Abstract
Three clinical Pseudomonas aeruginosa isolates (WCH2677, WCH2813, and WCH2837) isolated from the Women's and Children's Hospital, Adelaide, Australia, produced a metallo-β-lactamase (MBL)-positive Etest result. All isolates were PCR negative for known MBL genes. A gene bank was created, and an MBL gene, designated blaAIM-1, was cloned and fully characterized. The encoded enzyme, AIM-1, is a group B3 MBL that has the highest level of identity to THIN-B and L1. It is chromosomal and flanked by two copies (one intact and one truncated) of an ISCR element, ISCR15. Southern hybridization studies indicated the movement of both ISCR15 and blaAIM-1 within the three different clinical isolates. AIM-1 hydrolyzes most β-lactams, with the exception of aztreonam and, to a lesser extent, ceftazidime; however, it possesses significantly higher kcat values for cefepime and carbapenems than most other MBLs. AIM-1 was the first mobile group B3 enzyme detected and signals further problems for already beleaguered antimicrobial regimes to treat serious P. aeruginosa and other Gram-negative infections.
INTRODUCTION
The continuing increase in antibiotic resistance in Gram-negative bacteria is of concern, not least because of the increasing lack of therapeutic options available to treat infections caused principally by Pseudomonas aeruginosa and Acinetobacter baumannii (3, 17, 21). This phenomenon has been exacerbated by the dissemination of metallo-β-lactamases (MBLs) that can confer resistance to nearly all β-lactams, with the exception of aztreonam (4, 5, 43).
Like many resistance mechanisms, MBLs can be encoded by either genes ubiquitously carried on the chromosome or mobile genes (39). The latter genes now include the following subgroups: IMP (15), VIM (26), SPM-1 (36), GIM-1, SIM-1, KMH-1 (25), DIM-1 (20), and the recently described NDM-1 (44). So far, they all belong to MBL subgroup B1. MBL genes are often embedded in class 1 integrons and are carried as gene cassettes. It has also been shown that while many MBL genes are plasmid mediated, some are carried on the chromosome and can be associated with Tn21-like transposons or Tn402 transposons (30, 34). However, SPM-1 is not associated with a standard integron but is flanked by two genetic elements, designated ISCR4 (18). ISCR elements are IS91-like mobile elements and can potentially mobilize and duplicate blaSPM-1 via rolling-circle replication (33, 37).
The B3 subgroup MBLs have hitherto been reported for environmental bacteria, only some of which can cause opportunistic infections. These include Stenotrophomonas maltophilia (L1) (42), Janthinobacterium lividum (THIN-B) (23), Chryseobacterium meningosepticum (GOB-1) (1), Legionella gormanii (FEZ-1) (2), Caulobacter crescentus (CAU-1) (10), CAR-1 from Erwinia carotovora (29), POM-1 (32), and the recently reported ISCR1-associated SMB-1 (38). The MBL genes carried by these environmental bacteria encode subgroup B3 MBLs that are not closely related to the mobile B1 members (43). They are often GC rich and 2 to 3 kDa larger than the B1 subgroup members, although, with the exception of L1, which is a tetramer, they are all monomeric in structure (29).
Here we describe the full characterization of a new subclass of MBL, AIM-1 (Adelaide imipenemase), which was discovered in three P. aeruginosa isolates from Australia. Furthermore, we also demonstrate that a novel ISCR element, ISCR15, is implicated in the movement of blaAIM-1.
(Preliminary data were presented at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC], Chicago, IL, 2007 [45].)
MATERIALS AND METHODS
Clinical cases. (i) Clinical case 1.
Patient 1 was a healthy 18-year-old man from the Northern Territory, Australia, with newly diagnosed acute myeloid leukemia and admitted to the Royal Adelaide Hospital in March 2002. One week after presentation, he received broad-spectrum empirical antimicrobial therapy with intravenous ticarcillin-clavulanate and gentamicin, which was later changed to meropenem and vancomycin. On day 26, a multiresistant P. aeruginosa strain (WCH2677, designated the index isolate) was isolated from endotracheal aspirates, and the antimicrobial therapy was changed to intravenous amikacin and ticarcillin-clavulanate. The patient died on day 30 from acute respiratory distress syndrome and multiorgan failure.
(ii) Clinical case 2.
Patient 2 was a 59-year-old man with insulin-dependent type 2 diabetes and end-stage renal failure and was admitted at the same time as patient 1. Upon admission, he presented with necrotizing fasciitis of the anterior abdominal wall and was admitted to the intensive care unit (ICU), and empirical treatment with intravenous meropenem and vancomycin was commenced. Antibiotic therapy was modified to include intravenous amoxicillin, ciprofloxacin, and metronidazole. Superficial swabs of the wound taken on day 42 in the ICU identified a methicillin-resistant Staphylococcus aureus (MRSA) isolate and a multiresistant P. aeruginosa isolate (WCH2813). The patient continued to improve, and he was later discharged from the hospital on day 70.
