Abstract
A key challenge faced by promising antiviral drugs, such as iminosugars, is in vivo delivery to achieve effective levels of drug without toxicity. Four iminosugars, all deoxynojirimycin (DNJ) derivatives—N-butyl DNJ (NB-DNJ), N-nonyl DNJ, N-(9-methoxynonyl) DNJ, and N-(6′-[4″-azido-2″-nitrophenylamino]hexyl)-1-DNJ (NAP-DNJ)—potently inhibited both the percentage of cells infected with dengue virus and release of infectious virus from primary human monocyte-derived macrophages, demonstrating their efficacy in primary cells. In a lethal antibody-dependent enhancement mouse model of dengue pathogenesis, free NB-DNJ significantly enhanced survival and lowered viral load in organs and serum. Liposome-mediated delivery of NB-DNJ, in comparison with free NB-DNJ, resulted in a 3-log10 reduction in the dose of drug sufficient to enhance animal survival. The optimizing of the effective dose in this way could liberate the therapeutic potential of many cytotoxic antivirals against both dengue virus and a wide array of other viruses.
INTRODUCTION
Dengue is the most prevalent arthropod-borne viral infection in the world and a growing public health concern. Over the past 50 years, the increase in international travel, rapid urbanization, difficulties in vector control, and possibly climate change have promoted a 30-fold increase in reported dengue virus (DENV) infection (22). As such, the financial, social, and individual costs of dengue are significant, although underappreciated (41, 46). As an arthropod-borne disease, the prevention of transmission is difficult to achieve, although several genetic strategies targeting the mosquito vector have recently shown promise in pilot studies (19). Despite concerted effort for over 70 years, and more recently an increase in funding for vaccine research, there is still no licensed prophylactic vaccine for dengue. With no specific antiviral treatment available, management is presently supportive and focused on symptom control primarily through fluid replacement. Thus, there is significant need for a postexposure (i.e., therapeutic) antiviral.
Iminosugars, such as deoxynojirimycin (DNJ) and its N-alkylated derivatives, demonstrate antiviral activity against DENV by targeting host cellular factors required for viral morphogenesis (reviewed in reference 36). These molecules are analogues of glucose that inhibit host cell glucosidases required for folding and maturation of glycoproteins. Inhibition of these enzymes prevents correct glycoprotein folding and oligomerization and thereby inhibits assembly of infectious virions, as viral envelope glycoproteins cannot interact with endoplasmic reticulum (ER) chaperones (e.g., calnexin and calreticulin). As they target a host process, iminosugars are of use against a wide range of viral infections. Furthermore, they are effective against all 4 serotypes of DENV (45), exhibit low toxicity, and may prevent emergence of viral escape mutants.
The iminosugar N-butyl DNJ (NB-DNJ) has been licensed for use in humans for over 10 years for treatment of Gaucher disease; thus, its potential clinical use against dengue is highly feasible. In trials as an anti-HIV therapy, administration of free drug did not sustain sufficiently high serum concentrations to achieve an antiviral effect (14). Since that time, iminosugars with improved in vitro potency (but concurrently greater cellular toxicity) have been developed (1, 7, 9, 16, 24, 33). As a parallel approach to enhance NB-DNJ potency, liposome-mediated intracellular delivery of iminosugars shows great promise (30). Liposomes have been used for many years as drug delivery vehicles (39), and recently, phosphoethanolamine and cholesteryl hemisuccinate liposome formulations encapsulating NB-DNJ inhibited HIV-1 with a 100,000-fold enhancement at the 50% inhibitory concentration (IC50) relative to free NB-DNJ (30). Subsequently, ER-targeting liposomes have been developed which traffic to the ER following cellular uptake (29). In addition to the benefits of targeted drug delivery, similar liposomes have inherent antiviral properties mediated by their cholesterol-lowering effects in treated cells (31).
The presence of DENV nonstructural proteins in activated monocytes in the blood (12, 21) and in phagocytes in the lymph node and spleen (2) indicates that monocytes and macrophages are key targets of human DENV infection. These findings support the hypothesis that these cells play a central role in severe dengue disease as sources of both new virus and immune mediators that shape the course of the subsequent immune response (4, 5, 13, 17). In addition to direct infection, macrophages are susceptible to targeted DENV infection via Fc-mediated opsonization in a process known as antibody-dependent enhancement (ADE) (11, 27, 28, 37). In spite of the relevance of the system, antiviral activity of iminosugars against DENV has only once been tested in a human cell line (N-nonyl DNJ [NN-DNJ] in Huh-7 cells [45]), and iminosugars have not been tested against DENV infection in primary human monocyte-derived macrophages (MDMϕs).
