Abstract
ICESp1116, responsible for erm(B)-mediated, inducible erythromycin resistance in Streptococcus pyogenes, was comprehensively characterized, and its chromosomal integration site was determined. It displayed a unique mosaic organization consisting of a scaffold, related to TnGallo1 from Streptococcus gallolyticus, with two inserted fragments separated by IS1216. One fragment, containing erm(B), displayed high-level identity to a portion of the S. pyogenes plasmid pSM19035; the other, containing a truncated tet(M) gene, displayed high-level identity to the right-hand portion of Clostridium difficile Tn5397.
TEXT
Three erythromycin resistance methylase genes have so far been described in Streptococcus pyogenes: the classic, long-established erm(B) determinant, an erm(A) subclass commonly referred to as erm(TR), and the much less common erm(T) determinant (30). Whereas erm(TR) and erm(T) normally encode inducible erythromycin resistance, the erm(B) gene may encode constitutive resistance in some S. pyogenes isolates and inducible resistance in others.
Consistent with such heterogeneity of erm gene-encoded erythromycin resistance, a variety of erm-carrying genetic elements have been found and characterized in S. pyogenes, including diverse integrative and conjugative elements (ICEs) carrying erm(TR) (3, 9, 12) and closely related plasmids carrying erm(T) (11, 33). As regards erm(B), it is carried by different elements depending on whether it is expressed constitutively or inducibly. Tn916 family elements, such as Tn6002 (∼21 kb) (32) or Tn3872 (∼24 kb) (17), aside from rare plasmid locations, are primarily involved in the former case (7). When inducibly expressed, erm(B) has been shown to be carried by a different element, called Tn1116, of which only an ∼7-kb region—containing erm(B) and a fragment highly homologous to the right-hand portion of Clostridium difficile Tn5397 (18) — has so far been characterized (7). Tn5397 (∼21 kb; accession no. AF333235) is a unique Tn916 family element where the tndX (resolvase) gene replaces int (integrase) and xis (excisionase) (31).
Within the erythromycin-resistant S. pyogenes population circulating in Italy, isolates bearing inducible erm(B) represent a major subpopulation (13, 21, 27, 34) clearly prevailing among erm(B)-positive isolates (7, 24) and typically characterized by uniform susceptibility to tetracycline (7, 13, 21). In this study, Tn1116, renamed ICESp1116 (23), was comprehensively characterized (accession no. HE802677). ICESp1116 was uniformly distributed in S. pyogenes isolates with erm(B)-mediated, inducible erythromycin resistance.
ICESp1116 was investigated in S. pyogenes strain A-3 [erythromycin MIC > 128 μg/ml, inducible phenotype, erm(B) genotype; tetracycline MIC of 0.25 μg/ml, tet(M) genotype] (7). The principal oligonucleotide primer pairs used in PCR experiments are listed in Table 1. Most oligonucleotides for long PCR experiments were designed from the broad-host-range Gram-positive plasmid pSM19035 (∼29 kb; accession no. AY357120) (16) and from transposon TnGallo1 of Streptococcus gallolyticus UCN34 (∼41 kb; accession no. FN597254) (25). DNA sequencing and sequence analysis were performed as described elsewhere (9). A reported percentage of amino acid identity represents the mean value resulting from the comparison of deduced proteins encoded by individual open reading frames (ORFs) in the DNA fragments considered.
Table 1.