(iii) Clinical case 3.
Patient 3 was a 64-year-old woman with Streptococcus bovis endocarditis involving her prosthetic mitral valve and was admitted to the Royal Adelaide Hospital within 1 week of admission of patient 1. Empirical treatment with intravenous vancomycin and gentamicin was commenced. A skin swab from around her central line site grew a multiresistant P. aeruginosa strain (WCH2837) but without clinical evidence of any local or systemic infection. She recovered fully, without further complications, and was discharged home on day 22.
P. aeruginosa clinical strains.
Clinical isolates were identified by using the BD (Baltimore, MD) Phoenix automated microbiology system.
Susceptibility testing.
Susceptibility testing was performed by using the Phoenix 100 system (Becton Dickinson, Oxford, United Kingdom) and by using Etest strips (bioMérieux, La Plane, France), and results were interpreted according to European Committee on Antimicrobial Susceptibility Testing breakpoints (http://www.eucast.org/clinical_breakpoints/).
Phenotypic and molecular detections of MBL.
The Hodge test using MacConkey agar, an imipenem-EDTA double-disc synergy test, and MBL Etest strips (bioMérieux, La Plane, France) were used to screen for class B β-lactamase production (40). In addition, the carbapenemase activities of cell sonicates from broth cultures grown overnight were determined by spectrophotometric assays, which were carried out as previously described (6). The presence of known MBL genes (including blaVIM, blaIMP, blaSPM-1, blaGIM-1, blaSIM-1, blaDIM-1, and blaNDM-1) was screened for by PCR using primers designed for all known MBL subgroups and class 1 integron structures (20, 44). Characterized strains carrying known MBL genes were used as positive controls.
DNA cloning and sequence analysis.
Cloning experiments were performed by using cloning vector pK18 (35). Restriction endonucleases BamHI and Sau3AI and T4 ligase (Promega, Madison, WI) were used for cloning. Transformation was carried out by using electroporation and Escherichia coli TOP10 cells (Invitrogen Corp., Carlsbad, CA). The selection for transformants was performed on LB agar plates containing X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (30 μg/ml), ceftazidime (8 μg/ml), and kanamycin (50 μg/ml). Recombinant plasmids were recovered by using a QIAprep Spin miniprep kit (Qiagen, West Sussex, United Kingdom). The plasmid containing the MBL gene was designated pAIM-1 and contained an insert of 3.5 kb, and it was sequenced on both strands. The nucleotide sequences, deduced amino acid sequences, and phylogenetic relationships were analyzed by using the Lasergene software package (DNAStar, Madison, WI). The obtained sequences were compared to sequences available on the Internet (http://www.ebi.ac.uk/fasta33/).
Genomic DNA digestion and pulsed-field gel electrophoresis (PFGE).
The genomic DNA digestion of the clinical isolates was performed at 37°C overnight with PstI alone, PstI plus HincII, HindIII, BamHI, EcoRI, EcoRV, KpnI, or NdeI (Promega). The buffers were used according to the manufacturer's recommendations. Genomic DNA was prepared and digested with the restriction enzymes SpeI (Roche Diagnostics, Mannheim, Germany), I-CeuI (New England Biolabs, Beverly, MA), and S1 (Invitrogen, Abingdon, United Kingdom), and DNA fragments were separated as previously described (14).
Hybridization.
Hybridization was performed in gel. Briefly, the gel was dried for 5 h at 50°C and then rehydrated in double-distilled water for 5 min before 30-min incubations in denaturing solution (0.5 M NaOH, 1.5 M NaCl) and neutralizing solution (0.5 M Tris-HCl [pH 7.5], 1.5 M NaCl) at room temperature were performed. The gel was then prehybridized at 65°C using prehybridization solution (20 ml) (6× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.1% [wt/vol] polyvinylpyrrolidone 400, 0.1% [wt/vol] Ficoll, 0.1% [wt/vol] bovine serum albumin [BSA] [Cohn fraction V], 0.5% [wt/vol] SDS). The hybridization solution was the same as the prehybridization solution apart from 150 μg/ml denatured calf thymus DNA, which was added at least 4 h before the addition of the probe. The 32P-labeled probe was prepared by using the random primer technique (Stratagene, La Jolla, CA), as previously described (13). Gels were washed in 2% SSC followed by 0.1% SSC.
β-Lactamase purification and characterization.
