Mf50 (18, 20, 23, 49). Myx exhibits broad-spectrum antibacterial activity, with potent antibacterial activity against most Gram-positive species and some Gram-negative species. Myx is under investigation as a potential lead compound for broad-spectrum antibacterial therapy.
Myx functions by inhibiting bacterial RNA polymerase (RNAP) (3, 18, 20, 32, 49). The binding site on RNAP for Myx is located in the RNAP “switch region” and comprises the RNAP switch region structural elements termed “switch 1” and “switch 2,” (switch region SW1/SW2 subregion) (3, 18, 32, 49). The binding site on RNAP for Myx is different from the binding site on RNAP for the RNAP inhibitor in current use in broad-spectrum antibacterial therapy, rifampin (Rif) (3, 18, 32, 49). Accordingly, Myx exhibits no cross-resistance with Rif (18, 19, 32, 33, 49).
Previous studies have provided information about spontaneous resistance frequencies and resistance spectra for Myx (31, 32). However, the fitness costs of resistance have not previously been assessed.
In this work, we comprehensively evaluate the resistance properties of Myx in Staphylococcus aureus. We report (i) the spontaneous resistance rate for Myx in S. aureus, (ii) the spontaneous resistance spectrum of Myx in S. aureus, and (iii) the fitness costs of substitutions that confer Myx resistance.
MATERIALS AND METHODS
Materials.
(±)-Myx B was synthesized as described in Hu et al. (19). Rif was purchased from Sigma, Inc. Bacterial strains were obtained from the American Type Culture Collection.
MICs.
Minimal inhibitory concentrations (MICs) in column 2 of Table 1 were quantified using broth microdilution assays (8). MICs in column 3 of Table 1, and in Tables 3 and 4, were quantified using spiral gradient endpoint assays, essentially as described previously (38, 45, 54). Spiral gradient endpoint assays employed 150-mm by 4-mm exponential-gradient plates containing Mueller-Hinton II cation-adjusted agar and 0.5 to 100 μg/ml of Myx, 0.0008 to 0.2 μg/ml of Rif, or 0.2 to 40 μg/ml of Rif. Plates were prepared using an Autoplate 4000 spiral plater (Spiral Biotech, Inc.). Cells were grown to early log phase, adjusted to 1 × 108 CFU/ml, and swabbed radially onto plates. Plates were incubated for 16 h at 37°C. For each culture, the streak length was measured using a clear plastic template (Spiral Biotech, Inc.), the test compound concentration at the streak endpoint was calculated using the program SGE (Spiral Biotech, Inc.), and the MIC was defined as the calculated test compound concentration at the streak endpoint.
Table 1.
Antibacterial activity of Myx
| S. aureus strain | Myx MIC (μg/ml) by assay method |
Myx MBC (μg/ml) | |
|---|---|---|---|
| Broth microdilution | Spiral gradient endpoint | ||
| ATCC 12600 MSSA | 1.56 | 0.86 | 3.13 |
| ATCC 13709 MSSA | 1.56 | 0.86 | 6.25 |
| ATCC 29213 MSSA | 1.56 | 0.86 | 6.25 |
| BAA-1707 MRSA (MW2) | 0.78 | 0.80 | 3.13 |
| BAA-1717 MRSA (USA300) | 0.78 | 0.80 | 3.13 |
Table 3.
Sequences, resistance levels, and fitness costs of Myx-resistant mutants
| RNAP subunit and amino acid substitutiona | No. of independent isolates | MIC/MICwtb |
Fitness cost (% per generation [± SEM]) | |
|---|---|---|---|---|
| Myx | Rif | |||
| β Subunit (rpoB) | ||||
| 1080 (1275) Val → Ile | 1 | 20 | 1 | 8 (±2) |
| 1080 (1275) Val → Leu | 1 | 20 | 1 | 12 (±3) |
| 1084 (1279) Glu → Lys | 1 | >100 | 1 | 8 (±4) |
| 1101 (1296) Asp → Glu | 1 | 20 | 1 | 15 (±1) |
| 1127 (1322) Ser → Leu | 3 | >100 | 1 | 10 (±3) |
| 1127 (1322) Ser → Pro | 2 | >100 | 1 | 4 (±2) |
| β′ Subunit (rpoC) | ||||
| 334 (345) Lys → Asn | 3 | >100 | 1 | 6 (±2) |
| 925 (917) Thr → Lys | 1 | 20 | 1 | 7 (±1) |
| 925 (917) Thr → Arg | 1 | >100 | 1 | 5 (±2) |
| 1172 (1354) Gly → Cys | 1 | 20 | 1 | 13 (±1) |
| 1172 (1354) Gly → Asp | 2 | 20 | 1 | 14 (±1) |
Residues are numbered as in S. aureus RNAP and, in parentheses, as in E. coli RNAP.
MICs were determined using spiral gradient endpoint assays. The MIC of the wild-type parent (MICwt) is 0.86 μg/ml for Myx and 0.008 μg/ml for Rif.
Table 4.