Principal oligonucleotide primer pairs used
| Category and genea | Primer designation | Sequence (5′–3′) | Source or reference | Product size (bp) |
|---|---|---|---|---|
| Resistance genes | ||||
| erm(B) | ERMB1 | GAAAAGGTACTCAACCAAATA | 28 | 639 |
| ERMB2 | AGTAACGGTACTTAAATTGTTTAC | 28 | ||
| tet(M) | TETM2 | GAACTCGAACAAGAGGAAAGC | 19 | 740 |
| TETM3 | ATGGAAGCCCAGAAAGGAT | 19 | ||
| ICESp1116 mapping | ||||
| orf2 | ORF2-for | TTTTCCGAGTGCCGAAGGTGC | This study | 11,230 |
| orf11 | ORF11-rev | GCGTTACCCCTTTTTCTTTTCG | This study | |
| orf12 | ORF12-for | GACGGTTCCACCATCATTTATC | This study | 11,490 |
| orf23 | ORF23-rev | CTTTTTCTTCACTCGTAACCGT | This study | |
| orf24 | ORF24-for | AGACAATCAAGTTACTAATCAT | This study | 9,372 |
| orf31 | ORF31-rev | GTTTCCCATTTAGCCGTCA | This study | |
| orf31 | ORF31-for | GAAAAGAAACCCTGGTAAAAG | This study | 12,394 |
| Downstream of orf45 | 1116end-rev | GAGAATAATCATCAAGCCTAT | This study | |
| ICESp1116 junctions | ||||
| SPYALAB49_000242b | JUG-for | GCGTCTTGGAATCTGGTCA | This study | 621 |
| orf1 | ORF1-rev | CCTCTCTCAGCAAGAACTCGCCG | This study | |
| orf45 | ORF45-for | GTATGACAGGTATGAGATTTG | This study | 2,448 |
| SPYALAB49_000245b | EPI-rev | AGGCTCTTGCGGCGGGTAA | This study | |
| ICESp1116 circular form | ||||
| orf1 | ORF1-rev | CCTCTCTCAGCAAGAACTCGCCG | This study | 1,937 |
| orf45 | ORF45-for | GTATGACAGGTATGAGATTTG | This study | |
| Chromosomal empty target site | ||||
| SPYALAB49_000242b | JUG-for | GCGTCTTGGAATCTGGTCA | This study | 1,134 |
| SPYALAB49_000245b | EPI-rev | AGGCTCTTGCGGCGGGTAA | This study | |
| Inverse PCR assays | ||||
| orf2 | REPA-inv | GCACCTTCGGCACTCGGAAAA | This study | |
| REPA-for | CTAATGATACTGATATTAGT | This study | ||
| orf30 | BETA-inv | AGAAACGCCCTGTAACGCTT | This study | |
| BETA-for | GGAATCAGAACGCAAACG | This study | ||
| orf36 [erm(B)] | ERMB-inv | GTAAACAATTTAAGTACCGTTACT | This study | |
| ERMB-for | TATTTGGTTGAGTACTTTTTC | This study | ||
| orf44 (tndX) | TNDX-inv | CTTTGCTCGATAGGCTCTA | This study | |
| TNDX-for | CGGATTGATGAAATAGACAGT | This study |
Except for resistance genes and unless otherwise specified, designations are according to the ORF numbering of ICESp1116.
From the S. pyogenes Alab49 genome.
Genetic organization of ICESp1116.
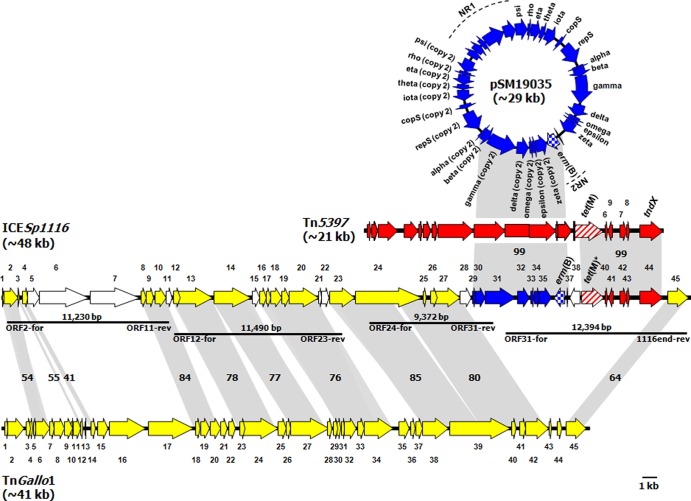
Most ORFs of ICESp1116 encoded proteins with various degrees of amino acid identity to those encoded by the corresponding ORFs of TnGallo1. Two fragments, separated by IS1216, were found to be inserted in a scaffold largely including TnGallo1-like sequences. One fragment contained erm(B) and displayed high-level identity to a portion of the S. pyogenes plasmid pSM19035 (16); the other contained a truncated tet(M) gene and displayed high-level identity to the right-hand portion of C. difficile transposon Tn5397 (18). ICESp1116 (size, 48,174 bp; G+C content, 35%; 45 ORFs) is shown in Fig. 1, where its ORF map is aligned with the ORF maps of TnGallo1, pSM19035, and Tn5397; the characteristics of the ORFs are detailed in the supplemental material (see Table S1). The organization of ICESp1116 is summarized below.