Cultures of E. coli carrying pAIM-1 were grown overnight at 37°C in 4 liters of LB broth. A periplasmic protein preparation (30 mM Tris [pH 8.0], as previously described [41]) was obtained, thereby discarding cytoplasmic proteins and, thus, 70% of E. coli proteins. This protein solution was treated with 30% and 60% ammonium sulfate solutions to precipitate the proteins, which were removed by centrifugation. The clarified supernatant was loaded onto a Q-Sepharose column (1.5 by 12 cm with a 25-ml bed volume; Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) equilibrated by running 50 volumes of 30 mM Tris (pH 8.0) at 2 ml/min at pH 7.5 through the column. The protein was eluted with a 0 to 500 mM NaCl gradient in 30 mM Tris (pH 8.0) at 2 ml/min. The AIM-1 enzyme eluted at <100 mM NaCl. Fractions possessing MBL activity were pooled and loaded onto a Sephacryl S 300 gel filtration column (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) preequilibrated with 50 mM Tris HCl with 250 mM NaCl buffer at 0.045 ml/min. Fractions showing the highest degree of carbapenemase activity against imipenem were pooled. An Amicon centrifugal filter (Ultracel-50k, -10k; Millipore, Carrigtwohill Co., Cork, Ireland) was used. Protein recovered in the filtrate and protein also retained in the filter were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After visualization with silver staining, the protein was deemed 99% pure by eye. The determination of the experimental pI of AIM-1 was undertaken, as previously described (19).
Determination of kinetic values.
Purified β-lactamase was used to determine the kinetic parameters kcat and Km. Reactions were performed at 22°C with 1 ml of assay buffer (50 mM cacodylate and 100 μM zinc chloride at pH 7.5) and carried out in duplicate. The standard deviation varied from 3 to 8.5%. The rate of hydrolysis of each β-lactam was calculated for at least 10 different concentrations of substrate based on the extinction coefficients for each substrate. The assays were performed with a Lambda 35 UV-visible (UV-Vis) spectrophotometer (Perkin-Elmer, Cambridge, United Kingdom), by observing the changes in absorption resulting from the opening of the β-lactam ring at the specific wavelengths for each of the 15 antimicrobial agents evaluated, as previously described (24).
Nucleotide sequence accession number.
The nucleotide sequence reported in the present study has been assigned EMBL nucleotide accession number AM998375.
RESULTS AND DISCUSSION
Relatedness and susceptibility profiles of the P. aeruginosa clinical isolates.
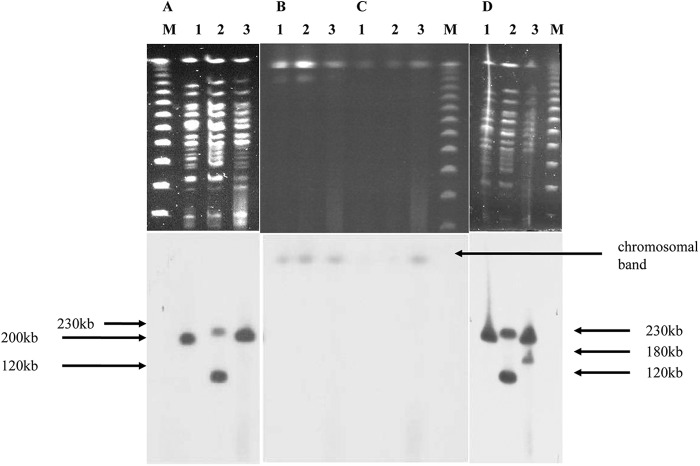
The susceptibility patterns of the three isolates WCH2677, WCH2813, and WCH2837 were found to be nearly identical (Table 1). Genomic DNA from WCH2677 was digested with PstI alone and in combination with a variety of restriction enzymes and revealed fragments of between 1.8 kb and 3.5 kb when hybridized with the blaAIM-1 probe (results not shown). The three P. aeruginosa isolates seem to be closely related clones, with fewer than 3 bands of difference between them in the PFGE analysis (Fig. 1).
Table 1.
Antimicrobial susceptibility patterns of the three AIM-1-producing isolates and E. coli TOP10 carrying blaAIM-1
| Antimicrobial agent | MIC (μg/ml) for isolate |
||||
|---|---|---|---|---|---|
| WCH2677 | WCH2713 | WCH2837 | E. coli TOP10 | E. coli TOP10 (pAIM-1) | |
| Penicillin | >256 | >256 | >256 | 24 | >256 |
| Ampicillin | >256 | >256 | >256 | 12 | >256 |
| Piperacillin | >256 | >256 | >256 | 0.5 | 12 |
| Cephalothin | >256 | >256 | >256 | 8 | >256 |
| Cefoxitin | >256 | >256 | >256 | 4 | 8 |
| Cefotaxime | >256 | >256 | >256 | 0.094 | 8 |
| Cefuroxime | >256 | >256 | >256 | 8 | 12 |
| Ceftazidime | 32 | 16 | 16 | 1 | 32 |
| Aztreonam | 6 | 4 | 4 | 0.094 | 0.125 |
| Cefepime | 6 | 8 | 8 | 0.032 | 0.19 |
| Imipenem | 512 | 512 | 512 | 0.094 | 0.25 |
| Meropenem | 128 | 128 | 256 | 0.064 | 0.25 |
| Ertapenem | 512 | 512 | 512 | 0.25 | 1 |
| Ciprofloxacin | >32 | >32 | >32 | 0.004 | 0.004 |
| Colistin | 4 | 1.5 | 4 | 0.38 | 0.38 |
Fig 1.