Here we report the antiviral activity of iminosugars against DENV using an in vitro human macrophage model of infection and an ADE mouse model of dengue pathogenesis. NB-DNJ was chosen as a model iminosugar for our experiments examining the ability of polyunsaturated ER-targeting liposomes (PERLs) to enhance iminosugar efficacy against DENV because NB-DNJ is clinically licensed, has relatively low cytotoxicity, and allows easy comparison to previous results obtained for HIV-1. Although this drug is a prototype for other iminosugar derivatives that have antiviral activity against DENV (36), to our knowledge, there are currently no published data on the antiviral activity of NB-DNJ against DENV in vitro or in vivo. We show that NB-DNJ delivered as either a free drug or encapsulated in PERLs enhanced survival in the ADE mouse model and that liposome-mediated delivery increased NB-DNJ potency 2,660-fold.
MATERIALS AND METHODS
Viruses and cells.
DENV2 strain D2S10 was derived from the parental DENV2 PL046 Taiwanese isolate, isolated by 10 cycles of peripheral passage in mice and C6/36 mosquito cells, as previously described (40). In comparison with PL046, at the consensus sequence level, D2S10 varies by 8 nucleotides, with only one amino acid change in domain II of the E protein (K128E) and one nucleotide substitution in the 3′ untranscribed region (3′ UTR). None of the N-linked glycosylation sites on prM, E, and NS1 are altered in this virus, in comparison to PL046 (40). DENV2 strain 16681 (a gift from E. Gould, Centre for Ecology and Hydrology, Oxford, United Kingdom) was propagated in the C6/36 cell line (a gift from the Armed Forces Research Institute of Medical Sciences, Thailand). Human monocytic cell lines U937 and HL60 (ATCC) were maintained in RPMI 1640 (Invitrogen) supplemented with 2 mM glutamine, 0.1 mg/ml streptomycin, 100 U/ml penicillin, and 10% heat-inactivated fetal calf serum (Gibco). Primary human MDMϕs were generated from human monocytes isolated from buffy coats (NHS Blood and Transplant, surplus to clinical requirements) as described previously (27) and incubated with 25 ng/ml recombinant human interleukin-4 (IL-4) (Peprotech) for 3 days prior to DENV infection to enhance their susceptibility to DENV infection. The use of human blood was approved by the NHS National Research Ethics Service (09/H0606/3).
Iminosugar derivatives.
The iminosugar compounds tested in this study include NB-DNJ (solubilized in phosphate-buffered saline [PBS]), NN-DNJ (solubilized in 83% dimethyl sulfoxide [DMSO]), N-(9-methoxynonyl) DNJ (MON-DNJ), and N-(6′-[4″-azido-2″-nitrophenylamino]hexyl)-1-DNJ (NAP-DNJ) (34) (solubilized in DMSO) (all gifts from United Therapeutics, Inc.).
Liposomes.
PERLs are composed of 1,2-dioleoyl-sn-glycerol-3-phosphoethanoloamine-N-[methoxy(polyethylene glycol)-2000] (ammonium salt), 1,2-didocosahexaenoyl-sn-glycero-3-phosphocholine, l-α-phosphatidylinositol, and l-α-phosphatidylserine, with a molar ratio of 1.5:1.5:1:1. All lipids were purchased from Avanti Polar Lipids. Fresh liposomes were prepared at 10 mM and diluted to 4 mM in PBS for injection for each experiment as described previously (30), and the distribution of liposome diameter (100 to 120 nm) was measured by dynamic light scattering. Inclusion of PEGylated phosphoethanolamine increases liposome circulation times in vivo (20). Calculation of the doses of iminosugar received in mice treated with 5 mM NB-DNJ PERLs was based on (i) an NB-DNJ encapsulation efficiency of 4.19% (95% confidence interval of ±1.49, n = 19) as established by measuring encapsulation of [14C]NB-DNJ (data not shown) and (ii) factoring in average lipid volume changes during purification of 1.31% (95% confidence interval of ±2.11, n = 72) as established using the Stewart assay for phospholipids (42; data not shown). The estimated dose was calculated using the average values above, while the “worst-case scenario” dose (with regard to fold enhancement calculations, i.e., if more drug was encapsulated and more lipid administered) was calculated using the average + 95% confidence intervals.
Cytotoxicity assay.
Cell viability was measured using the Promega CellTitre 96 Aqueous One solution cell proliferation MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay according to the manufacturer's instructions. Cell viability was calculated as a percentage of the untreated cells. The values representing the concentration of iminosugar causing a 50% reduction in cell viability as a percentage of control, untreated cells (CC50 values) were calculated using the line of best fit on graphs of drug concentration versus percentage of cell viability.
Inhibition of DENV infection of human macrophages.