Sequences, resistance levels, and fitness costs of Rif-resistant mutants
| RNAP β subunit (rpoB) mutationa | No. of independent isolates | MIC/MICwtb |
Fitness cost (% per generation [± SEM])c | |
|---|---|---|---|---|
| Rif | Myx | |||
| 464 (509) Ser → Pro | 1 | 2,000 | 1 | 13 (±2) |
| 471 (516) Asp → Gly | 7 | 60 | 1 | 0 (−5 ± 7) |
| 471 (516) Asp → Tyr | 2 | 100 | 1 | 0 (±3) |
| 477 (522) Ala → Asp | 3 | >5,000 | 1 | 9 (±6) |
| 477 (522) Ala → Val | 1 | 100 | 1 | 10 (±5) |
| 481 (526) His → Asn | 1 | 300 | 1 | 0 (−1 ± 2) |
| 481 (526) His → Arg | 1 | >5,000 | 1 | 6 (±4) |
| 481 (526) His → Tyr | 6 | >5,000 | 1 | 14 (±1) |
| 486 (531) Ser → Leu | 2 | >5,000 | 1 | 13 (±6) |
Residues are numbered as in S. aureus RNAP and, in parentheses, as in E. coli RNAP.
MICs were determined using spiral gradient endpoint assays. The Myx MIC for the wild type (MICwt) is 0.86 μg/ml, and the Rif MICwt is 0.008 μg/ml.
Observed fitness costs of the Rif-resistant mutants of ≤0 are shown as 0 and are highlighted in boldface. Observed fitness costs of <0 are shown in parentheses ± standard errors of the means.
MBCs.
Minimal bactericidal concentrations (MBCs) were determined as follows. Cells (5 × 105 CFU/ml, diluted from log-phase cultures) were incubated for 16 h at 37°C in 100 μl of Mueller-Hinton II cation-adjusted broth containing amounts of test compound equivalent to 0× MIC, 0.5× MIC, 1× MIC, 2× MIC, or 4× MIC. Samples were diluted 1:1,000; aliquots were applied to Mueller-Hinton II cation-adjusted agar plates, plates were incubated for 16 h at 37°C, and colonies were counted. The MBC was defined as the lowest concentration of test compound that resulted in a ≥99.9% reduction in colony count.
Spontaneous resistance rates.
Resistance rates were determined using fluctuation assays (14, 24, 26, 57). Defined numbers of cells of S. aureus ATCC 12600 (1 × 109 CFU/plate) were plated on Mueller-Hinton II cation-adjusted agar containing amounts of test compound equivalent to 1× MIC, 2× MIC, 4× MIC, 8× MIC, or 16× MIC, and numbers of colonies were counted after 24 h at 37°C (at least five independent determinations for each concentration of each test compound). Resistance rates and 95% confidence intervals were calculated using the Ma-Sandri-Sarkar maximum-likelihood estimator (MSS-MLE) (27, 44, 50) as implemented on the Fluctuation Analysis Calculator (FALCOR [http://www.keshavsingh.org/protocols/FALCOR.html]) (16). Sampling correction was performed as described previously (22, 51).
Sequencing of resistant mutants.
Cells were lysed using 1 mg/ml lysozyme and 1 mg/ml lysostaphin (Sigma, Inc.). Genomic DNA was isolated using the Wizard Genomic DNA Purification Kit (Promega, Inc.) according to the procedures specified by the manufacturer, and genomic DNA was quantified by measurement of UV absorbance. The rpoB gene and the rpoC gene were PCR amplified in reaction mixtures containing 0.2 μg of genomic DNA, 0.4 μM forward and reverse oligonucleotide primers (5′-CGTTAAATAGATAAGTTAATTAAGAATAAATATAGAATCG-3′ and 5′-TGGCTTAAAGTACTAAACTGAATCATC-3′ for rpoB; 5′-GCCATTTTAAATAAATGCAAATCAATCAAATAGC-3′ and 5′-CCTTTAATATATTAACATTGAACAAGAGAATTCG-3′ for rpoC), 5 U of Taq DNA polymerase (Genscript, Inc.), and 800 μM deoxynucleoside triphosphate (dNTP) mix (200 μM each dNTP; Agilent, Inc.). The PCR program consisted of an initial denaturation step of 5 min at 94°C, followed by 30 cycles of 30 s at 94°C, 45 s at 48°C, and 4 min at 72°C, with a final extension step of 10 min at 72°C. PCR products containing the rpoB gene (3.5 kb) or the rpoC gene (3.6 kb) were isolated by electrophoresis on 0.8% agarose, extracted from gel slices using a Gel/PCR DNA Fragments Extraction Kit (IBI Scientific, Inc.) according to the procedures specified by the manufacturer, and sequenced (Sanger sequencing; eight sequencing primers per gene).
Resistance fitness costs.