Fig 1.
ORF map and genetic organization of ICESp1116 from S. pyogenes strain A-3 (accession no. HE802677) and its alignment with the ORF maps of S. gallolyticus transposon TnGallo1 (accession no. FN597254), S. pyogenes plasmid pSM19035 (accession no. AY357120), and C. difficile transposon Tn5397 (accession no. AF333235). The ORFs, indicated as arrows pointing in the direction of transcription, are numbered consecutively in ICESp1116 (orf1 to orf45, with predicted functions reported in Table S1 in the supplemental material) and in TnGallo1 (orf1 to orf45). In pSM19035, where the two nonrepeated sequences NR1 and NR2 are marked by broken lines, the ORFs are numbered according to the specific designations reported in GenBank (the designations of the NR1 ORFs are not indicated). In Tn5397, the ORFs are also numbered according to the specific designations reported in GenBank [those upstream of tet(M) are not indicated]. TnGallo1 ORFs and related ORFs of ICESp1116 are depicted as yellow arrows. pSM19035 ORFs and ICESp1116 ORFs of the pSM19035 fragment are depicted as blue arrows [with erm(B) checkered]. Tn5397 ORFs and ICESp1116 ORFs of the Tn5397 fragment are depicted as red arrows [with tet(M) striped]. The other ICESp1116 ORFs are depicted as white arrows. Gray areas between ORF maps denote amino acid identities as reported (%). Horizontal bars indicate the amplicons (each with the relevant primer pair and size) allowing PCR mapping.
(i) orf1-orf28 (bp 1 to 32,827; 36% G+C).
Most ORFs in this region encoded proteins with various degrees of amino acid identity (41% to 85%) to TnGallo1. The specific functions associated with some such ORFs (orf2, orf13, orf16, orf20, orf23, and orf27) (see Table S1 in the supplemental material) are presumably involved in ICE transfer. Among TnGallo1-unrelated ORFs, orf5-orf7 is a cluster that does not occur in TnGallo1 but does occur in the genomes of closely related Streptococcus species which, like S. gallolyticus, result from the recent reclassification of Streptococcus bovis (26); the highest degree of amino acid identity (96%) was with the proteins encoded by the corresponding three-ORF cluster of Streptococcus macedonicus (accession no. HE613569). Special functions appear to be associated with orf6 and orf7. The former encodes a putative SspB protein, a large multidomain protein of the AgI/II family, i.e., cell surface proteins that may be key factors in the formation of streptococcal biofilms and that play multiple roles in streptococcal adherence, colonization, and microbial community development (6). orf7 encodes a bacteriocin-processing endopeptidase belonging to the peptidase family C39 (14).
(ii) orf29-orf37 (pSM19035 fragment) (bp 32,828 to 39,611; 34% G+C).
This region, initially explored by inverse PCR, was formed by an ∼6.8-kb erm(B)-containing fragment whose ORF encoded proteins with 99% amino acid identity to those encoded by a corresponding portion of pSM19035. This plasmid has extraordinarily long inverted repeats (∼80% of the plasmid genome) separated by two nonrepeated sequences (NR1 and NR2) (5). The pSM19035 fragment comprises seven ORFs (orf29-orf35) from one of the inverted repeats (from alpha [copy 2] to zeta [copy 2]) plus NR2 [containing erm(B) (orf36) and its leader peptide (orf37)]. Interestingly, orf33 (omega [copy 2]), orf34 (epsilon [copy 2]), and orf35 (zeta [copy 2]) correspond to a pSM19035 omega-epsilon-zeta operon constituting a plasmid addiction system where the epsilon and zeta genes encode antitoxin and toxin, respectively, while omega plays an autoregulatory function (35). Accordingly, the zeta-epsilon toxin-antitoxin cassette might contribute to stable maintenance of ICESp1116 in the bacterial population.