MDMϕs were infected with DENV2 strain 16681 at a multiplicity of infection of 1 for 90 min, and then virus containing supernatant was replaced with X-VIVO medium (Cambrex) supplemented with 1% autologous human plasma and serial dilutions of iminosugar. Cells were incubated for 2 days, and then the cell culture supernatant was removed, centrifuged for 5 min at 400 × g to pellet any cells, and stored at −80°C. Cells were fixed with 4% paraformaldehyde. Infectious viral titers were obtained by viral plaque assay (27) on LLC-MK2 monkey kidney cells (limit of plaque detection of 33 PFU/ml), and neither the presence of NB-DNJ nor that of liposomes in cell supernatants affected DENV titers (see Table S1 in the supplemental material). The percentage of infected cells was measured by immunofluorescence (27) using an anti-DENV2 E protein monoclonal antibody, with the average percentage of cells infected being 20.9% ± 13.5% (n = 22). IC50s, averaged for multiple donors, were expressed as means ± standard deviations (SDs). Selectivity indices (SIs) were calculated as follows: SI1 = CC50/IC50 and the more stringent SI2 = CC10/IC90.
In vivo ADE mouse model of DENV infection.
All experimental procedures were preapproved by the UC Berkeley Animal Care and Use Committee and were performed according to the guidelines of the UC Berkeley Animal Care and Use Committee and of the United State Public Health Service and the USDA Animal Welfare Act. AG129 mice (lacking alpha/beta and gamma interferon receptors) were primed with an enhancing concentration of anti-DENV monoclonal antibody prior to infection with DENV in an in vivo model of ADE as described previously (3). Specifically, mice were injected intraperitoneally with 2 μg of 4G2, a pan-flavivirus monoclonal antibody against E protein, 20 to 24 h prior to intravenous infection with 105 PFU DENV D2S10. Beginning on the same day as infection, mice were treated intraperitoneally with 100 μl PBS (no-drug controls) (twice a day), 100 μl 100 mg/ml NB-DNJ (twice a day), 100 μl 4 mM PERLs (once a day), 100 μl 4 mM PERLs encapsulating 5 mM NB-DNJ (once a day) (calculated to contain an equivalent daily dose of NB-DNJ as 200 μl 8.8-μg/ml NB-DNJ), 100 μl 8.8-μg/ml NB-DNJ (twice a day), or 100 μl 25-mg/ml NB-DNJ (twice a day). The group of mice administered free NB-DNJ received injections twice daily to increase the stability of drug levels due to efficient clearance times in vivo (half-life of 0.68 h in mice following intravenous injection [P. Laing, unpublished data]). For survival experiments, treatment continued up to 7 days, and mice were monitored four times daily for signs of morbidity and were euthanized if moribund, as approved through the animal protocol. Mice surviving at day 7 were monitored regularly for an additional 38 days (total of 45 days postinfection). Twelve of 28 animals (43%) showed no signs of disease or illness at day 45. Sixteen of 28 mice developed paralysis between days 11 and 43 postinfection and were euthanized. For viral load experiments, mice were euthanized at day 3.5, and virus was quantified in the liver, small intestine, and spleen by plaque assay and in serum by quantitative reverse transcription-PCR (qRT-PCR) (3). Viremia is expressed as PFU equivalents/ml, which was calculated by dividing the genomic RNA copy number in each sample by the genome/PFU ratio of C6/36-derived virus as determined by plaque assay and qRT-PCR. No mouse lost more than 18% of its starting body weight, including those euthanized when moribund.
Statistical analysis.
Data from in vitro assays were analyzed using KaleidaGraph 4.0.3 (Synergy Software). Briefly, several logarithmic sigmoidal curve types were fitted to each data set with weighting applied based on precision of measurements (coefficient of variance). The curve providing the best description of the data (i.e., lowest χ2) was used to calculate relevant values (IC50, IC90, etc.). Error bars represent standard deviations (SDs) unless otherwise noted. For a detailed description of methods used to analyze in vitro data, see the experimental procedures in the supplemental material. The Mantel-Cox log rank test was used to analyze the animal survival study.
RESULTS
NB-DNJ has low cytotoxicity for human cells.
For improved drug selectivity, DNJ has been derivatized (particularly by addition of variable-length N-linked alkyl chains), leading to changes in bioavailability, pharmacokinetics, and toxicity. We initially assessed the in vitro cytotoxicity of NB-DNJ, NN-DNJ, MON-DNJ, and NAP-DNJ in primary human macrophages by determining the concentration of iminosugar causing a 50% reduction in cell viability in comparison to control, untreated cells (CC50) (Table 1). Notably, no effect on cell viability was observed for NB-DNJ concentrations up to 31.6 mM for 5 donors tested. Further, NB-DNJ in human monocytic cell lines (U937 and HL60) and primary human monocytes displayed no detectable cytotoxicity following 2 days of treatment at drug concentrations up to 5 mM (see Table S2 in the supplemental material).