Resistance fitness costs were quantified using pairwise-competition fitness assays, essentially as described previously (25, 55). Equal numbers of log-phase cells of an antibacterial-compound-resistant mutant and the isogenic wild-type parent (∼1 × 103 CFU each) were mixed in 5 ml of Mueller-Hinton II cation-adjusted broth, and the mixed culture was incubated for 20 h (∼20 to ∼23 doubling times for the isogenic wild-type parent) at 37°C with shaking. At time zero and again at 20 h, the numbers of cells of the antibacterial-compound-resistant mutant and the isogenic wild-type parent were quantified by plating, in parallel, on Mueller-Hinton II cation-adjusted agar containing the antibacterial compound (6.25 μg/ml Myx or 0.012 μg/ml Rif) and on Mueller-Hinton II cation-adjusted agar without the antibacterial compound, followed by incubation for 20 to 40 h at 37°C and colony counting. Numbers of cells of the antibacterial-compound-resistant mutant were determined from the colony counts on plates containing the antibacterial compound. Numbers of cells of the isogenic wild-type parent were determined by subtracting the colony counts on plates containing the antibacterial compound from the colony counts on plates lacking the antibacterial compound.
The numbers of generations of the resistant mutant (Gmut) and of the wild-type parent (Gwt) were calculated according to Wichelhaus et al. (55):
| (1) |
| (2) |
where Amut and Awt are the numbers of CFU/ml for the mutant and wild-type, respectively, at time zero, and Bwt and Bmut are the numbers of CFU/ml for the mutant and wild-type, respectively, at 20 h.
The fitness cost (FC) was calculated according to Sander et al. (42):
| (3) |
Pairwise-competition fitness assays directly comparing Myx-resistant mutants and Rif-resistant mutants were performed in an analogous manner, mixing equal numbers of log-phase cells of a Myx-resistant mutant and an Rif-resistant mutant (∼1 × 103 CFU each) and quantifying numbers of cells of the Myx-resistant mutant and the Rif-resistant mutant by plating on Mueller-Hinton II cation-adjusted agar containing 6.25 μg/ml Myx and on Mueller-Hinton II cation-adjusted agar containing 0.012 μg/ml Rif, respectively.
RESULTS
Antibacterial activity of Myx in S. aureus MSSA and S. aureus MRSA.
To assess antibacterial activity, we performed MIC assays and minimal bactericidal concentration (MBC) assays. Results are presented in Table 1. The results indicate, consistent with previous reports (19, 20, 31, 33, 49), that Myx exhibits potent antibacterial activity against both methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) (MICs of 0.78 to 1.56 μg/ml). Results of spiral gradient endpoint MIC assays are identical, within one 2-fold serial dilution interval, to results of broth microdilution MIC assays. The results further indicate that Myx is bactericidal against both MSSA and MRSA at concentrations approximately 2× to 3× MIC (MBC of 3.13 to 6.25 μg/ml).
Myx resistance in S. aureus: resistance rate.
To assess the spontaneous resistance rate for Myx, we performed fluctuation assays (14, 24, 26, 57), plating defined numbers of cells (∼109 CFU/plate) of S. aureus ATCC 12600 on agar containing 1×, 2×, 4×, 8×, or 16× MIC of Myx, counting numbers of resistant colonies after 24 h of incubation at 37°C, and calculating resistance rates using the MSS-MLE method (16, 22, 27, 44, 50, 51). For comparison, we assayed the resistance rate for Rif under the identical experimental conditions. The results are presented in Table 2. The observed resistance rates for Myx at 1×, 2×, 4×, 8×, and 16× MIC were 7 × 10−8, 7 × 10−8, 6 × 10−8, 4 × 10−8, and 4 × 10−8 per generation, respectively. The observed resistance rates for Rif at 1×, 2×, 4×, 8× and 16× MIC were 10 × 10−8, 6 × 10−8, 6 × 10−8, 3 × 10−8, and 3 × 10−8, respectively, per generation, consistent with previously reported values for Rif (30, 34). Within experimental error, the observed resistance rates for Myx and Rif were identical. We conclude, consistent with previous work (31), that the resistance rates for Myx and Rif are equal or comparable.
Table 2.
Resistance rates for Myx and Rif
| Inhibitor | Concn (× MIC) | Resistance rate per generation (95% confidence interval) |
|---|---|---|
| Myx | 1 | 7 (4–11) × 10−8 |
| 2 | 7 (4–10) × 10−8 | |
| 4 | 6 (3–8) × 10−8 | |
| 8 | 4 (2–7) × 10−8 | |
| 16 | 4 (2–7) × 10−8 | |
| Rif | 1 | 10 (6–14) × 10−8 |
| 2 | 6 (3–9) × 10−8 | |
| 4 | 6 (5–8) × 10−8 | |
| 8 | 3 (1–6) × 10−8 | |
| 16 | 3 (1–6) × 10−8 |
Myx resistance in S. aureus: resistance spectrum.
To define the resistance spectrum for Myx, we isolated and sequenced the rpoB and rpoC genes, encoding the RNAP β and β′ subunits, from each of 17 independent spontaneous Myx-resistant mutants identified in the experiments described in the preceding section. For comparison, to define the resistance spectrum for Rif under identical experimental conditions, we isolated and sequenced the rpoB gene from each of 24 independent spontaneous Rif-resistant mutants identified in the experiments described in the preceding section.