Evidence of movement of ISCR15 and blaAIM-1, and relatedness of AIM-1-positive strains. All panels a marker (lane M), WCH2677 (lane 1), WCH2813 (lane 2), and WCH2837 (lane 3). (A) SpeI digestion. (B) I-CeuI digestion. (C) S1 digestion and probing with blaAIM-1. (D) SpeI digestion and probing with ISCR15.
Phenotypic and molecular screening for MBLs.
The results of MBL screening tests were positive using the imipenem-EDTA double-disc synergy test, MBL Etest strips, and determinations of carbapenemase activities by spectrophotometry (data not shown) (43). However, PCR analysis failed to detect previously known MBL genes (blaVIM, blaIMP, blaSPM-1, blaGIM-1, blaKMH-1, blaSIM-1, blaDIM-1, and blaNDM-1) as well as integrons.
Nucleotide and deduced amino acid sequences of blaAIM-1.
DNA from P. aeruginosa WCH2677, the index strain, was used to construct the DNA genomic library. One colony was isolated from the genomic library, and E. coli TOP10 carrying the recombinant had a ceftazidime MIC of 32 μg/ml and gave a positive Etest MBL result (phantom zone). The 3.5-kb cloned fragment contained an open reading frame (ORF) encoding a putative protein of 303 amino acids with a molecular mass of approximately 32 kDa. The predicted pI of the protein is 6.0, and the experimental pI is 6.3 (data not shown). The protein is designated AIM-1 (Adelaide imipenemase). AIM-1 possesses a leader peptide with the probable cleavage site occurring at position 22 between an alanine and a serine, as was shown previously for L1 (42).
The amino acid sequence displayed the highest level of identity with THIN-B (42.1%) (22), followed by L1 (31.4%) (42), and is placed phylogenetically within the B3 subgroup cluster (29). The predicted amino acid sequence showed that the active site of AIM-1 has amino acid motifs that are broadly conserved throughout the MBL family of enzymes (Fig. 2), i.e., the zinc binding motif HXHXD (residues 116 to 120) and other residues involved in the coordination of the two Zn2+ ions (His196 and His263, according to the BBL numbering system [11]). While AIM-1 shows reasonable similarity to L1, it lacks the leucines at positions 5 and 8 and the methionine at position 140 essential for polymerization into a tetramer (Fig. 2) (12, 27). These data are consistent with the fact that AIM-1 behaved as a monomeric protein throughout the gel filtration process (data not shown).
Fig 2.
Alignment of the amino acid sequence of AIM-1 with those of L1 and THIN-B. Differences in the amino acid sequences are noted by a single letter representing the amino acid change within a particular sequence. Conserved residues and residues involved in the coordination of the zinc ions are denoted with asterisks. Numbering is according to the updated BBL scheme (11).
Susceptibility profiles of E. coli (pAIM-1).
The MICs of E. coli TOP10 harboring the cloned AIM-1 gene (pAIM-1) are reported in Table 1. pAIM-1 mediated resistance to penicillin, ampicillin, piperacillin, cephalothin, cefoxitin, cefotaxime, cefuroxime, and ceftazidime but did not confer resistance to the β-lactams aztreonam, cefepime, imipenem, and meropenem. Most MBL-positive P. aeruginosa strains (and the majority of Acinetobacter and Enterobacteriaceae strains) usually exhibit ceftazidime MICs of >100 μg/ml, which has become a signature for MBL detection, as the carbapenem MICs vary significantly and are an unreliable indication of the presence of an MBL (43). Thus, it is worrisome that this rule no longer holds true and that clinically significant MBLs could be missed upon screening due to their lack of resistance to in vitro ceftazidime, as seen in Enterobacteriaceae (8, 31).
Genetic context of blaAIM-1.
