Abstract
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) has spread rapidly throughout the world in the last decade. We sought to demonstrate the impact of the emergence of CA-MRSA in Western Canada on physician visits, incision-and-drainage procedures, and antibiotic prescribing for skin and soft tissue infections (SSTI). We used the provincial physician billing system to determine the rate of physician visits (per 1,000 population per year) of SSTI and incision-and-drainage procedures. A database capturing all outpatient prescriptions in the province was anonymously linked to associated physician billing codes to quantify prescriptions associated with SSTI. Antibiotic prescriptions (overall and class specific) were expressed as their defined daily dose (DDD) per 1,000 inhabitants per day. Between 1996 and 2008, the rate of visits for all SSTI increased by 15%, and the majority of visits did not include an incision-and-drainage procedure. The rate of antibiotic prescribing for SSTI increased by 49%. The majority of this increase was attributable to the higher rates of use of clindamycin (627%), trimethoprim-sulfamethoxazole (380%), cephalosporins (160%), and amoxicillin-clavulanate (627%). Health care utilization and antibiotic prescribing rates for SSTI, but not incision-and-drainage procedures, have increased in association with the CA-MRSA epidemic. While much of the increase in antibiotic use reflects an appropriate change to trimethoprim-sulfamethoxazole, there is room for education regarding the limitations of cephalosporins and clindamycin, given current susceptibility profiles.
INTRODUCTION
Staphylococcus aureus has proven to be highly adaptable in the antibiotic age. This organism became resistant to penicillin shortly after the introduction of the antibiotic (4), which prompted the use of methicillin and oxacillin for the treatment of Staphylococcus aureus infections. However, the development of resistance to those beta-lactams was seen in the early 1960s, just 2 years after their introduction (4a). Between the 1960s and the 1990s, the majority of methicillin-resistant Staphylococcus aureus (MRSA) infections were seen in hospitals (7, 12, 39). Studies have clearly demonstrated that hospital-associated methicillin-resistant Staphylococcus aureus (HA-MRSA) infections result in excess mortality in hospitalized patients (6, 17). Although strict infection control, hand hygiene, and antibiotic use have kept the incidence of HA-MRSA infections steady within hospitals, a number of countries around the world, including Canada, have now reported increasing rates of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infections (16, 30, 32, 35).
Like their hospital counterparts, infections related to CA-MRSA also cause skin and soft tissue infections (SSTI) but in individuals within the larger community and without prior exposure to a hospital or health care setting (10, 28). Since the early 1990s, epidemics of CA-MRSA have occurred in Western Australia, Taiwan, Canada, and the United States (3, 13, 19, 29, 37, 38).
In British Columbia, dramatic increases in the proportions of S. aureus isolates resistant to methicillin and carrying the CA-MRSA phenotype have been observed in community laboratories since 2002 (1, 5, 24). Strains causing SSTI in Western Canada were more likely to be of the CA-MRSA-10 (USA300) strain type, whereas in Eastern Canada, the strain type was predominately CA-MRSA-2 (USA100/800) (35). Given this difference, we felt that it was important to characterize the impact of SSTI in Western Canada on health care and antibiotic utilization.
MATERIALS AND METHODS
The Ministry of Health in British Columbia houses several health care-related databases, which have comprehensive information on the majority of the population who live in British Columbia (population of 4.6 million). One of these systems is PharmaNet, a centralized data system that links all pharmacies with every prescription dispensed through community pharmacies in British Columbia. All antimicrobials are recorded in this system, except those used for the treatment of sexually transmitted infections and HIV. We also used the Medical Service Plan (MSP) billing system, which records all reimbursement claims submitted by health practitioners for medically required services provided to British Columbia residents, including diagnostic codes. We used the MSP database, which serves general practitioners (GPs) and, therefore, supplies billing codes for hospitalized patients, and those treated within the emergency department (ED) were not included. Antibiotic prescriptions from 1996 to 2008 were extracted from PharmaNet with anonymous patient and prescriber identifiers that were then used to match to the MSP billing system for indication (i.e., diagnostic codes). The prescription was matched to patient and prescriber identifiers and a dispensing date that was within 5 days after the physician visit. If more than one visit met the matching criteria, only the visit closest to the dispensing date was used. If more than one diagnostic code was recorded for the same visit, all the diagnostic codes were used.
Antibiotics were classified based on the anatomical therapeutic chemical (ATC) classification system developed by World Health Organization (WHO). The defined daily dose (DDD) was used to calculate consumption rates as DDD per 1,000 population per day for individuals aged 15 years and older, and prescription rates per 1,000 population per day were used for those under 15 years of age (as weight-based dosing in children precludes the use of DDD). Population estimates were obtained from PEOPLE 33, revised (Population Extrapolation for Organizational Planning with Less Error) (34). The MSP diagnostic codes were based on the ninth revision of the international classification of diseases developed by the WHO, commonly referred to as ICD9 (http://icd9cm.chrisendres.com/). We grouped several indications within the classification of SSTI, including cellulitis and abscess (ICD9 682), erysipelas (ICD9 035), cellulitis and abscess of finger and toe (ICD9 681), impetigo (ICD9 684), carbuncles and furuncles (ICD9 680), and other local infections of the skin and subcutaneous tissue (ICD9 686).