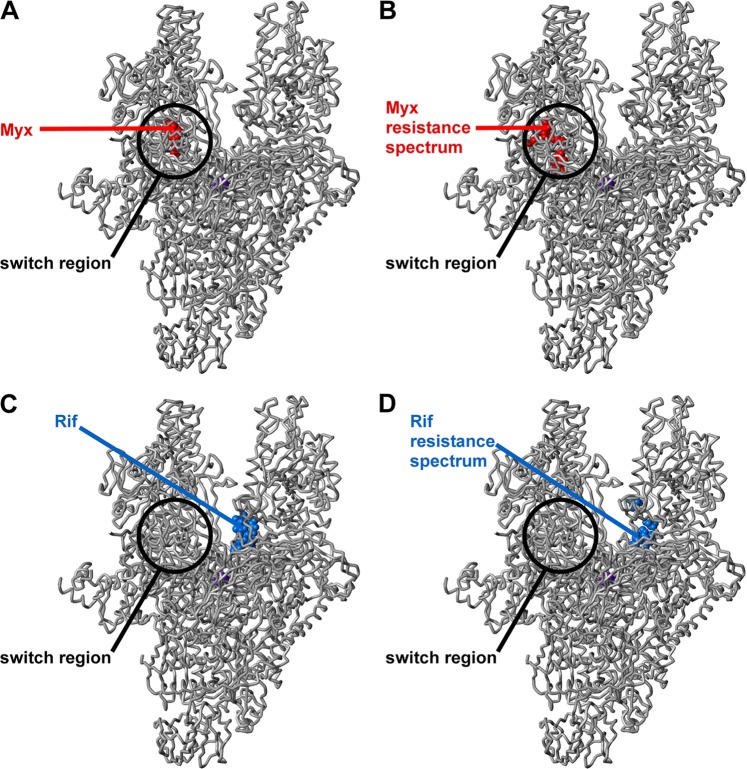
The results for Myx resistance are presented in Table 3 and Fig. 1B. All Myx-resistant mutants were found to contain mutations in rpoB or rpoC, confirming that RNAP is the primary or exclusive functional cellular target of Myx in S. aureus. All 17 Myx-resistant mutants were single-substitution mutants. Six substitutions conferring Myx resistance were identified in the RNAP β subunit [V1080(1275)I, V1080(1275)L, E1084(1279)K, D1101(1296)E, S1127(1322)L, and S1127(1322)P], and five substitutions conferring Myx resistance were identified in the RNAP β′ subunit [K334(345)N, T925(917)K, T925(917)R, G1172(1354)C, and G1172(1354)D] (residues numbered as in S. aureus RNAP and, in parentheses, as in Escherichia coli RNAP). Four of the substitutions previously were reported as Myx-resistant substitutions in E. coli [E1084(1279)K and S1127(1322)P in the RNAP β subunit; K334(345)N and G1172(1354)C in the RNAP β′ subunit] (32), and three of the substitutions previously were reported as Myx-resistant substitutions in S. aureus [S1127(1322)L in the RNAP β subunit; K334(345)N and T925(917)R in the RNAP β′ subunit] (31). All sites of substitutions conferring Myx resistance mapped to the binding site on RNAP for Myx, located in the SW1/SW2 subregion of the RNAP switch region (Fig. 1A and B). None overlapped the binding site on RNAP for Rif (Fig. 1C) or the resistance spectrum on RNAP for Rif (Fig. 1D). Resistance and cross-resistance levels were quantified using spiral gradient endpoint assays (38, 45, 54). Six substitutions resulted in 20-fold resistance to Myx, and five substitutions resulted in >100-fold resistance to Myx (Table 3). (Compound availability precluded assays at >100× MIC.) The median resistance level was 20-fold. Consistent with the absence of overlap with the binding site for Rif, no Myx-resistant substitution conferred cross-resistance to Rif (Table 3).
Fig 1.
Locations of binding sites and sites of resistance substitutions for Myx and Rif in the structure of bacterial RNAP. (A) Binding site for Myx (Myx shown in red) (32). (B) Resistance spectrum for Myx in S. aureus (sites of Myx-resistant substitutions identified in this study shown in red) (Table 3). (C) Binding site for Rif (Rif shown in blue) (6). (D) Resistance spectrum for Rif in S. aureus (sites of Rif-resistant substitutions identified in this study shown in blue) (Table 4). Atomic coordinates for RNAP and Myx are from a crystal structure of the Thermus thermophilus RNAP-Myx complex (32) (Protein Data Bank [PDB] accession code 3DXJ; σ subunit and β′ subunit nonconserved regions omitted for clarity). Atomic coordinates for Rif are from the crystal structure of the Thermus aquaticus RNAP-Rif complex (6) (PDB accession code 1I6V). The RNAP active-center Mg2+ is shown as a violet sphere for reference.