Table 1.
Anti-DENV activity and cytotoxicity of iminosugars in primary human macrophagesa
| Drug | CC, μM (n)b |
IC, μM (n)c |
SId |
|||
|---|---|---|---|---|---|---|
| 50% | 10% | 50% | 90% | 1 | 2 | |
| NB-DNJ | 24,903 ± 10,506e (3) | 837 ± 428 | 6.00 ± 7.31 (5) | 62.1 ± 60.7 (4) | 4,151 | 13.5 |
| NN-DNJ | 317 (1) | 42 | 0.91 ± 0.40 (4) | 8.02 ± 4.14 (3) | 348 | 5.2 |
| MON-DNJ | 3,150 ± 1,211 (7) | 837 ± 178 (4) | 3.09 ± 3.93 (5) | 7.74 ± 3.63 (5) | 1,019 | 108.1 |
| NAP-DNJ | 300 ± 123 (2) | 10 | 0.04 ± 0.01 (3) | 0.28 ± 0.14 (3) | 7,500 | 35.7 |
Throughout, n represents the number of monocyte donors tested.
50% and 10%, CC50 and CC10, respectively. Human monocyte-derived macrophages from multiple donors were incubated for 2 days with serial dilutions of iminosugars, alongside controls containing equivalent medium and DMSO concentrations, as appropriate. Cell viability was measured as described in Materials and Methods and calculated as a percentage of that of the untreated cells. CC50 values were calculated using the line of best fit on graphs of drug concentration versus the percentage of cell viability as a percentage of the control. Means and standard deviations are shown.
50% and 90%, IC50 and IC90, respectively. Data were calculated for inhibition of release of infectious virus.
Selectivity indices (SIs) were calculated as follows: SI1 = CC50/IC50, and the more stringent SI2 = CC10/IC90.
Cells from 5/8 donors showed no toxicity of NB-DNJ when tested at up to 31.6 mM and are not included in the calculation of this value.
Iminosugars inhibit multiple aspects of DENV infection of human macrophages.
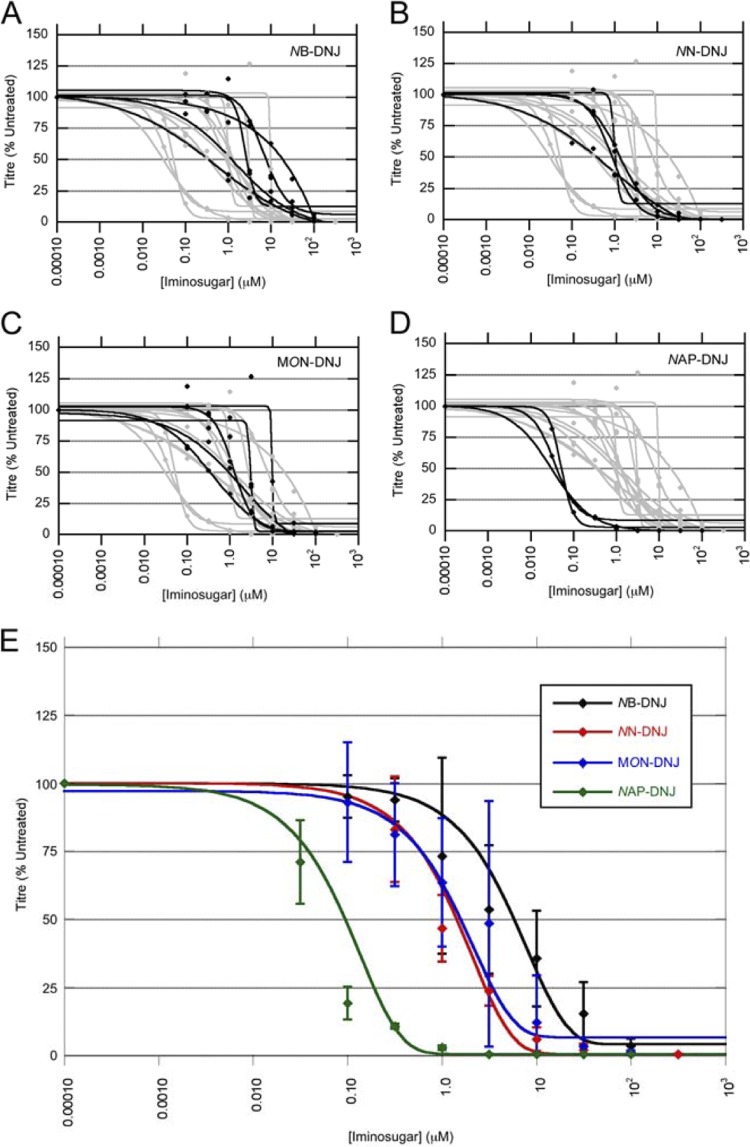
We next tested the ability of NB-DNJ, NN-DNJ, MON-DNJ, and NAP-DNJ to inhibit the release of infectious DENV from primary human macrophages as a measure of antiviral activity. Untreated cells released 103 to 105 PFU/ml (data not shown). The antiviral effect was normalized to untreated cells, and curves of best fit (for a full description of the methodology, see the experimental procedures in the supplemental material) are shown for results from cells of individual donors (Fig. 1). All iminosugars tested reduced both the release of infectious virus from the infected macrophages (Fig. 1) and the percentage of cells infected (Fig. 2). Curves for individual donors demonstrate the variation between donors in potency of antiviral activity of the iminosugars, particularly for NB-DNJ (Fig. 1A to D). The concentration of drug required to inhibit the titer of virus released to 50% of that of the untreated control (IC50) varied for the iminosugars tested, ranging from 6.0 μM for NB-DNJ to 0.04 μM for NAP-DNJ (Table 1).