We analyzed the data first with respect to overall rate of antibiotic use for SSTI in British Columbia and then according to the use of the six major classes (tetracyclines [J01A], penicillins [J01C], cephalosporins [J01D], trimethoprim-sulfamethoxazole [J01E], macrolides [J01FA], and lincosamides [J01FF]). We also evaluated clinically relevant antibiotics being used within each class, such as tetracycline and doxycycline for the class of tetracyclines (J01A); penicillin V, amoxicillin, ampicillin, amoxicillin-clavulanate, and cloxacillin within the class of penicillins (J01C); cephalexin and cefuroxime axetil for the class of cephalosporins (J01D); trimethoprim-sulfamethoxazole (J01E); erythromycin, clarithromycin, and azithromycin for the class of macrolides (J01FA); and clindamycin for the class of lincosamides (J01FF). For all of the above-described groupings, we also evaluated changes in use by gender and by age group (<1, 1 to 4, 5 to 9, 10 to 14, 15 to 19, 20 to 39, 40 to 59, and 60+ years old). All analyses were performed by using SAS 9.2. The direct age standardization method was used to calculate rates of physician visits for each year, using the 2008 population structure to make rates comparable over time.
RESULTS
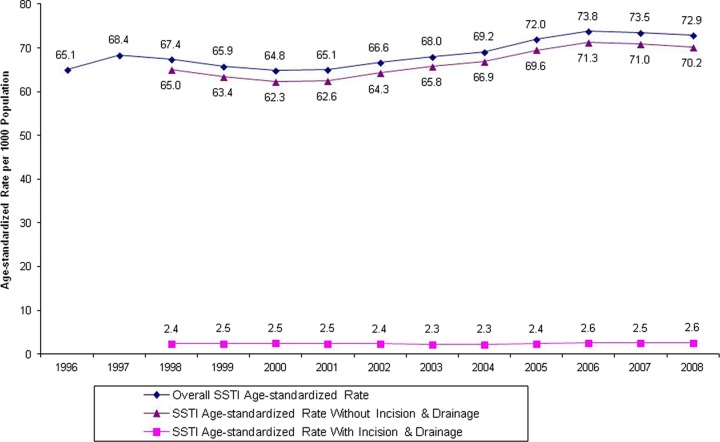
The age-standardized rate of physician visits for SSTI increased by 12% from 1996 to 2008 (65 to 73 visits/1,000 population/year) (Fig. 1). The actual number of visits increased more markedly, by 30%, from 245,433 to 319,323, but this was against a backdrop of a growing population. The majority of these visits did not involve an incision-and-drainage procedure (less than 4% per year), regardless of the year, between 1996 and 2008.
Fig 1.
Age-standardized rates of SSTI-related physician visits from 1996 to 2008.
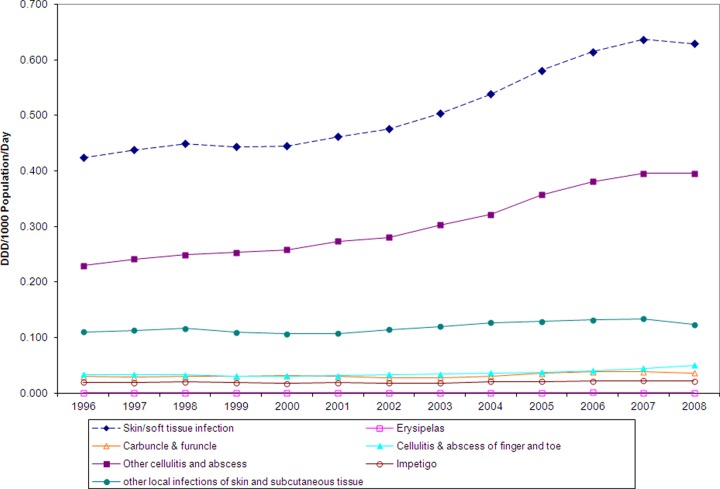
The linked database contained 26,064,428 prescription records for the years between 1996 and 2008. The rate of antibiotic consumption for SSTI increased by 49%, from 0.42 DDD per 1,000 population per day to 0.63 DDD per 1,000 population per day (Fig. 2), and was associated mostly with the treatment of cellulitis and abscesses. Other indications within the SSTI category remained fairly steady over time, and limited amounts of antibiotics were used for the treatment of these infections (Fig. 2).
Fig 2.
Overall antibiotic use for treatment of skin and soft tissue infections, by category of infection, from 1996 to 2008.
Table 1 shows the breakdown of antibiotics into the six different classes for the population of British Columbia. The greatest change over time was observed for the use of clindamycin, which increased by 627% (0.007 to 0.051 DDD/1,000 population/day), followed by a 380% increase in the use of trimethoprim-sulfamethoxazole (0.010 to 0.048 DDD/1,000 population/day). The use of the cephalosporin class increased 160%, from 0.115 to 0.300 DDD/1,000 population/day, with the majority of this being related to cephalexin. As expected, the use of penicillins declined by 33%, from 0.180 to 0.121 DDD/1,000 population/day, despite a 157% increase in the use of amoxicillin-clavulanate (0.014 to 0.037 DDD/1,000 population/day). The use of cloxacillin declined by 60% (0.126 to 0.051 DDD/1,000 population/day), while the use of penicillin V decreased by 54% (0.014 to 0.006 DDD/1,000 population/day). The use of macrolides declined by 27%. The majority of these changes took place after 2003.