The results for Rif are presented in Table 4 and Fig. 1D. All Rif-resistant mutants contained mutations in rpoB, consistent with previous reports for Rif resistance in S. aureus and in other bacterial species (2, 4, 7, 10–13, 15, 17, 21, 29, 35–37, 40, 41, 43, 46–48, 52, 53, 55, 56). All identified Rif-resistant mutants were single-substitution mutants. Nine substitutions conferring Rif resistance were identified in the RNAP β subunit [S464(509)P, D471(516)G, D471(516)Y, A477(522)D, A477(522)V, H481(526)N, H481(526)R, H481(526)Y, and S486(531)L]. One Rif-resistant substitution resulted in 60-fold resistance, two resulted in 100-fold resistance, one resulted in 300-fold resistance, and all others resulted in ≥2,000-fold resistance (Table 4). The median resistance level was 2,000-fold. Consistent with the absence of overlap with the binding site for Myx (Fig. 1), no Rif-resistant substitution conferred cross-resistance to Myx (Table 4).
We conclude that the Myx resistance spectrum in S. aureus comprises substitutions of residues of the Myx binding site located in the SW1/SW2 subregion of the RNAP switch region and that the Myx resistance spectrum does not overlap the Rif resistance spectrum (Tables 3 and 4; Fig. 1). We conclude further that Myx-resistant mutants exhibit generally lower resistance levels than Rif-resistant mutants (with at least 6 of the 11 Myx-resistant mutants exhibiting resistance levels lower than any of the 9 Rif-resistant mutants and with a multiple-order-of-magnitude difference in median resistance levels: i.e., 20-fold versus 2,000-fold).
Myx resistance in S. aureus: resistance fitness costs.
To assess the fitness cost of Myx-resistant mutants, we performed pairwise-competition fitness assays, essentially as described by Lenski and by Wichelhaus et al. (25, 55). For each identified Myx-resistant mutant, we mixed equal numbers of cells of the Myx-resistant mutant and the isogenic wild-type parent (1 × 103 CFU of each), incubated the mixed culture for 20 h at 37°C (20 to 23 doubling times for the wild-type parent), quantified Myx-resistant and wild-type cells by plating cultures in parallel to medium with Myx and without Myx, and calculated the fitness cost per generation (see Materials and Methods). For comparison, we assessed the fitness cost, under identical experimental conditions, for each identified Rif-resistant mutant.
The results for Myx-resistant mutants are presented in Table 3. All Myx-resistant mutants exhibited nonzero, >0% per generation, fitness costs. The lowest and highest fitness costs were 4% per generation and 15% per generation, respectively. The mean and median fitness costs were 9% per generation and 8% per generation, respectively. Growth rates of three mutants with substitutions of the RNAP switch region previously have been assessed (28), but the data in the present study provide the first quantitative measurements of fitness costs of any mutant with substitutions of the RNAP switch region.
The results for Rif are presented in Table 4, column 5. Three Rif-resistant mutants have fitness costs of zero or less than zero: D471(516)G, D471(516)Y, and H481(526)N (Table 4, boldface). The observed fitness costs for Rif-resistant mutants in S. aureus are in agreement with previously reported data (55). The conclusion that a subset of Rif-resistant mutants exhibit fitness costs of zero or less than zero is in agreement with previous work for multiple bacterial species, including S. aureus (55), Enterococcus faecium (11), Mycobacterium tuberculosis (4, 15), and E. coli (41).
To assess the significance of the observed difference in fitness costs of Myx-resistant mutants and Rif-resistant mutants, we performed direct pairwise-competition fitness assays between the two lowest-fitness-cost Myx-resistant mutants [β S1127(1322)P and β′ T925(917)R; fitness costs of 4% per generation and 5% per generation, respectively] and a zero-fitness cost Rif-resistant mutant [β H481(526)N]. The results are presented in Table 5. The results confirm that the two lowest-fitness-cost Myx-resistant mutants are significantly less fit than the zero-fitness-cost Rif-resistant mutant. The observed relative fitness costs are equal within error to the predicted relative fitness costs calculated as the difference between the fitness costs of the Myx-resistant mutants and the Rif-resistant mutant. We conclude that all Myx-resistant mutants—but not all Rif-resistant mutants—have significant nonzero fitness costs.
Table 5.
Direct determination of relative fitness costs of lowest-fitness-cost Myx-resistant mutants and zero-fitness-cost Rif-resistant mutant
| Myx-resistant mutant (RNAP subunit and mutation)a | Rif-resistant mutant (RNAP subunit and mutation)a | Relative fitness cost of Myx resistance vs Rif-resistance (% per generation [± SEM]) |
|
|---|---|---|---|
| Predicted valueb | Observed valuec | ||
| β 1127 (1322) Ser → Pro | β 481 (526) His → Asn | 5 (±4) | 6 (±2) |
| β′ 925 (917) Thr → Lys | β 481 (526) His → Asn | 8 (±3) | 8 (±1) |
Residues are numbered as in S. aureus RNAP and, in parentheses, as in E. coli RNAP.
Predicted relative fitness costs are calculated as the fitness cost of the Myx-resistant mutant (Table 3) minus the fitness cost of the Rif-resistant mutant (Table 4).
Observed relative fitness costs are from pairwise-competition fitness assays directly comparing the Myx-resistant mutant and the Rif-resistant mutant.
To assess whether fitness costs of Myx-resistant mutants are stable or whether fitness costs decrease due to the emergence of compensatory mutations, we measured fitness costs of representative Myx-resistant mutants [β V1080(1275)Ile and β′ K334(345)N; fitness costs of 8% per generation and 6% per generation, respectively] after 0, 1, and 2 serial passages. Fitness costs did not change after up to 2 serial passages (Table 6).