Fig 1.
Iminosugars inhibit release of infectious virus from DENV-infected macrophages. Primary human macrophages were infected with DENV and then incubated with medium containing NB-DNJ (A), NN-DNJ (B), MON-DNJ (C), or NAP-DNJ (D) for 2 days, with each drug concentration measured in triplicate. (A to D) Release of infectious virus from DENV-infected macrophages was measured by plaquing supernatants of cells treated with a drug. Plaques were counted and averaged for each drug concentration, and the percentage of infectious virus released was calculated as a percentage of the PFU/ml calculated from untreated cells. Curves of best fit are shown for cells of individual donors (black), with inhibition curves for other iminosugars shown in gray for comparison. (E) Iminosugar inhibition of release of infectious virus, with curves representing averages for multiple donors. Each line represents the results from cells of multiple donors. For detailed descriptions of how curves were derived, see the experimental procedures in the supplemental material. Error bars represent the SDs.
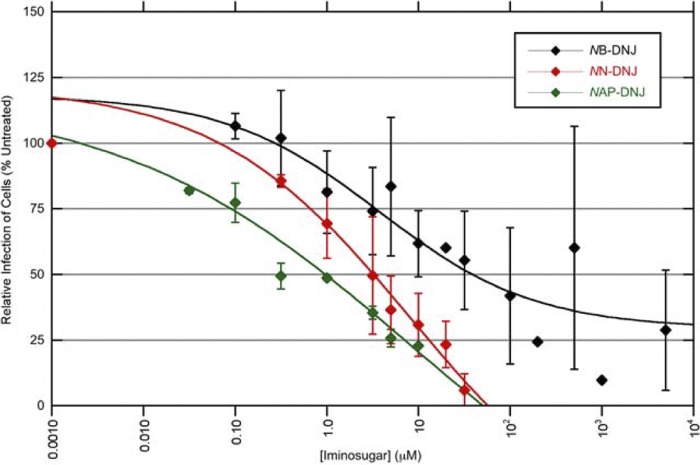
Fig 2.
Iminosugars inhibit infection of DENV-infected macrophages. Primary human macrophages were infected with DENV and then incubated with medium containing NB-DNJ, NN-DNJ, or NAP-DNJ for 2 days, with each drug concentration measured in triplicate. Each line represents average immunofluorescent results from cells of multiple donors. For detailed descriptions of how curves were derived, see the experimental procedures in the supplemental material. Error bars represent the SDs.
PERLs demonstrate low cytotoxicity for human monocytic cell lines and primary human macrophages.
ER-targeting liposomes deliver NB-DNJ intracellularly, which leads to enhanced antiviral activity against HIV (29). To assess the possibility of using polyunsaturated ER-targeting liposomes (PERLs) to enhance the anti-DENV activity of iminosugars, we first investigated their cytotoxicity for uninfected cells. PERLs encapsulating either PBS or 5 mM NB-DNJ showed equivalent cytotoxicity at day 2 for primary human macrophages (CC50 values around 500 μM) (see Table S1 in the supplemental material), similar to results with Huh-7.5 cells (S. Pollock, unpublished data). At concentrations up to 100 μM, PBS-containing PERLs enhanced primary human macrophage viability to more than 200% of that of untreated controls (data not shown), possibly by acting as a source of lipid. This enhancing effect was not observed with primary human monocytes, although cytotoxicity remained low (see Table S1). The inclusion of 10 to 50% autologous human plasma in the cytotoxicity assay further reduced PERL cytotoxicity 2- to 4-fold (data not shown).