Table 1.
Types of antibiotic classes used for treatment of skin and soft tissue infections between 1996 and 2008a
| Yr | DDD per 1,000 population per day |
||||||
|---|---|---|---|---|---|---|---|
| Overall (J01) | Tetracyclines (J01A) | Penicillins (J01C) | Cephalosporins (J01D) | Sulfonamides-trimethoprim (J01E) | Macrolides (J01FA) | Clindamycin (J01FF) | |
| 1996 | 0.424 | 0.037 | 0.180 | 0.115 | 0.010 | 0.045 | 0.007 |
| 1997 | 0.438 | 0.034 | 0.179 | 0.135 | 0.010 | 0.044 | 0.009 |
| 1998 | 0.449 | 0.035 | 0.172 | 0.150 | 0.009 | 0.042 | 0.012 |
| 1999 | 0.443 | 0.033 | 0.155 | 0.163 | 0.008 | 0.040 | 0.014 |
| 2000 | 0.445 | 0.032 | 0.143 | 0.174 | 0.007 | 0.040 | 0.016 |
| 2001 | 0.462 | 0.031 | 0.140 | 0.189 | 0.007 | 0.040 | 0.020 |
| 2002 | 0.476 | 0.032 | 0.140 | 0.202 | 0.006 | 0.039 | 0.024 |
| 2003 | 0.504 | 0.032 | 0.141 | 0.220 | 0.007 | 0.040 | 0.028 |
| 2004 | 0.538 | 0.032 | 0.143 | 0.248 | 0.009 | 0.042 | 0.030 |
| 2005 | 0.581 | 0.036 | 0.136 | 0.283 | 0.013 | 0.040 | 0.037 |
| 2006 | 0.615 | 0.038 | 0.131 | 0.304 | 0.023 | 0.039 | 0.044 |
| 2007 | 0.637 | 0.043 | 0.124 | 0.308 | 0.040 | 0.036 | 0.050 |
| 2008 | 0.629 | 0.045 | 0.121 | 0.300 | 0.048 | 0.033 | 0.051 |
Percent changes from 1996 to 2008 were 48.5% overall (J01), 23.4% for tetracyclines (J01A), −33.0% for penicillins (J01C), 160.4% for cephalosporins (J01D), 379.9% for sulfonamides-trimethoprim (J01E), −26.8% for macrolides (J01FA), and 626.9% for clindamycin (J01FF).
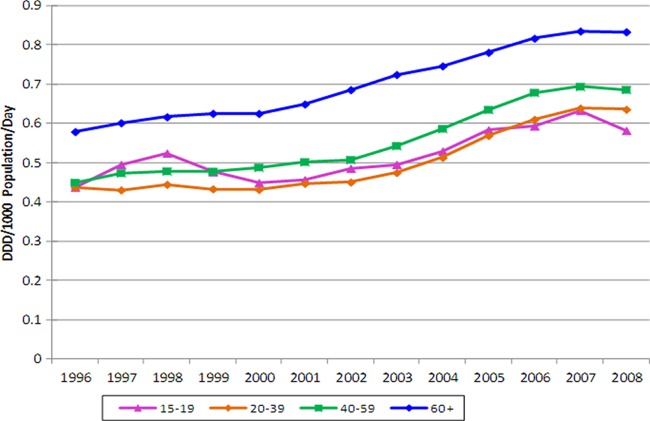
Table 2 shows overall antibiotic use for individuals 15 years of age or older. Between 1996 and 2008, the overall antibiotic use increased 48.3%, with clindamycin accounting for the greatest increase. The use of trimethoprim-sulfamethoxazole (394.5%) and cephalosporins (158.3%) also increased greatly, while there was a modest increase in the use of tetracyclines (21.2%). Both penicillin and macrolide usage declined by 34.3% and 26.1%, respectively. Figure 3 shows antibiotic usage broken down by age. An increase in antibiotic use was seen across all age categories after 2003, with the largest increase seen in the 40- to 59-year age group (52.8%). Furthermore, antibiotic use increased over time for both males (0.44 to 0.67 DDD/1,000 population/day) and females (0.40 to 0.59 DDD/1,000 population/day) from 1996 to 2008, representing 51.4% and 45.7% increases, respectively. Throughout the study period, adults over the age of 60 years had the highest levels of consumption, and by 2008, their utilization was 0.83 DDD/1,000 population/day, followed by individuals 40 to 59 years of age (0.69 DDD/1,000 population/day), 20 to 39 years of age (0.64 DDD/1,000 population/day), and 15 to 19 years of age (0.58 DDD/1,000 population/day).
Table 2.