Table 6.
Fitness costs of Myx-resistant mutants after 0, 1, and 2 serial passages
| RNAP subunit and mutationa | No. of serial passages | Fitness cost (% per generation [± SEM]) |
|---|---|---|
| β 1080 (1275) Val → Ile | 0 | 8 (±2) |
| 1 | 9 (±1) | |
| 2 | 9 (±1) | |
| β¢ 334 (345) Lys → Asn | 0 | 6 (±2) |
| 1 | 6 (±1) | |
| 2 | 8 (±1) |
Residues are numbered as in S. aureus RNAP and, in parentheses, as in E. coli RNAP.
DISCUSSION
Our results show that Myx has bactericidal activity in S. aureus (Table 1) and define the resistance properties of Myx in S. aureus (Tables 2 to 4; Fig. 1). Our results indicate that the spontaneous resistance rate for Myx is equal or comparable to that for Rif (Table 2), that the resistance spectrum for Myx does not overlap the resistance spectrum for Rif (Tables 3 and 4; Fig. 1B and D), that the resistance levels of Myx-resistant mutants generally are lower than those of Rif-resistant mutants (Tables 3 and 4), and that the resistance fitness costs of all Myx-resistant mutants—but not all Rif-resistant mutants—are greater than zero (Tables 3 and 4).
The finding that the resistance levels of Myx-resistant mutants generally are lower than those of Rif-resistant mutants, together with the finding that fitness costs of all Myx-resistant mutants—but not all Rif-resistant mutants—are greater than zero, supports the viability of Myx as a lead compound for antibacterial drug development.
The finding that all Myx-resistant mutants, but not all Rif-resistant mutants, have significant nonzero fitness costs is particularly noteworthy. Previous work has established a strong correlation between the fitness cost of antibacterial-agent-resistant mutants and the clinical prevalence of antibacterial-agent-resistant mutants (1, 4, 5, 9, 10, 15, 35, 42, 55). For Rif, this correlation is especially strong (4, 10, 15, 35, 55). Rif-resistant substitutions that have zero or subzero fitness costs are highly significantly overrepresented in Rif-resistant clinical isolates across multiple bacterial species (4, 10, 15, 35, 55). The three Rif-resistant substitutions that we have found to have zero fitness costs in S. aureus [D(516)G, D(516)Y, and H(526)N] account for more than 50% of sequenced Rif-resistant clinical isolates of S. aureus (2, 17, 29, 35, 46, 52, 53, 56) and more than 50% of sequenced Rif-resistant clinical isolates of Streptococcus pneumoniae (7, 12, 13, 37). The three Rif-resistant substitutions that we have found to have zero fitness costs in S. aureus [D(516)G, D(516)Y, and H(526)N], together with another substitution found to have zero fitness cost in M. tuberculosis [S(531)L] (4, 15), account for more than 35% of sequenced Rif-resistant clinical isolates of M. tuberculosis (40, 43).
The difference in fitness costs of Myx resistance and Rif resistance presumably relates to the fact that the binding site on RNAP for Myx (the RNAP switch region SW1/SW2 subregion) performs critical functions in opening and closing the RNAP active-center cleft and in DNA binding (3, 18, 32, 39, 49), whereas the binding site on RNAP for Rif performs no critical functions (18). Accordingly, substitutions in the SW1/SW2 subregion of the RNAP switch region presumably are more likely to impair RNAP function and thereby to impair viability. Since the nonzero fitness costs are likely to be a property of the switch region SW1/SW2 subregion, it is likely that nonzero fitness costs will be observed not only with Myx but also with other RNAP inhibitors that function through the switch region SW1/SW2 subregion, including corallopyronin and ripostatin (32, 49).
ACKNOWLEDGMENTS
This work was supported by NIH grants AI072766 and AI90837, a Global Alliance for TB Drug Development contract, and a Howard Hughes Medical Institute Investigatorship to R.H.E.