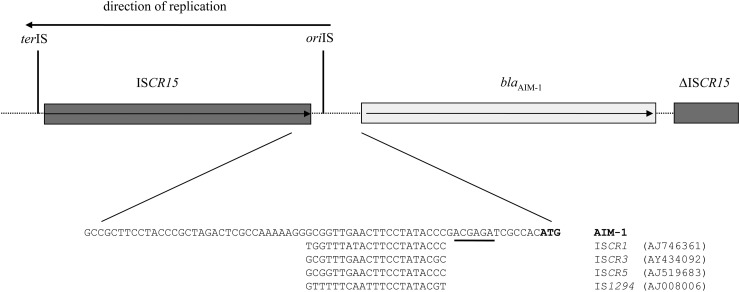
Given that blaAIM-1 was not part of a standard class 1 integron, the upstream and downstream sequences were determined. Upstream of blaAIM-1, there is an ORF, which we have designated ISCR15A, which displays 93.7% nucleotide identity to ISCR5 (Fig. 3). Thus, unlike the majority of MBL genes, blaAIM-1 was not found on a class 1 integron, but instead, it is flanked by two ISCR elements (Fig. 1), rather like blaSPM-1 with ISCR4 and blaOXA-45 with ISCR5. The spacing between the ISCR15A and blaAIM-1 ORFs is 270 bp (Fig. 3). Two hundred thirty-six base pairs downstream from the 3′ end of the ISCR15 element is the signature origin of the insertion sequence (oriIS), 5′-GCGTTTGAACTTCCTATACCC-3′ (boldface type indicates consensus sequence), which is strikingly similar to the oriIS sequences of IS1294, ISCR1, ISCR3, and ISCR5. Immediately upstream of the blaAIM-1 structural gene is a putative ribosomal binding site (RBS), which is only a single base pair downstream of the oriIS site, indicating that the promoter for blaAIM-1 lies within the 3′ end of the ISCR element (Fig. 3), which has been shown for other ISCR1 and other antibiotic resistance genes. A truncated ISCR15-like sequence (designated ISCR15BΔ) of 185 bp was found downstream of blaAIM-1, which has a nucleotide identity of 96.7% to ISCR5 (16). However, the mobility of blaAIM-1 is unlikely to be dependent on ISCR15A, as this element is in the wrong orientation and ISCR15BΔ is truncated despite being in the correct orientation. However, the transposase of ISCR15A may still use the intact oriIS site of the truncated ISCR15B element and thus may still be capable of mobilizing blaAIM-1 in trans (37). The oriIS of ISCR15A is positioned just 13 bp upstream of the start codon of blaAIM-1. Figure 3 shows a creditable RBS, but we cannot identify an appropriate −10 or −35 promoter. However, given the close proximity of the start of blaAIM-1 to the end of ISCR15A (as depicted by the oriIS), the promoter for blaAIM-1 expression must lie within the transposase, as was demonstrated previously for ISCR1 and downstream resistance genes (22, 33). blaAIM-1 and ISCR15 have GC ratios of 69.6% and 68.8%, respectively, indicting that they are likely derived from similar sources but also confirming their nonpseudomonal origin, which normally has a GC ratio of 66%.
Fig 3.
Schematic representation of WCH2677 carrying blaAIM-1 (the arrows in the gene boxes indicate the direction of transcription). The sequence upstream of blaAIM-1 and the oriIS for ISCR1, ISCR3, ISCR5, and IS1294 is highlighted. The putative ribosomal binding site is underlined, and the start codon of blaAIM-1 is indicated in boldface type. GenBank accession numbers are in parentheses.
However, the Southern blot patterns using the blaAIM-1 and ISCR15 probes were not identical. These two genes were used as DNA probes to determine their genetic locations and examine any differences between the strains. While WCH2677 and WCH2837 hybridized as a single band with the blaAIM-1 probe, WCH2813 gave a double band, indicating two copies of the MBL gene (Fig. 1). Since the ISCR15BΔ element is truncated, this evidence may indicate that ISCR15A has mobilized both itself and blaAIM-1 in WCH2813 but has replicated only itself in WCH2837. However, the possibility exists that in strain WCH2837, ISCR15 could have replicated both probes but that the second copy of blaAIM-1 may have been deleted via a recombination event or, indeed, that the genes have been mobilized by another mobile element resident in these isolates (37). Interestingly, when these same digests were probed with ISCR15, only WCH2677 gave the single band, and WCH2837 had an additional band, suggesting the movement of ISCR15 within this isolate. The probing of genomic DNA with SpeI, S1, and I-CeuI indicated that blaAIM-1 is carried on the chromosome in all three strains (Fig. 1).
Functional properties of AIM-1.