Types of antibiotic classes used for treatment of skin and soft tissue infections between 1996 and 2008 in individuals aged 15 years or oldera
| Yr | DDD per 1,000 population per day |
||||||
|---|---|---|---|---|---|---|---|
| Overall (J01) | Tetracyclines (J01A) | Penicillins (J01C) | Cephalosporins (J01D) | Sulfonamides-trimethoprim (J01E) | Macrolides (J01FA) | Clindamycin (J01FF) | |
| 1996 | 0.47009 | 0.04287 | 0.19512 | 0.12798 | 0.01098 | 0.04816 | 0.00855 |
| 1997 | 0.48504 | 0.03989 | 0.19414 | 0.14858 | 0.01076 | 0.04595 | 0.01146 |
| 1998 | 0.49817 | 0.04082 | 0.18626 | 0.16587 | 0.01009 | 0.04476 | 0.01481 |
| 1999 | 0.49215 | 0.03764 | 0.16779 | 0.18015 | 0.00915 | 0.04328 | 0.01639 |
| 2000 | 0.49326 | 0.03742 | 0.15496 | 0.19231 | 0.00790 | 0.04299 | 0.01900 |
| 2001 | 0.51016 | 0.03601 | 0.15080 | 0.20837 | 0.00776 | 0.04271 | 0.02381 |
| 2002 | 0.52445 | 0.03728 | 0.14906 | 0.22198 | 0.00709 | 0.04172 | 0.02793 |
| 2003 | 0.55587 | 0.03643 | 0.15091 | 0.24273 | 0.00808 | 0.04228 | 0.03288 |
| 2004 | 0.59345 | 0.03645 | 0.15243 | 0.27341 | 0.00995 | 0.04427 | 0.03489 |
| 2005 | 0.64253 | 0.04132 | 0.14352 | 0.31300 | 0.01497 | 0.04239 | 0.04399 |
| 2006 | 0.68171 | 0.04385 | 0.13946 | 0.33723 | 0.02633 | 0.04178 | 0.05113 |
| 2007 | 0.70478 | 0.04894 | 0.13197 | 0.33969 | 0.04548 | 0.03806 | 0.05893 |
| 2008 | 0.69727 | 0.05195 | 0.12811 | 0.33063 | 0.05428 | 0.03560 | 0.05963 |
Percent changes from 1996 to 2008 were 48.3% overall (J01), 21.2% for tetracyclines (J01A), −34.3% for penicillins (J01C), 158.3% for cephalosporins (J01D), 394.5% for sulfonamides-trimethoprim (J01E), −26.1% for macrolides (J01FA), and 597.2% for clindamycin (J01FF).
Fig 3.
Overall antibiotic use for treatment of skin and soft tissue infections in the 15-year-old or older age group, from 1996 to 2008.
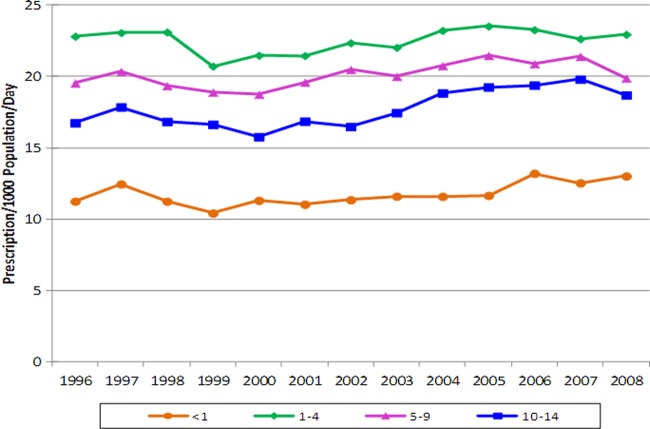
Table 3 shows trends in antibiotic use for children. Between 1996 and 2008, overall antibiotic use in children less than 15 years of age increased by only 4.6%. Once again, the majority of this increase was seen in the use of clindamycin (556.5%), followed by trimethoprim-sulfamethoxazole (108.7%) and cephalosporins (92.4%). Both penicillin and macrolide use decreased by approximately 50%, although it should be noted that amoxicillin-clavulanate use increased by 49.1%. Figure 4 shows the usages by various age cohorts. After 2003, the largest increase was seen for children less than 1 year old (12.2%), followed by children 10 to 14 years old (7.1%). In 2008, children between 1 and 4 years of age had the highest prescription rate (23.0 per 1,000 population/day), while those less than 1 year old had the lowest prescription rate (13.0 per 1,000 population/day).
Table 3.