(iii) orf38 (bp 39,612 to 40,420; 36% G+C).
orf38 encoded the transposase of IS1216, found mainly in enterococcal genomes but also in other Gram-positive bacteria and frequently associated with antibiotic resistance genes in streptococci (2, 15, 29).
(iv) orf39-orf44 (Tn5397 fragment) (bp 40,421 to 46,047; 36% G+C).
This region was formed by an ∼5.6-kb fragment whose ORF encoded proteins with 99% amino acid identity to those encoded by the right-hand portion of Tn5397. The fragment starts with a truncated tet(M) gene (orf39), resulting from the insertion of IS1216 in the tet(M) coding sequence. Clearly, the truncated tet(M) gene was silent, in agreement with the tetracycline susceptibility of S. pyogenes A-3. The next ORFs of the fragment (orf40-orf44) were ∼99% identical to the corresponding ORFs of Tn5397, the last one (orf44) being tndX.
(v) orf45 (bp 46,048 to 48,174; 31% G+C).
We expected that tndX, the last ORF of Tn5397, was also the last ORF of ICESp1116. However, inverse PCR assays revealed an additional ICE ORF (orf45) encoding a protein with 64% amino acid identity to that encoded by the last ORF of TnGallo1, which encodes a DDE transposase.
Chromosomal integration of ICESp1116.
The left junction of ICESp1116 was identified by inverse PCR. ICESp1116 was found to be integrated at the 3′ end of an ORF, detected in all S. pyogenes genomes sequenced so far, whose highest degree of DNA identity (99%) was with the SPYALAB49_000243 gene from S. pyogenes Alab49 (accession no. CP003068) (4).
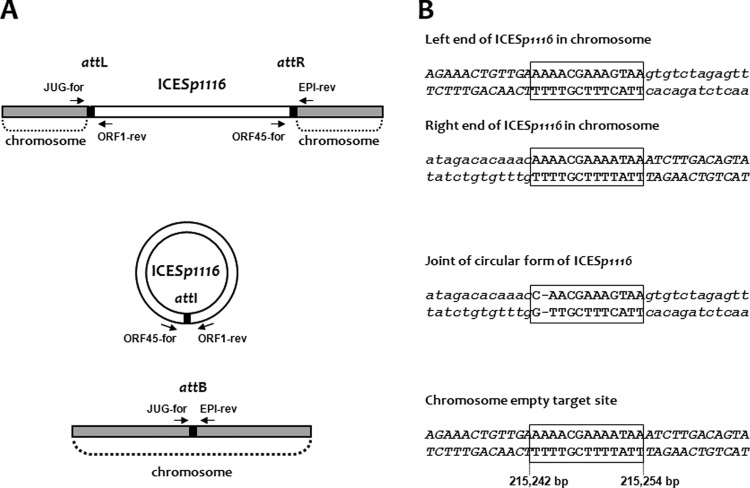
Detection of the circular form and of the core integration site of ICESp1116.
The ICESp1116 circular form was detected using an appropriate outward-directed primer pair (ORF45-for/ORF1-rev) (Fig. 2A). Sequence analysis of the amplicons from the integrated (containing attL and attR) and circular (containing attI) forms of ICESp1116 and from the empty chromosome (containing attB) of S. pyogenes A-3 led to the identification of a 13-bp putative core site (corresponding to bases 215,242 to 215,254 of the S. pyogenes Alab49 genome) (Fig. 2B).
Fig 2.