Analysis of purified AIM-1 by SDS-PAGE showed a single band corresponding to a molecular mass of 32 kDa (data not shown). Under our experimental conditions, AIM-1 readily hydrolyzed most compounds, with the exception of aztreonam and clavulanic acid (Table 2). Apart from the values for ceftazidime, these values are broadly similar to those of the L1 enzyme. The turnover rates for the carbapenems are higher than those for most other MBLs due to the very high kcat values of AIM-1. However, despite the high level of carbapenem hydrolysis, the recombinant clone in E. coli could not confer resistance to either carbapenem (MICs of 0.25 μg/ml for both imipenem and meropenem), which is consistent with other reports of MBL genes such as blaVIM and blaIMP types (43).
Table 2.
Steady-state kinetic constants of AIM-1, L1, THIN-B, IMP-1, and VIM-2e
| Compound | AIM-1a |
L1b |
THIN-B |
IMP-1 |
VIM-2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| kcat (s−1) | Km (μM) | kcat/Km (s−1/M) | kcat (s−1) | Km (μM) | kcat/Km (s−1/M) | kcat (s−1) | Km (μM) | kcat/Km (s−1/M) | kcat (s−1) | Km (μM) | kcat/Km (s−1/M) | kcat (s−1) | Km (μM) | kcat/Km (s−1/M) | |
| Penicillin G | 778 | 31 | 2.6 × 107 | 410 | 75 | 5.5 × 106 | NR | 320 | 520 | 6.2 × 105 | 56 | 49 | 1.1 × 106 | ||
| Ampicillin | 594 | 41 | 1.4 × 106 | 580 | 300 | 1.9 × 106 | 480 | 1300 | 3.7 × 105 | 950 | 200 | 4.8 × 106 | NR | ||
| Piperacillin | 337 | 17 | 2 × 107 | 420 | 200 | 2.1 × 106 | 100 | 500 | 2 × 105 | ND | ND | ND | 33 | 72 | 4.5 × 105 |
| Cephalothin | 529 | 38 | 1.4 × 107 | 65 | 43 | 1.5 × 106 | NR | 48 | 21 | 2.4 × 106 | 57 | 44 | 1.3 × 106 | ||
| Cefoxitin | 145 | 26 | 5.7 × 106 | 2.2 | 3.3 | 6.7 × 105 | NR | 16 | 8 | 2 × 106 | 3 | 24 | 1.2 × 105 | ||
| Cefotaxime | 609 | 49 | 1.2 × 107 | 140 | 160 | 8.8 × 105 | 80 | 40 | 2 × 106 | 1.3 | 4 | 3.5 × 105 | 28 | 32 | 8.6 × 105 |
| Cefuroxime | 292 | 29 | 9.9 × 106 | 53 | 130 | 4.1 × 105 | 140 | 50 | 2.8 × 106 | 8 | 37 | 2.2 × 105 | 12 | 22 | 5.5 × 105 |
| Ceftazidime | 7 | 148 | 4.9 × 104 | 27 | 145c | 0.2 × 106 | 20 | 140 | 1.4 × 105 | 8 | 44 | 1.8 × 105 | 89 | 98 | 9.0 × 105 |
| Cefepime | 93 | 594 | 1.6 × 105 | 0.33 | 130b | 2.5 × 104 | >2.3 | >300 | 7.9 × 103 | 7 | 11 | 6.6 × 105 | 5 | 184 | 3 × 104 |
| Aztreonam | ND | ND | ND | >1,000 | >0.01 | >1,000 | <0.0001 | <0.5 | ND | ND | |||||
| Imipenem | 1,700 | 97 | 1.7 × 107 | 384 | 48c | 8 × 106 | 120 | 80 | 1.5 × 106 | 46 | 39 | 1.2 × 106 | 10 | 10 | 1.0 × 106 |
| Meropenem | 1,000 | 163 | 6.8 × 106 | 77 | 13d | 5.9 × 106 | 200 | 40 | 5 × 106 | 50 | 10 | 1.2 × 105 | 1.4 | 5 | 2.8 × 105 |
AIM-1 is very different from other group B3 MBLs in that it is able to be mobilized. The fact that ISCR elements are now implicated in the mobilization of three MBL genes, blaSPM-1 (18), blaNDM-1 (44), and now blaAIM-1, indicates that these elements will contribute to the growing trend of resistance among clinically important opportunistic pathogens. During the writing of the manuscript, the MBL gene blaSMB-1 was reported to be adjacent to the ISCR1 complex class 1 integron (38).
ACKNOWLEDGMENTS
This work was funded by European Union grant LSHM-CT-2005-018705, European Union FP7 grant PAR, and Wellcome Trust grant 084627/Z/08/Z.