Types of antibiotic classes used for treatment of skin and soft tissue infections between 1996 and 2008 in individuals aged less than 15 yearsa
| Yr | No. of prescriptions per 1,000 population per day |
||||||
|---|---|---|---|---|---|---|---|
| Overall (J01) | Tetracyclines (J01A) | Penicillins (J01C) | Cephalosporins (J01D) | Sulfonamides-trimethoprim (J01E) | Macrolides (J01FA) | Clindamycin (J01FF) | |
| 1996 | 0.05164 | 0.00036 | 0.02682 | 0.01661 | 0.00146 | 0.00604 | 0.00016 |
| 1997 | 0.05399 | 0.00032 | 0.02582 | 0.02008 | 0.00150 | 0.00584 | 0.00028 |
| 1998 | 0.05193 | 0.00040 | 0.02365 | 0.02090 | 0.00126 | 0.00529 | 0.00027 |
| 1999 | 0.04949 | 0.00041 | 0.02042 | 0.02229 | 0.00104 | 0.00487 | 0.00030 |
| 2000 | 0.04899 | 0.00032 | 0.01887 | 0.02352 | 0.00094 | 0.00473 | 0.00042 |
| 2001 | 0.05086 | 0.00028 | 0.01850 | 0.02567 | 0.00099 | 0.00473 | 0.00051 |
| 2002 | 0.05189 | 0.00032 | 0.01872 | 0.02672 | 0.00087 | 0.00451 | 0.00058 |
| 2003 | 0.05219 | 0.00040 | 0.01781 | 0.02748 | 0.00094 | 0.00478 | 0.00060 |
| 2004 | 0.05487 | 0.00031 | 0.01785 | 0.02985 | 0.00102 | 0.00491 | 0.00076 |
| 2005 | 0.05629 | 0.00030 | 0.01777 | 0.03177 | 0.00107 | 0.00436 | 0.00081 |
| 2006 | 0.05597 | 0.00031 | 0.01625 | 0.03248 | 0.00144 | 0.00422 | 0.00107 |
| 2007 | 0.05631 | 0.00037 | 0.01480 | 0.03362 | 0.00248 | 0.00381 | 0.00106 |
| 2008 | 0.05401 | 0.00036 | 0.01425 | 0.03196 | 0.00304 | 0.00323 | 0.00108 |
Percent changes from 1996 to 2008 were 4.6% overall (J01), 0.5% for tetracyclines (J01A), −46.9% for penicillins (J01C), 92.4% for cephalosporins (J01D), 108.7% for sulfonamides-trimethoprim (J01E), −46.6% for macrolides (J01FA), and 556.5% for clindamycin (J01FF).
Fig 4.
Overall antibiotic use for treatment of skin and soft tissue infections in the less-than-15-year-old age group, from 1996 to 2008.
DISCUSSION
Our study is the first in Canada to characterize the incidence of skin and soft tissue infections, incision-and-drainage procedures, and antibiotic treatment of SSTI at the population level. There was a striking increase in both the incidence of patient visits for SSTI and the use of antibiotics, which coincides with the CA-MRSA epidemic in British Columbia (1, 2, 5, 24). Also, our study shows that clinicians are using cephalosporins for the treatment of SSTI, while the use of clindamycin, amoxicillin-clavulanate, trimethoprim-sulfamethoxazole, and tetracycline classes is also increasing. The clonal expansion of CA-MRSA is clearly doing more than changing laboratory susceptibility profiles and showing up in hospitals; it is accounting for an excess of tens of thousands of physician visits each year in British Columbia.
Our findings are similar to those of previous studies that have shown an increase in the incidence of SSTI in the CA-MRSA era. These studies have shown anywhere from 25% to 50% increases in SSTI rates since 2003, with clindamycin accounting for the majority of the antibiotic use (9, 18, 26, 33). The increase in the incidence of SSTI seen in our study (15%) was not as dramatic as that reported previously in the literature, and this may be related to the fact that we did not have data on emergency department (ED) visits within our database. Given that some SSTI treatments, including incision-and-drainage procedures, are conducted in the ED, we are likely underestimating our findings.
Previous work, including a Canadian surveillance study on pediatric MRSA, also showed an increase in the number of children requiring treatment for SSTI with time (13–15, 21, 22). For example, a recent U.S. study showed that in 2000, there were 17,525 ± 838 admissions for SSTI, which represented 0.65% of all pediatric hospitalizations and corresponded to a rate of 23.2 SSTI hospitalizations per 100,000 children per year. However, by 2006, there were 48,228 ± 2,223 admissions for SSTI, which represented 1.77% of all pediatric hospitalizations and corresponded to a rate of 62.7 SSTI hospitalizations per 100,000 children per year (22). In our study, we also found an increase in antibiotic use over time, but it was not as dramatic as those seen in the U.S. populations. We believe that the reason for our small number of pediatric cases may be related to the fact that recent studies reported in the literature (13–15, 21, 22) evaluated children with SSTI presenting to the emergency department or admitted to a hospital for intravenous antibiotics; hospital-based prescriptions are not included in PharmaNet, and thus, our study would have underestimated the effect of CA-MRSA on the pediatric population. This was verified by a recent Canadian surveillance study which evaluated laboratory data for hospitalized children and showed that overall MRSA rates per 10,000 patient days increased from 0.08 (1995) to 3.88 (2007), with the majority of the increase being accounted for by CA-MRSA, while HA-MRSA rates remained stable (25).
Although we do not have linked microbiological data to confirm etiology, the increasing incidence of SSTI shown in our population-based study correlates with the increase in institutionally based surveillance data for MRSA (1, 5). This suggests that CA-MRSA is driving the increased incidence of SSTI seen in our study. In 2007, the most common genotype (47% of isolates) was CA-MRSA-2 (USA100/800), while CA-MRSA-10 (USA300) was the second most common strain type (27% of isolates) (34, 36). More recently, CA-MRSA genotypes in Canadian hospitals increased from 19.5% of MRSA isolates in 2007 to 31.9% in 2009 (P < 0.001) (31). Much of the recent change is linked to the CA-MRSA-10 (USA300) genotype, which contains staphylococcal cassette chromosome mec element (SCCmec) type IVa and is Panton-Valentine leukocidin (PVL) positive (31, 36).