Detection of excision of ICESp1116 from the genome. (A) Scheme of primer binding sites on the element and the genome. The chromosomal region is shown in gray, and ICESp1116 is in white. The integrated (top) and circular (center) forms of the element are shown, as is the regenerated target after excision (bottom). The core integration site is shown as a black box. Oligonucleotide primers and their direction of priming are represented by arrows. The primer pairs JUG-for/ORF1-rev and ORF45-for/EPI-rev detect the junctions between the genome and ICESp1116 (attL and attR, respectively), ORF45-for/ORF1-rev detects the circular form (containing attI) of ICESp1116, and JUG-for/EPI-rev detects the empty target site (containing attB). (B) Partial nucleotide sequences of the ICESp1116 integrated form at the left and right junctions (top), of the circular form (center), and of the chromosomal empty target site (bottom), showing the putative core sites (boxed sequences in uppercase roman letters), corresponding to bases 215,242 to 215,254 of the S. pyogenes Alab49 genome (accession no. CP003068). Nucleotides belonging to ICESp1116 are in lowercase italics; nucleotides belonging to the S. pyogenes A-3 chromosome are in uppercase italics.
Distribution of ICESp1116 in other S. pyogenes isolates with erm(B)-mediated, inducible erythromycin resistance.
Twenty additional S. pyogenes test strains—randomly selected among isolates having inducible erythromycin resistance mediated by the erm(B) gene, collected in 1998 to 2007—displayed uniform antibiotic susceptibility patterns: all were highly resistant to 14-, 15-, and 16-membered macrolides (MIC, >128 μg/ml); all were susceptible to clindamycin without induction (MIC, 0.03 to 0.5 μg/ml) but resistant after induction with erythromycin (MIC, >128 μg/ml); and all were susceptible to tetracycline (MIC, ≤0.125 to 0.5 μg/ml) in spite of (similar to the case with strain A-3) a tet(M) genotype. The 20 strains were subjected to PCR mapping using four primer pairs, designed from the sequence of ICESp1116, yielding amplicons covering the entire element (Fig. 1). While 19 strains produced amplicons of the expected size with all four primer pairs, the 20th gave no amplification with the fourth primer pair, although it did yield a regular amplicon when ORF31-for was paired with a primer internal to orf45. All strains gave positive PCRs with the primer pairs targeting the left and the right junctions, respectively (Fig. 2A). These data confirm that ICESp1116 is typically harbored by S. pyogenes isolates with erm(B)-mediated, inducible erythromycin resistance.
Conclusions.
ICESp1116 shares with other streptococcal elements involved in macrolide resistance a mosaic structure resulting from the insertion of particular DNA fragments into a particular scaffold. These DNA fragments are of the most varied origins and often carry other antibiotic resistance genes—besides erythromycin resistance determinants—and other genes encoding products which may increase bacterial fitness. The scaffolds are also of various origins and natures. In the case of ICESp1116, the scaffold is related to a streptococcal transposon (TnGallo1); in other cases, it may consist of a Tn916 family transposon (10, 22, 30), a clostridial ICE (9), or a bacteriophage (1, 8, 10, 20, 30).
A unique feature of ICESp1116 is its chromosomal integration site. The relevant chromosomal gene (highest identity, the SPYALAB49_000243 gene from S. pyogenes Alab49) occurs in all the S. pyogenes genomes sequenced so far but has never been shown to represent the chromosomal integration site of a genetic element. It may be hypothesized that orf45 of ICESp1116 plays a role in such a specific chromosomal integration.
The occurrence of IS1216 between the pSM19035 fragment and the Tn5397 fragment suggests that the insertion sequence might have been involved in early mobilization of one of the two fragments to form ICESp1116. IS1216 cuts off the coding sequence of tet(M), resulting in loss of the 5′ end of the gene (besides, of course, the remaining upstream portion of Tn5397) and in a silent tet(M). A subpopulation harboring ICESp1116, sharing a number of typing characters, is highly prevalent in Italy among erythromycin-resistant S. pyogenes isolates (7, 13, 21, 24, 27, 34). The success of this subpopulation may have been favored by the acquisition of specific genetic traits, such as orf6, whose product, the cell wall-anchored adhesin SspB, may have provided ICESp1116-harboring isolates with an adaptive advantage and enhanced their survival through a greater ability to compete with indigenous commensal streptococci in colonizing host surfaces (6).
Supplementary Material
ACKNOWLEDGMENT
This work was partly supported by the Italian Ministry of Education, University and Research (PRIN 200929YFMK).