Footnotes
Published ahead of print 24 September 2012
REFERENCES
- 1. Andersson DI. 2006. The biological cost of mutational antibiotic resistance: any practical conclusions? Curr. Opin. Microbiol. 9:461–465 [DOI] [PubMed] [Google Scholar]
- 2. Aubry-Damon H, Soussy CJ, Courvalin P. 1998. Characterization of mutations in the rpoB gene that confer rifampin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 42:2590–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Belogurov GA, et al. 2009. Transcription inactivation through local refolding of the RNA polymerase structure. Nature 457:332–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Billington OJ, McHugh TD, Gillespie SH. 1999. Physiological cost of rifampin resistance induced in vitro in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 43:1866–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Böttger EC, Springer B, Pletschette M, Sander P. 1998. Fitness of antibiotic-resistant microorganisms and compensatory mutations. Nat. Med. 4:1343–1344 [DOI] [PubMed] [Google Scholar]
- 6. Campbell EA, et al. 2001. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 104:901–912 [DOI] [PubMed] [Google Scholar]
- 7. Chen JY, et al. 2004. Mutations of the rpoB gene in rifampicin-resistant Streptococcus pneumoniae in Taiwan. J. Antimicrob. Chemother. 53:375–378 [DOI] [PubMed] [Google Scholar]
- 8. Clinical and Laboratory Standards Institute 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M07-A8, 8th ed Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9. Cohen T, Murray M. 2004. Modeling epidemics of multidrug-resistant M. tuberculosis of heterogeneous fitness. Nat. Med. 10:1117–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Comas I, et al. 2012. Whole-genome sequencing of rifampicin-resistant Mycobacterium tuberculosis strains identifies compensatory mutations in RNA polymerase genes. Nat. Genet. 44:106–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Enne VI, Delsol AA, Roe JM, Bennett PM. 2004. Rifampicin resistance and its fitness cost in Enterococcus faecium. J. Antimicrob. Chemother. 53:203–207 [DOI] [PubMed] [Google Scholar]
- 12. Enright M, Zawadski P, Pickerill P, Dowson CG. 1998. Molecular evolution of rifampicin resistance in Streptococcus pneumoniae. Microb. Drug Resist. 4:65–70 [DOI] [PubMed] [Google Scholar]
- 13. Ferrándiz MJ, et al. 2005. New mutations and horizontal transfer of rpoB among rifampin-resistant Streptococcus pneumoniae from four Spanish hospitals. Antimicrob. Agents Chemother. 49:2237–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foster PL. 2006. Methods for determining spontaneous mutation rates. Methods Enzymol. 409:195–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gagneux S, et al. 2006. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science 312:1944–1946 [DOI] [PubMed] [Google Scholar]
- 16. Hall BM, Ma CX, Liang P, Singh KK. 2009. Fluctuation analysis CalculatOR: a web tool for the determination of mutation rate using Luria-Delbrück fluctuation analysis. Bioinformatics 25:1564–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hellmark B, Söderquist B, Unemo M. 2009. Simultaneous species identification and detection of rifampicin resistance in staphylococci by sequencing of the rpoB gene. Eur. J. Clin. Microbiol. Infect. Dis. 28:183–190 [DOI] [PubMed] [Google Scholar]
- 18. Ho MX, Hudson BP, Das K, Arnold E, Ebright RH. 2009. Structures of RNA polymerase-antibiotic complexes. Curr. Opin. Struct. Biol. 19:715–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu T, et al. 1998. Total synthesis and preliminary antibacterial evaluation of the RNA polymerase inhibitors (±)-myxopyronin A and B. J. Org. Chem. 63:2401–2406 [Google Scholar]
- 20. Irschik H, Gerth K, Höfle G, Kohl W, Reichenbach H. 1983. The myxopyronins, new inhibitors of bacterial RNA synthesis from Myxococcus fulvus (Myxobacterales). J. Antibiot. (Tokyo) 36:1651–1658 [DOI] [PubMed] [Google Scholar]
- 21. Jin DJ, Gross CA. 1988. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J. Mol. Biol. 202:45–58 [DOI] [PubMed] [Google Scholar]
- 22. Jones ME. 1993. Accounting for plating efficiency when estimating spontaneous mutation rates. Mutat. Res. 292:187–189 [DOI] [PubMed] [Google Scholar]
- 23. Kohl W, Irschik H, Reichenbach H, Höfle G. 1983. Antibiotika aus Gleitenden Bakterien, XVII. Myxopyronin A und B—zwei neue Antibiotika aus Myxococcus fulvus Stamm Mx f50. Liebigs Ann. Chemie 1983:1656–1667 [Google Scholar]
- 24. Lea D, Coulson C. 1949. The distribution of the numbers of mutants in bacterial populations. J. Genet. 49:264–285 [DOI] [PubMed] [Google Scholar]
- 25. Lenski RE. 1988. Experimental studies of pleiotropy and epistasis in Escherichia coli. I. Variation in competitive fitness among mutants resistant to virus T4. Evolution 42:425–432 [DOI] [PubMed] [Google Scholar]
- 26. Luria SE, Delbrück M. 1943. Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28:491–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma WT, Sandri GV, Sarkar S. 1992. Analysis of the Luria-Delbrück distribution using discrete convolution powers. J. Appl. Probab. 29:255–267 [Google Scholar]
- 28. Mariner K, et al. 2011. Activity of and development of resistance to corallopyronin A, an inhibitor of RNA polymerase. Antimicrob. Agents Chemother. 55:2413–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mick V, et al. 2010. Molecular characterization of resistance to rifampicin in an emerging hospital-associated methicillin-resistant Staphylococcus aureus clone ST228, Spain. BMC Microbiol. 10:68 doi:10.1186/1471-2180-10-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moorman DR, Mandell GL. 1981. Characteristics of rifampin-resistant variants obtained from clinical isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 20:709–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moy TI, et al. 2011. Evaluating the activity of the RNA polymerase inhibitor myxopyronin B against Staphylococcus aureus. FEMS Microbiol. Lett. 319:176–179 [DOI] [PubMed] [Google Scholar]