Changes in susceptibility have been evident in Canadian CA-MRSA isolates with increasing resistance to clindamycin and macrolides over time. This has been especially evident in the CA-MRSA-10 (USA300) strains (1, 27, 32). Over the course of the CA-MRSA epidemic, the rate of clindamycin resistance in British Columbia (reports from many community laboratories across British Columbia) has increased to 36.4% of isolates (laboratory data do not characterize isolates as inducible or not), but rates of resistance have remained at 2.2% for trimethoprim-sulfamethoxazole and 2.0% for doxycycline (5). Clindamycin resistance is an emerging issue for CA-MRSA and was recently described for populations of men who have sex with men in San Francisco, CA, and Boston, MA, where CA-MRSA-10 (USA300) strains harbored large conjugative plasmid genes that encoded multidrug resistance, including clindamycin, mupirocin, and macrolide resistances (11, 34). Clearly, further work needs to be done to characterize clindamycin-resistant strains; whether this is due to a clonal multidrug-resistant strain originally introduced or pressured by the inappropriate use of clindamycin needs to be elucidated.
In our study, we saw very little use of incision and drainage and certainly no increase over time. This is surprising, since Infectious Diseases Society of America (IDSA) guidelines suggest incision and drainage as the primary treatment for a cutaneous abscess (23). Having said that, we also saw very few cases coded as having furuncles, which usually require an incision-and-drainage procedure, but this may have been due to the fact that many of these types of procedures are carried out in emergency departments, and we would have missed these in our analysis.
Antibiotic therapy is recommended for the rapid progression of cellulitis, systemic signs and symptoms, associated comorbidities, immunosuppression, very young or elderly persons, difficult-to-drain abscesses, associated septic phlebitis, a lack of a response to a previous incision-and-drainage procedure, and severe disease (23). Suggestions for antibiotics to be used include cephalosporins (for susceptible S. aureus strains and nonpurulent cellulitis), clindamycin, and tetracyclines (23), and in special circumstances only, due to cost, linezolid is a viable option (23). For severe infections, vancomycin continues to be the drug of choice (8, 10). This is good general advice, but clinicians in Western Canada need to consider the unique trends in resistance seen in the CA-MRSA-10 (USA300) strains. Trimethoprim-sulfamethoxazole or doxycycline may be better initial choices for suspected cases of CA-MRSA infection in our setting.
The findings of our study are worrisome. Not only is the incidence of SSTI in British Columbia increasing, likely due to the CA-MRSA-10 (USA300) strain of CA-MRSA, but clinicians appear not to be aware that the incision-and-drainage procedure is first-line therapy, nor are they aware that our strain in Western Canada differs in susceptibility patterns from the common CA-MRSA strain present in the rest of Canada. The frequent use of clindamycin may suggest that physicians in British Columbia, while cognizant of the increase in the rate of CA-MRSA infections, may not also be aware of the increasing clindamycin resistance in this organism when choosing empirical therapy. Furthermore, the inappropriate use of antibiotics can potentially drive resistance rates up and is associated with potentially serious adverse effects, including those due to Clostridium difficile disease. The CA-MRSA-10 (USA300) strain continues to remain susceptible to trimethoprim-sulfamethoxazole, tetracyclines, linezolid, daptomycin, and vancomycin. If trimethoprim-sulfamethoxazole is being used for the treatment of either undrained abscesses or streptococcal SSTI, its use as a single agent would not be considered optimal. In our study, we are unable to delineate the use of antibiotics for simple cellulitis versus CA-MRSA infection; macrolides and cephalosporins may have been used appropriately for the former indication.
Our study had several limitations that must be acknowledged. Since CA-MRSA is not a reportable condition, we used overall skin and/or soft tissue infections in the community to look for changes as CA-MRSA entered our communities. In doing so, we possibly overestimated the incidence of CA-MRSA, given that some of these infections would be methicillin sensitive and others would be due to streptococcal infections. However, there have been no independently observed epidemiological changes in methicillin-sensitive S. aureus (MSSA) and streptococcal disease to explain the widespread increase in numbers of physician visits for SSTI. Miscoding is always a possibility when administrative data are used, especially when related to coding for physician office visits. This could have resulted in an over- or underestimation of the number of skin and/or soft tissue infections and probably led to an underestimation of the number of abscess drainage procedures, as the physicians may have billed for a general visit instead. However, we have not identified any factors that would have increased or decreased the use of appropriate codes over time. Our data set did not include patients who were seen in hospital-associated outpatient clinics or within hospital emergency departments in British Columbia. As such, if there was abscess drainage or treatment in those two settings, we would not have captured this in our analysis. This study was conducted using the population from British Columbia, so the results should be directly generalizable to other parts of Western Canada. However, due to differences in the predominant strains and health care systems, the results may not be applicable to the United States. A major strength of this study is the use of the administrative data set with its large sample size, with a population-based cohort of more than 130,000 people who were prescribed antibiotics for an SSTI. Moreover, the use of administrative databases provides the opportunity to obtain data on dispensed medications collected prospectively and independently of the outcomes, avoiding recall bias.