Footnotes
Published ahead of print 17 September 2012
REFERENCES
- 1. Bellais S, Aubert D, Naas T, Nordmann P. 2000. Molecular and biochemical heterogeneity of class B carbapenem-hydrolyzing beta-lactamases in Chryseobacterium meningosepticum. Antimicrob. Agents Chemother. 44:1878–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boschi L, et al. 2000. The Legionella (Fluoribacter) gormanii metallo-beta-lactamase: a new member of the highly divergent lineage of molecular-subclass B3 beta-lactamases. Antimicrob. Agents Chemother. 44:1538–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bush K. 2010. Alarming beta-lactamase-mediated resistance in multidrug-resistant Enterobacteriaceae. Curr. Opin. Microbiol. 13:558–564 [DOI] [PubMed] [Google Scholar]
- 4. Bush K. 2010. Bench-to-bedside review: the role of beta-lactamases in antibiotic-resistant Gram-negative infections. Crit. Care 14:224 doi:10.1186/cc8892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bush K, Fisher JF. 2011. Epidemiological expansion, structural studies and clinical challenges of new beta-lactamases from Gram-negative bacteria. Annu. Rev. Microbiol. 65:455–478 [DOI] [PubMed] [Google Scholar]
- 6. Castanheira M, Toleman MA, Jones RN, Schmidt FJ, Walsh TR. 2004. Molecular characterization of a beta-lactamase gene, blaGIM-1, encoding a new subclass of metallo-beta-lactamase. Antimicrob. Agents Chemother. 48:4654–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crowder MW, Walsh TR, Banovic L, Pettit M, Spencer J. 1998. Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 42:921–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daikos GL, et al. 2007. Activity of imipenem against VIM-1 metallo-beta-lactamase-producing Klebsiella pneumoniae in the murine thigh infection model. Clin. Microbiol. Infect. 13:202–205 [DOI] [PubMed] [Google Scholar]
- 9. Docquier JD, et al. 2004. Biochemical characterization of the THIN-B metallo-beta-lactamase of Janthinobacterium lividum. Antimicrob. Agents Chemother. 48:4778–4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Docquier JD, et al. 2002. CAU-1, a subclass B3 metallo-beta-lactamase of low substrate affinity encoded by an ortholog present in the Caulobacter crescentus chromosome. Antimicrob. Agents Chemother. 46:1823–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galleni M, et al. 2001. Standard numbering scheme for class B beta-lactamases. Antimicrob. Agents Chemother. 45:660–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu Z, Gunasekera TS, Spadafora L, Bennett B, Crowder MW. 2008. Metal content of metallo-beta-lactamase L1 is determined by the bioavailability of metal ions. Biochemistry 47:7947–7953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumarasamy KK, et al. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 10:597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laraki N, et al. 1999. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-beta-lactamase IMP-1 produced by Escherichia coli. Antimicrob. Agents Chemother. 43:902–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lauretti L, et al. 1999. Cloning and characterization of blaVIM, a new integron-borne metallo-beta-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 43:1584–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li H, Walsh TR, Toleman MA. 2009. Molecular analysis of the sequences surrounding blaOXA-45 reveals acquisition of this gene by Pseudomonas aeruginosa via a novel ISCR element, ISCR5. Antimicrob. Agents Chemother. 53:1248–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Louie A, et al. 2010. Impact of different carbapenems and regimens of administration on resistance emergence for three isogenic Pseudomonas aeruginosa strains with differing mechanisms of resistance. Antimicrob. Agents Chemother. 54:2638–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poirel L, Magalhaes M, Lopes M, Nordmann P. 2004. Molecular analysis of metallo-beta-lactamase gene bla(SPM-1)-surrounding sequences from disseminated Pseudomonas aeruginosa isolates in Recife, Brazil. Antimicrob. Agents Chemother. 48:1406–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poirel L, et al. 2000. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-beta-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother. 44:891–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poirel L, Rodriguez-Martinez JM, Al Naiemi N, Debets-Ossenkopp YJ, Nordmann P. 2010. Characterization of DIM-1, an integron-encoded metallo-beta-lactamase from a Pseudomonas stutzeri clinical isolate in the Netherlands. Antimicrob. Agents Chemother. 54:2420–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rice LB. 2007. Emerging issues in the management of infections caused by multidrug-resistant gram-negative bacteria. Cleve. Clin. J. Med. 74(Suppl 4):S12–S20 doi:10.3949/ccjm.74.suppl_4.512 [DOI] [PubMed] [Google Scholar]