While NB-DNJ-containing PERLs are antiviral in vitro (antiviral effect observed in cells from 5 out of 5 human donors), tests using cells from 10 donors showed that PERLs alone are only antiviral against dengue virus in cells from half the donors. The interesting donor-linked factors influencing dengue virus susceptibility to inhibition by PERLs are now under investigation in our lab using human macrophages. Testing of the antiviral effects of PERLs ± NB-DNJ in vitro never resulted in enhanced infectious virus release (data not shown).
PERLs enhance the antiviral activity of NB-DNJ in vivo.
A recently developed ADE mouse model of dengue disease (3, 47) was used to compare the abilities of free NB-DNJ and PERL-encapsulated NB-DNJ to protect mice from a lethal DENV infection using daily treatment. The maximum PERL dose tested in toxicity studies caused no adverse effects or toxicity in mice (U. Ramstedt, unpublished data), and hence this dose (100 μl of 4 mM PERLS) was used in our studies. All mice treated with either PBS or PBS-containing PERLs died between days 4 and 5 (Fig. 3). Free NB-DNJ administered at 1,000 mg/kg of body weight/day significantly (P < 0.0001, Mantel-Cox log rank test) enhanced survival (Fig. 3) and reduced morbidity (data not shown). When the dose of free NB-DNJ administered was reduced 4-fold (250 mg/kg body weight/day), only 20% of mice survived (P = 0.043 compared to PBS-treated mice, Mantel-Cox log rank test). Mice treated with NB-DNJ-containing PERLs (labeled as NB-DNJ PERLs) also showed significantly enhanced survival (P = 0.007, Mantel-Cox log rank test) in comparison with PBS-treated mice.
Fig 3.
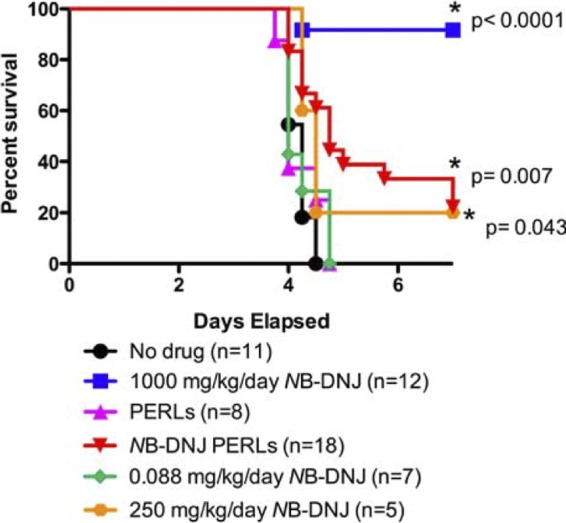
PERLs enhance the antiviral effects of NB-DNJ in vivo. Mice were administered 2 μg anti-DENV MAb (4G2) intraperitoneally 24 h prior to infection. On day 0, mice were challenged intravenously with 105 PFU of DENV2 strain D2S10, followed by daily treatment with PBS (no drug), 1,000 mg/kg/day NB-DNJ, PERLs, or NB-DNJ PERLs or 0.088 mg/kg/day NB-DNJ and were monitored for survival. Kaplan-Meier survival curves and the numbers of mice per group are shown.
We could not directly address what dose of PERL-encapsulated NB-DNJ would give equivalent protection to 1,000 mg/kg/day free NB-DNJ, as the required dose of PERLs would exceed the maximum doses tested and shown to have no toxicity (U. Ramstedt, unpublished data), so alternately we treated mice with an equivalent dose of NB-DNJ to that encapsulated in the PERLs. Initially assuming an encapsulation efficiency of 4% (based on measuring entrapment of self-quenching calcein [S. Pollock, unpublished data]), we conservatively calculated that daily treatment of mice with NB-DNJ PERLs (100 μl of 4 mM PERLs encapsulating 5 mM NB-DNJ) was equivalent to 0.088 mg/kg/day NB-DNJ. Subsequently, we measured the encapsulation efficiency of NB-DNJ itself using [14C]NB-DNJ. The measured value of 4.19% encapsulation efficiency was close to that of calcein and allowed us to calculate that mice treated daily with NB-DNJ PERLs received an equivalent dose of 0.094 mg/kg/day NB-DNJ (see Materials and Methods). Mice treated with a dose of free NB-DNJ (0.088 mg/kg/day) closely equivalent to that administered encapsulated in PERLs (0.094 mg/kg/day) did not show any enhanced survival in comparison with those in either the PBS- or empty-PERL-treated groups. These results indicate that when delivered via PERLs, 2,660-fold less NB-DNJ (250 mg/kg/day versus 0.094 mg/kg/day) provided a survival benefit against a lethal challenge dose of DENV in mice. Using a more conservative estimate, where the 95% confidence intervals for average percentages of encapsulation and losses/gains in lipid concentration are taken into account, PERL encapsulation provided a >1,900-fold reduction in the dose of drug able to enhance animal survival.