Conclusions.
The rates of physician visits for SSTI and the associated antibiotic use have increased in association with the CA-MRSA epidemic in British Columbia. The first-line treatment of abscesses remains incision and drainage, and cultures should be obtained from SSTI, where possible, to guide subsequent therapy. When antibiotic treatment is required for SSTI suspected to be due to CA-MRSA, trimethoprim-sulfamethoxazole plus a cephalosporin or doxycycline should be used in our population, rather than clindamycin.
ACKNOWLEDGMENTS
Funding for this project was obtained from the British Columbia Michael Smith Foundation for Health Research and the British Columbia Ministry of Health Pharmaceutical Service Branch.
We thank the Ministry of Health for providing us with the data required to conduct this analysis.
None of the authors of the manuscript have received funds for speaking at symposia organized on behalf of industry, nor have they received funds for research from industry. None of the authors hold any stocks in industry.
Fawziah Marra was responsible for the design, implementation, and supervision of the study. All authors contributed to the study concept and design. Methodological advice and statistical analysis were provided by Mei Chong and Rachel McKay. Data were acquired by Mei Chong and Fawziah Marra. Fawziah Marra drafted the manuscript. All authors contributed to the revision of the manuscript for important content.
Footnotes
Published ahead of print 24 September 2012
REFERENCES
- 1. Al-Rawahi GN, et al. 2010. Community-associated CMRSA-10 (USA-300) is the predominant strain among methicillin-resistant Staphylococcus aureus strains causing skin and soft tissue infections in patients presenting to the emergency department of a Canadian tertiary care hospital. J. Emerg. Med. 38:6–11 [DOI] [PubMed] [Google Scholar]
- 2. Al-Rawahi GN, et al. 2008. Methicillin-resistant Staphylococcus aureus nasal carriage among injection drug users: six years later. J. Clin. Microbiol. 46:477–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baggett HC, et al. 2003. An outbreak of community-onset methicillin resistant Staphylococcus aureus skin infections in southwestern Alaska. Infect. Control Hosp. Epidemiol. 24:397–402 [DOI] [PubMed] [Google Scholar]
- 4. Barber M. 1947. Staphylococcal infection due to penicillin-resistant strains. Br. Med. J. ii:863–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a. Barber M. 1961. Methicillin-resistant staphylococci. J. Clin. Pathol. 14:385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. BC Centre for Disease Control 2012. Antimicrobial resistance trends in the province of British Columbia: 2011. Communicable Disease Prevention Services, BCCDC, Vancouver, British Columbia, Canada: http://www.bccdc.ca/prevention/AntibioticResistance Accessed 6 June 2012 [Google Scholar]
- 6. Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. 2002. Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch. Intern. Med. 162:2229–2235 [DOI] [PubMed] [Google Scholar]
- 7. Boyce JM. 1990. Increasing prevalence of methicillin-resistant Staphylococcus aureus in the United States. Infect. Control Hosp. Epidemiol. 11:639–642 [DOI] [PubMed] [Google Scholar]
- 8. Chua K, Howden BP. 2009. Treating Gram-positive infections: vancomycin update and the whys, wherefores and evidence base for continuous infusion of anti-Gram-positive antibiotics. Curr. Opin. Infect. Dis. 22:525–534 [DOI] [PubMed] [Google Scholar]
- 9. Dalager-Pedersen M, Søgaard M, Schønheyder HC. 2011. Staphylococcus aureus skin and soft tissue infections in primary healthcare in Denmark: a 12-year population-based study. Eur. J. Clin. Microbiol. Infect. Dis. 30:951–956 [DOI] [PubMed] [Google Scholar]
- 10. Deleo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated methicillin-resistant Staphylococcus aureus. Lancet 375:1557–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diep BA, et al. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367:731–739 [DOI] [PubMed] [Google Scholar]
- 12. Embil J, et al. 1994. Methicillin-resistant Staphylococcus aureus in tertiary care institutions on the Canadian prairies 1990–1992. Infect. Control Hosp. Epidemiol. 15:646–651 [DOI] [PubMed] [Google Scholar]
- 13. Fergie JE, Purcell K. 2001. Community-acquired methicillin-resistant Staphylococcus aureus infections in south Texas children. Pediatr. Infect. Dis. J. 20:860–863 [DOI] [PubMed] [Google Scholar]
- 14. Frei CR, Makos BR, Daniels KR, Oramasionwu CU. 2010. Emergence of community-acquired methicillin-resistant Staphylococcus aureus skin and soft tissue infections as a common cause of hospitalization in United States children. J. Pediatr. Surg. 45:1967–1974 [DOI] [PubMed] [Google Scholar]
- 15. Gerber JS, Coffin SE, Smathers SA, Zaoutis TE. 2009. Trends in the incidence of methicillin-resistant Staphylococcus aureus infection in children's hospitals in the United States. Clin. Infect. Dis. 49:65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Golding GR, et al. 2011. High rates of Staphylococcus aureus USA400 infection, Northern Canada. Emerg. Infect. Dis. 17:722–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanberger H, et al. 2011. Increased mortality associated with methicillin-resistant Staphylococcus aureus (MRSA) infection in the intensive care unit: results from the EPIC II study. Int. J. Antimicrob. Agents 38:331–335 [DOI] [PubMed] [Google Scholar]
- 18. Hersh AL, Chambers HF, Maselli JH, Gonzales R. 2008. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch. Intern. Med. 168:1585–1591 [DOI] [PubMed] [Google Scholar]
- 19. Hussain FM, Boyle-Vavra S, Daum RS. 2001. Community-acquired methicillin-resistant Staphylococcus aureus colonization in healthy children attending an outpatient pediatric clinic. Pediatr. Infect. Dis. J. 20:763–767 [DOI] [PubMed] [Google Scholar]
- 20. Reference deleted.