Footnotes
Published ahead of print 1 October 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Banks DJ, Porcella SF, Barbian KD, Martin JM, Musser JM. 2003. Structure and distribution of an unusual chimeric genetic element encoding macrolide resistance in phylogenetically diverse clones of group A Streptococcus. J. Infect. Dis. 188:1898–1908 [DOI] [PubMed] [Google Scholar]
- 2. Barile S, Devirgiliis C, Perozzi G. 2012. Molecular characterization of a novel mosaic tet(S/M) gene encoding tetracycline resistance in foodborne strains of Streptococcus bovis. Microbiology 158:2353–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beres SB, Musser JM. 2007. Contribution of exogenous genetic elements to the group A Streptococcus metagenome. PLoS One 2:e800 doi:10.1371/journal.pone.0000800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bessen DE, et al. 2011. Whole-genome association study on tissue tropism phenotypes in group A Streptococcus. J. Bacteriol. 193:6651–6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boitsov AS, et al. 1979. Inverted repeats on plasmids determining resistance to MLS antibiotics in group A streptococci. FEMS Microbiol. Lett. 6:11–14 [Google Scholar]
- 6. Brady LJ, et al. 2010. The changing faces of Streptococcus antigen I/II polypeptide family adhesins. Mol. Microbiol. 77:276–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brenciani A, et al. 2007. Genetic elements carrying erm(B) in Streptococcus pyogenes, and association with tet(M) tetracycline resistance gene. Antimicrob. Agents Chemother. 51:1209–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brenciani A, et al. 2010. Characterization of Φm46.1, the main Streptococcus pyogenes element carrying mef(A) and tet(O) genes. Antimicrob. Agents Chemother. 54:221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brenciani A, et al. 2011. Two distinct genetic elements are responsible for erm(TR)-mediated erythromycin resistance in tetracycline-susceptible and tetracycline-resistant strains of Streptococcus pyogenes. Antimicrob. Agents Chemother. 55:2106–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Del Grosso M, et al. 2011. Genetic resistance elements carrying mef subclasses other than mef(A) in Streptococcus pyogenes. Antimicrob. Agents Chemother. 55:3226–3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DiPersio LP, DiPersio JR, Beach JA, Loudon AM, Fuchs AM. 2011. Identification and characterization of plasmid-borne erm(T) macrolide resistance in group B and group A Streptococcus. Diagn. Microbiol. Infect. Dis. 71:217–223 [DOI] [PubMed] [Google Scholar]
- 12. Giovanetti E, Brenciani A, Tiberi E, Bacciaglia A, Varaldo PE. 2012. ICESp2905, the erm(TR)-tet(O) element of Streptococcus pyogenes, is formed by two independent integrative and conjugative elements. Antimicrob. Agents Chemother. 56:591–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giovanetti E, Montanari MP, Mingoia M, Varaldo PE. 1999. Phenotypes and genotypes of erythromycin-resistant Streptococcus pyogenes strains in Italy and heterogeneity of inducibly resistant strains. Antimicrob. Agents Chemother. 43:1935–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Håvarstein LS, Diep DB, Nes IF. 1995. A family of bacteriocin ABC transporters carry out proteolytic processing of their substrates concomitant with export. Mol. Microbiol. 16:229–240 [DOI] [PubMed] [Google Scholar]
- 15. Liu LC, et al. 2008. Identification of tet(S) gene area in tetracycline-resistant Streptococcus dysgalactiae subsp. equisimilis clinical isolates. J. Antimicrob. Chemother. 61:453–455 [DOI] [PubMed] [Google Scholar]
- 16. Malke H. 1974. Genetics of resistance to macrolide antibiotics and lincomycin in natural isolates of Streptococcus pyogenes. Mol. Gen. Genet. 135:349–367 [DOI] [PubMed] [Google Scholar]
- 17. McDougal LK, et al. 1998. Detection of Tn917-like sequences within a Tn916-like conjugative transposon (Tn3872) in erythromycin-resistant isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2312–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mullany P, Pallen M, Wilks M, Stephen JR, Tabaqchali S. 1996. A group II intron in a conjugative transposon from the gram-positive bacterium, Clostridium difficile. Gene 174:145–150 [DOI] [PubMed] [Google Scholar]