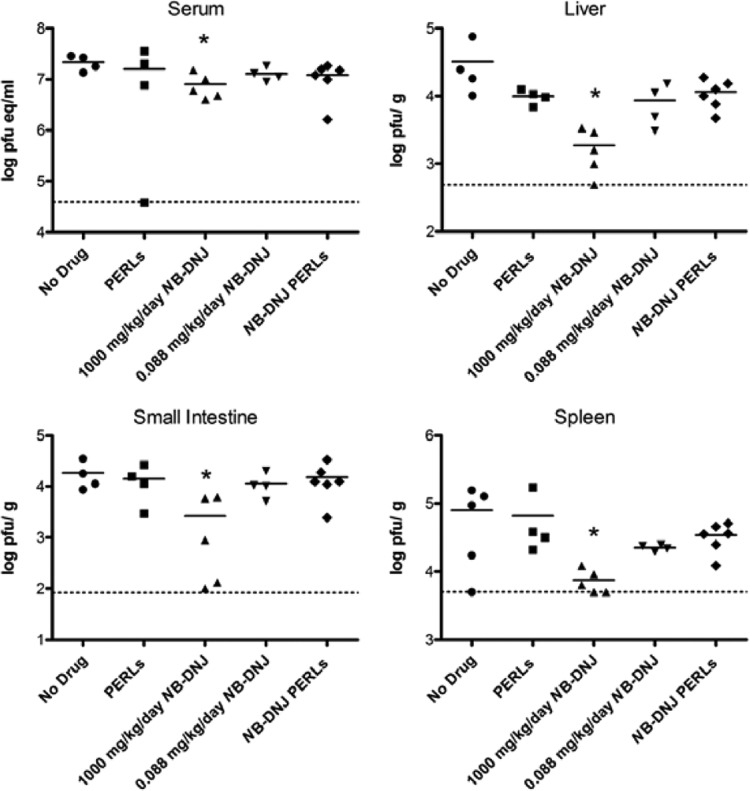
As an additional measure of antiviral effect, we compared the viral load in the same treatment groups as assessed above by euthanizing the mice at day 3.5 postinfection, just prior to commencement of severe illness (except for the 250-mg/kg/day treatment, for which these data were not available). Serum and tissues from various organs were assessed for viral load by plaque assay or qRT-PCR. As shown in Fig. 4, treatment with 1,000 mg/kg/day NB-DNJ resulted in a significant decrease in the viral load in liver, small intestine, sera, and spleen. However, treatment with NB-DNJ PERLs resulted in a small (not significant) decrease in viral load (in liver and spleen) in comparison with PBS-treated mice (Fig. 4), despite improved survival (Fig. 3). Treatment of the mice with 0.088 mg/kg/day NB-DNJ or administration of empty PERLs also resulted in a slight (although not significant) decrease in virus titers in liver and spleen, in comparison with those from PBS-treated (“no-drug”) mice.
Fig 4.
Effect of PERLs and NB-DNJ on viral load. Mouse treatment groups were as described in the legend to Fig. 3. Viral burden was measured in the indicated tissues at day 3.5 postinfection by qRT-PCR (serum) or plaque assay (all other tissues) as described by Balsitis et al. (2). Symbols indicate values in individual mice. The limit of detection is represented by dashed lines. eq, equivalents. Pairwise comparisons were performed with Mann-Whitney two-tailed P values, and asterisks show groups in which the average viral titer is significantly (P ≤ 0.05) different from that of the no-drug-treatment group.
DISCUSSION
This study advances the field on a number of fronts. This is the first description of PERL-mediated delivery of iminosugars in vivo, demonstrating that this system results in a >3-log10 reduction in the dose of drug sufficient to enhance animal survival. Furthermore, the lowering of the effective antiviral dose achieved by PERL-mediated drug delivery provides a potentially effective mechanism to reduce cytotoxicity of drugs with greater in vitro potency against DENV than those tested in this study. The use of a mouse ADE model of DENV infection to test antivirals extends previous data (36, 38, 45) to demonstrate an anti-DENV effect of NB-DNJ in vivo. Although previous studies evaluated DNJ (45) and NN-DNJ (38), we demonstrate here for the first time the antiviral activity of NB-DNJ against DENV in cell lines, primary cells, and an in vivo model.
NB-DNJ has high in vitro selectivity (SI = 4,151). This impressive SI is largely due to the low cytotoxicity observed in this assay—a result consistent with the current safe use of NB-DNJ in humans. Other DNJ derivatives with more potent antiviral activity than NB-DNJ (shown in studies by ourselves and others) (7, 9, 36) generally show greater toxic effects on cells in vitro. In support of this trend, the cytotoxicity of the four iminosugars we tested (NB-DNJ, NN-DNJ, N9-MON-DNJ, and NAP-DNJ) was correlated with antiviral activity. However, due to the acute nature of DENV disease, short treatment periods (<5 days) are likely, and therefore, slightly higher host-associated toxicity of the more potently antiviral DNJ derivatives (if it occurs) may be more acceptable than in the context of chronic diseases, such as those caused by hepatitis C virus (HCV) or HIV/AIDS, for example, where much longer treatment periods are necessary. A shorter treatment period also reduces the probability of the development of DNJ-resistant mutants.