- 21. Karamatsu ML, Thorp AW, Brown L. 2012. Changes in community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections presenting to the pediatric emergency department: comparing 2003 to 2008. Pediatr. Emerg. Care 28:131–135 [DOI] [PubMed] [Google Scholar]
- 22. Lautz TB, Raval MV, Barsness KA. 2011. Increasing national burden of hospitalizations for skin and soft tissue infections in children. J. Pediatr. Surg. 46:1935–1941 [DOI] [PubMed] [Google Scholar]
- 23. Liu C, et al. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:285–292 [DOI] [PubMed] [Google Scholar]
- 24. Lloyd-Smith E, et al. 2011. Screening for methicillin-resistant Staphylococcus aureus (MRSA) in community-recruited injection drug users: are throat swabs necessary? Epidemiol. Infect. 13:1721–1724 [DOI] [PubMed] [Google Scholar]
- 25. Matlow A, et al. 2012. National surveillance of methicillin-resistant Staphylococcus aureus among hospitalized pediatric patients in Canadian acute care facilities, 1995-2007. Pediatr. Infect. Dis. J. 31:814–820 [DOI] [PubMed] [Google Scholar]
- 26. McCaig LF, McDonald LC, Mandal S, Jernigan DB. 2006. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerg. Infect. Dis. 12:1715–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McDougal LK, et al. 2010. Emergence of resistance among USA300 methicillin-resistant Staphylococcus aureus isolates causing invasive disease in the United States. Antimicrob. Agents Chemother. 54:3804–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moellering RC., Jr 2012. MRSA: the first half century. J. Antimicrob. Chemother. 67:4–11 [DOI] [PubMed] [Google Scholar]
- 29. Mulvey MR, et al. 2005. Community-associated methicillin-resistant Staphylococcus aureus, Canada. Emerg. Infect. Dis. 11:844–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Naimi TS, et al. 2003. Comparison of community and healthcare-associated methicillin-resistant Staphylococcus aureus infection. JAMA 290:2976–2984 [DOI] [PubMed] [Google Scholar]
- 31. Nichol KA, et al. 2011. Comparison of community-associated and health care-associated methicillin-resistant Staphylococcus aureus in Canada: results of the CANWARD 2007-2009 study. Diagn. Microbiol. Infect. Dis. 69:320–325 [DOI] [PubMed] [Google Scholar]
- 32. Nichol KA, et al. 2009. Comparison of community-associated and healthcare associated methicillin-resistant Staphylococcus aureus in Canada: results from CANWARD. Can. J. Infect. Dis. Med. Microbiol. 20(Suppl A):31–36 [DOI] [PubMed] [Google Scholar]
- 33. Pallin DJ, et al. 2008. Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann. Emerg. Med. 51:291–298 [DOI] [PubMed] [Google Scholar]
- 34. Robicsek A, et al. 2008. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann. Intern. Med. 148:409–418 [DOI] [PubMed] [Google Scholar]
- 35. Simor AE, et al. 2010. Methicillin-resistant Staphylococcus aureus colonization or infection in Canada: national surveillance of changing epidemiology, 1995-2007. Infect. Control Hosp. Epidemiol. 31:348–356 [DOI] [PubMed] [Google Scholar]
- 36. Simor AE, et al. 2010. Antimicrobial susceptibilities of health care-associated and community-associated strains of methicillin-resistant Staphylococcus aureus from hospitalized patients in Canada, 1995 to 2008. Antimicrob. Agents Chemother. 54:2265–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tenover FC, et al. 2008. Characterization of Staphylococcus aureus isolates from nasal cultures collected from individuals in the United States in 2001 to 2004. J. Clin. Microbiol. 46:2837–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Udo EE, Pearman JW, Grubb WB. 1993. Genetic analysis of community isolates of methicillin-resistant Staphylococcus aureus in Western Australia. J. Hosp. Infect. 25:97–108 [DOI] [PubMed] [Google Scholar]
- 39. Witte W, Kresken M, Braulke C, Cuny C. 1997. Increasing incidence and widespread dissemination of methicillin-resistant Staphylococcus aureus (MRSA) in hospitals in central Europe, with special reference to German hospitals. Clin. Microbiol. Infect. 3:414–422 [DOI] [PubMed] [Google Scholar]