- 19. Olsvik B, Olsen I, Tenover FC. 1995. Detection of tet(M) and tet(O) using the polymerase chain reaction in bacteria isolated from patients with periodontal disease. Oral Microbiol. Immunol. 10:87–92 [DOI] [PubMed] [Google Scholar]
- 20. Palmieri C, Princivalli MS, Brenciani A, Varaldo PE, Facinelli B. 2011. Different genetic elements carrying the tet(W) gene in two human clinical isolates of Streptococcus suis. Antimicrob. Agents Chemother. 55:631–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ripa S, et al. 2001. SmaI macrorestriction analysis of Italian isolates of erythromycin-resistant Streptococcus pyogenes and correlations with macrolide-resistance phenotypes. Microb. Drug Resist. 7:65–71 [DOI] [PubMed] [Google Scholar]
- 22. Roberts AP, Mullany P. 2011. Tn916-like genetic elements: a diverse group of modular mobile elements conferring antibiotic resistance. FEMS Microbiol. Rev. 35:856–871 [DOI] [PubMed] [Google Scholar]
- 23. Roberts AP, et al. 2008. Revised nomenclature for transposable genetic elements. Plasmid 60:167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rombini S, et al. 2011. A study on erm(B)-mediated MLS resistance in Streptococcus pyogenes clinical isolates. Diagn. Microbiol. Infect. Dis. 70:387–394 [DOI] [PubMed] [Google Scholar]
- 25. Rusniok C, et al. 2010. Genome sequence of Streptococcus gallolyticus: insights into its adaptation to the bovine rumen and its ability to cause endocarditis. J. Bacteriol. 192:2266–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schlegel L, Grimont F, Ageron E, Grimont PA, Bouvet A. 2003. Reappraisal of the taxonomy of the Streptococcus bovis/Streptococcus equinus complex and related species: description of Streptococcus gallolyticus subsp. gallolyticus subsp. nov., S. gallolyticus subsp. macedonicus subsp. nov. and S. gallolyticus subsp. pasteurianus subsp. nov. Int. J. Syst. Evol. Micribiol. 53:631–645 [DOI] [PubMed] [Google Scholar]
- 27. Spinaci C, et al. 2004. Genetic diversity of cell-invasive erythromycin-resistant and -susceptible group A streptococci determined by analysis of the RD2 region of the prtF1 gene. J. Clin. Microbiol. 42:639–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sutcliffe J, Grebe T, Tait-Kamradt A, Wondrack L. 1996. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 40:2562–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsai JC, et al. 2005. The erm(T) gene is flanked by IS1216V in inducible erythromycin-resistant Streptococcus gallolyticus subsp. pasteurianus. Antimicrob. Agents Chemother. 49:4347–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Varaldo PE, Montanari MP, Giovanetti E. 2009. Genetic elements responsible for erythromycin resistance in streptococci. Antimicrob. Agents Chemother. 53:343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang H, Mullany P. 2000. The large resolvase TndX is required and sufficient for integration and excision of derivatives of the novel conjugative transposon Tn5397. J. Bacteriol. 182:6577–6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Warburton PJ, Palmer RM, Munson MA, Wade WG. 2007. Demonstration of in vivo transfer of doxycycline resistance mediated by a novel transposon. J. Antimicrob. Chemother. 60:973–980 [DOI] [PubMed] [Google Scholar]
- 33. Woodbury RL, et al. 2008. Plasmid-borne erm(T) from invasive, macrolide-resistant Streptococcus pyogenes strains. Antimicrob. Agents Chemother. 52:1140–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zampaloni C, Cappelletti P, Prenna M, Vitali LA, Ripa S. 2003. emm gene distribution among erythromycin-resistant and -susceptible Italian isolates of Streptococcus pyogenes. J. Clin. Microbiol. 41:1307–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zielenkiewicz U, Ceglowski P. 2005. The toxin-antitoxin system of the streptococcal plasmid pSM19035. J. Bacteriol. 187:6094–6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.