- 22. Rodriguez-Martinez JM, Poirel L, Canton R, Nordmann P. 2006. Common region CR1 for expression of antibiotic resistance genes. Antimicrob. Agents Chemother. 50:2544–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rossolini GM, et al. 2001. Metallo-beta-lactamase producers in environmental microbiota: new molecular class B enzyme in Janthinobacterium lividum. Antimicrob. Agents Chemother. 45:837–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Samuelsen O, Castanheira M, Walsh TR, Spencer J. 2008. Kinetic characterization of VIM-7, a divergent member of the VIM metallo-beta-lactamase family. Antimicrob. Agents Chemother. 52:2905–2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sekiguchi J, et al. 2008. KHM-1, a novel plasmid-mediated metallo-beta-lactamase from a Citrobacter freundii clinical isolate. Antimicrob. Agents Chemother. 52:4194–4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Senda K, et al. 1996. PCR detection of metallo-beta-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum beta-lactams. J. Clin. Microbiol. 34:2909–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simm AM, et al. 2002. Characterization of monomeric L1 metallo-beta-lactamase and the role of the N-terminal extension in negative cooperativity and antibiotic hydrolysis. J. Biol. Chem. 277:24744–24752 [DOI] [PubMed] [Google Scholar]
- 28. Spencer J, Clarke AR, Walsh TR. 2001. Novel mechanism of hydrolysis of therapeutic beta-lactams by Stenotrophomonas maltophilia L1 metallo-beta-lactamase. J. Biol. Chem. 276:33638–33644 [DOI] [PubMed] [Google Scholar]
- 29. Stoczko M, Frere JM, Rossolini GM, Docquier JD. 2008. Functional diversity among metallo-beta-lactamases: characterization of the CAR-1 enzyme of Erwinia carotovora. Antimicrob. Agents Chemother. 52:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tato M, Coque TM, Baquero F, Canton R. 2010. Dispersal of carbapenemase blaVIM-1 gene associated with different Tn402 variants, mercury transposons, and conjugative plasmids in Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 54:320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tato M, et al. 2010. Carbapenem heteroresistance in VIM-1-producing Klebsiella pneumoniae isolates belonging to the same clone: consequences for routine susceptibility testing. J. Clin. Microbiol. 48:4089–4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thaller MC, et al. 2011. Metallo-β-lactamase production by Pseudomonas otitidis: a species-related trait. Antimicrob. Agents Chemother. 55:118–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Toleman MA, Bennett PM, Walsh TR. 2006. ISCR elements: novel gene-capturing systems of the 21st century? Microbiol. Mol. Biol. Rev. 70:296–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Toleman MA, Biedenbach D, Bennett DM, Jones RN, Walsh TR. 2005. Italian metallo-beta-lactamases: a national problem? Report from the SENTRY Antimicrobial Surveillance Programme. J. Antimicrob. Chemother. 55:61–70 [DOI] [PubMed] [Google Scholar]
- 35. Toleman MA, Rolston K, Jones RN, Walsh TR. 2004. blaVIM-7, an evolutionarily distinct metallo-beta-lactamase gene in a Pseudomonas aeruginosa isolate from the United States. Antimicrob. Agents Chemother. 48:329–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Toleman MA, et al. 2002. Molecular characterization of SPM-1, a novel metallo-beta-lactamase isolated in Latin America: report from the SENTRY antimicrobial surveillance programme. J. Antimicrob. Chemother. 50:673–679 [DOI] [PubMed] [Google Scholar]
- 37. Toleman MA, Walsh TR. 2011. Combinatorial events of insertion sequences and ICE in Gram-negative bacteria. FEMS Microbiol. Rev. 35:912–935 [DOI] [PubMed] [Google Scholar]
- 38. Wachino J, et al. 2011. SMB-1, a novel subclass B3 metallo-beta-lactamase, associated with ISCR1 and a class 1 integron, from a carbapenem-resistant Serratia marcescens clinical isolate. Antimicrob. Agents Chemother. 55:5143–5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Walsh TR. 2010. Emerging carbapenemases: a global perspective. Int. J. Antimicrob. Agents 36(Suppl 3):S8–S14 doi:10.1086/S0924-8579(10)70004-2 [DOI] [PubMed] [Google Scholar]
- 40. Walsh TR, Bolmstrom A, Qwarnstrom A, Gales A. 2002. Evaluation of a new Etest for detecting metallo-beta-lactamases in routine clinical testing. J. Clin. Microbiol. 40:2755–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Walsh TR, Gamblin S, Emery DC, MacGowan AP, Bennett PM. 1996. Enzyme kinetics and biochemical analysis of ImiS, the metallo-beta-lactamase from Aeromonas sobria 163a. J. Antimicrob. Chemother. 37:423–431 [DOI] [PubMed] [Google Scholar]
- 42. Walsh TR, et al. 1994. Sequence analysis of the L1 metallo-beta-lactamase from Xanthomonas maltophilia. Biochim. Biophys. Acta 1218:199–201 [DOI] [PubMed] [Google Scholar]
- 43. Walsh TR, Toleman MA, Poirel L, Nordmann P. 2005. Metallo-beta-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yong D, et al. 2009. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 53:5046–5054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yong D, et al. Abstr. 47th Annu. Intersci. Conf. Antimicrob. Agents Chemother., abstr C1-593.2007. [Google Scholar]