- 32. Mukhopadhyay J, et al. 2008. The RNA polymerase “switch region” is a target for inhibitors. Cell 135:295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Neill A, et al. 2000. RNA polymerase inhibitors with activity against rifampin-resistant mutants of Staphylococcus aureus. Antimicrob. Agents Chemother. 44:3163–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O'Neill AJ, Cove JH, Chopra I. 2001. Mutation frequencies for resistance to fusidic acid and rifampicin in Staphylococcus aureus. J. Antimicrob. Chemother. 47:647–650 [DOI] [PubMed] [Google Scholar]
- 35. O'Neill AJ, Huovinen T, Fishwick CW, Chopra I. 2006. Molecular genetic and structural modeling studies of Staphylococcus aureus RNA polymerase and the fitness of rifampin resistance genotypes in relation to clinical prevalence. Antimicrob. Agents Chemother. 50:298–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ovchinnikov YA, et al. 1983. RNA polymerase rifampicin resistance mutations in Escherichia coli: sequence changes and dominance. Mol. Gen. Genet. 190:344–348 [DOI] [PubMed] [Google Scholar]
- 37. Padayachee T, Klugman KP. 1999. Molecular basis of rifampin resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:2361–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paton JH, Holt AH, Bywater MJ. 1990. Measurement of MICs of antibacterial agents by spiral gradient endpoint compared with conventional dilution method. Int. J. Exp. Clin. Chemother. 3:31–38 [Google Scholar]
- 39. Pupov D, et al. 2010. Multiple roles of the RNA polymerase β′ SW2 region in transcription initiation, promoter escape, and RNA elongation. Nucleic Acids Res. 38:5784–5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramaswamy S, Musser JM. 1998. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber. Lung Dis. 79:3–29 [DOI] [PubMed] [Google Scholar]
- 41. Reynolds MG. 2000. Compensatory evolution in rifampin-resistant Escherichia coli. Genetics 156:1471–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sander P, et al. 2002. Fitness cost of chromosomal drug resistance-conferring mutations. Antimicrob. Agents Chemother. 46:1204–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sandgren A, et al. 2009. Tuberculosis drug resistance mutation database. PLoS Med. 6:e2 doi:10.1371/journal.pmed.1000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sarkar S, Ma WT, Sandri GvH. 1992. On fluctuation analysis: a new, simple and efficient method for computing the expected number of mutants. Genetica 85:173–179 [DOI] [PubMed] [Google Scholar]
- 45. Schalkowsky S. 1994. Measures of susceptibility from a spiral gradient of drug concentrations. Adv. Exp. Med. Biol. 349:107–120 [DOI] [PubMed] [Google Scholar]
- 46. Sekiguchi J, et al. 2006. Emergence of rifampicin resistance in methicillin-resistant Staphylococcus aureus in tuberculosis wards. J. Infect. Chemother. 12:47–50 [DOI] [PubMed] [Google Scholar]
- 47. Severinov K, Soushko M, Goldfarb A, Nikiforov V. 1993. Rifampicin region revisited. New rifampicin-resistant and streptolydigin-resistant mutants in the beta subunit of Escherichia coli RNA polymerase. J. Biol. Chem. 268:14820–14825 [PubMed] [Google Scholar]
- 48. Severinov K, Soushko M, Goldfarb A, Nikiforov V. 1994. RifR mutations in the beginning of the Escherichia coli rpoB gene. Mol. Gen. Genet. 244:120–126 [DOI] [PubMed] [Google Scholar]
- 49. Srivastava A, et al. 2011. New target for inhibition of bacterial RNA polymerase: “switch region.” Curr. Opin. Microbiol. 14:532–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stewart FM. 1994. Fluctuation tests: how reliable are the estimates of mutation rates? Genetics 137:1139–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stewart FM, Gordon DM, Levin BR. 1990. Fluctuation analysis: the probability distribution of the number of mutants under different conditions. Genetics 124:175–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van Rensburg MJ, Whitelaw A, Elisha B. 2012. Genetic basis of rifampicin resistance in methicillin-resistant Staphylococcus aureus suggests clonal expansion in hospitals in Cape Town, South Africa. BMC Microbiol. 12:46 doi:10.1186/1471-2180-12-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Villar M, et al. 2011. Epidemiological and molecular aspects of rifampicin-resistant Staphylococcus aureus isolated from wounds, blood and respiratory samples. J. Antimicrob. Chemother. 66:997–1000 [DOI] [PubMed] [Google Scholar]
- 54. Wallace AS, Corkill JE. 1989. Application of the spiral plating method to study antimicrobial action. J. Microbiol. Methods 10:303–310 [Google Scholar]
- 55. Wichelhaus TA, et al. 2002. Biological cost of rifampin resistance from the perspective of Staphylococcus aureus. Antimicrob. Agents Chemother. 46:3381–3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wichelhaus TA, Schäfer V, Brade V, Böddinghaus B. 1999. Molecular characterization of rpoB mutations conferring cross-resistance to rifamycins on methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 43:2813–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Young K. 2006. In vitro antibacterial resistance selection and quantitation. Curr. Protoc. Pharmacol., chapter 13, unit 13A.6. doi:10.1002/0471141755.ph13a06s34 [DOI] [PubMed] [Google Scholar]