A >2-log10-fold reduction of infectious DENV released from primary human macrophages was achieved by free NB-DNJ, NN-DNJ, MON-DNJ, and NAP-DNJ. The 1- to 2-log range in inhibition curves for individual donors indicates biological variation in humans (e.g., variation in expression or function of DENV receptors, ER α-glucosidases, and/or molecules involved in antiviral defense, etc.). Because severe dengue disease in humans has been associated with 10- to 100-fold-higher viremia than dengue fever (25, 44), reduction of viral replication may be instrumental to limit the risk of developing severe disease. Supporting this hypothesis, mice treated with 1000 mg/kg/day NB-DNJ showed 92% survival in the mouse model and had significantly decreased viral loads in the liver, small intestine, serum, and spleen. In contrast, mice administered NB-DNJ PERLs, which also exhibited increased survival, showed a maximally 3-fold drop in viral titer in the organs at day 3.5 following treatment. Similar reductions in virus levels were seen in the mice treated with 0.088 mg/kg/day NB-DNJ, which was not protective here. Thus, the enhanced survival of mice treated with NB-DNJ PERLs in comparison with untreated mice and mice treated with 0.088 mg/kg/day NB-DNJ suggests that the PERLs are delivering the drug in such a way as to enhance its antiviral potential, although the mechanism remains unclear. This may be via improved retention in cells, more efficient delivery to the ER, and/or increased in vivo circulation times (due to PEGylation of liposomes), which will be studied in the future. No in vivo toxicity or adverse effects were seen at the highest liposome doses tested (U. Ramstedt, unpublished data)—the same doses as those used in the present study. In ongoing work, we are quantifying the effects of higher doses of liposomes in mice in order to determine the dose of PERL-encapsulated NB-DNJ that provides 90% survival in the DENV ADE mouse model. This will further our understanding of the potential for liposomes to enhance drug delivery in vivo.
In humans, however, the immunopathogenic reaction, rather than the viral load directly (15), is hypothesized to be responsible for the clinical manifestations of severe dengue. Thus, differential triggering of the inflammatory cascade (in particular tumor necrosis factor alpha [TNF-α] in the ADE mouse model) may explain increased survival in NB-DNJ PERL-treated mice. Of note, the kinetics of the entire infection and disease period is compacted in the mice, in comparison to humans; thus, it is possible that if viral load were measured earlier, then correlations between viral load and survival among all groups may have been apparent. Alternatively, our data could be explained by an indirect mechanism of action of iminosugars on factors that could influence disease outcome, other than viral load or TNF-α. This intriguing possibility is under investigation in our group.
NB-DNJ has high oral bioavailability (10), is already clinically licensed, and has been used to treat Gaucher's disease patients for over 10 years without any serious adverse effects reported other than those listed in the original labeling. It was also approved for treatment in Niemann-Pick disease in 2009. Chang et al. observed 100% protection of DENV-infected AG129 mice, when treated orally with 150 mg/kg/day DNJ derivative iminosugar CM-10-18 (8). As iminosugars target host-based pathways, emergence of treatment-resistant viral strains is predicted to be delayed, if not abolished. These make iminosugars strong candidates for clinical development as anti-DENV drugs. A safe and efficacious anti-DENV drug could potentially be used to reduce progression to more severe dengue disease (dengue hemorrhagic fever [DHF] and dengue shock syndrome [DSS]) in patients living in regions of endemicity and presenting symptoms of DENV infection, as well as in hospitalized patients with laboratory-diagnosed DENV infection. Our and others' results demonstrate that iminosugars have considerable potential to meet these needs; however, as viral load generally peaks at or prior to hospitalization (18, 26, 43), a therapeutic drug targeted at reducing viral replication has only a short time (24 to 48 h) within which to act. Studies testing the window following exposure, during which iminosugars are successful as postexposure therapeutics, should be performed in the future. The infection and disease period in the ADE mouse model is shorter than that in humans; in light of the narrow window for effectiveness, the protection we observed from lethality is promising for its potential efficacy in humans.
Although much work will be necessary on the stability of the liposomes, as well as cargo loading and retention, these liposomes indicate that this area is worth pursuing. PERL-mediated delivery of iminosugars may significantly improve drug delivery (and therefore activity) while decreasing the necessary concentration (and therefore toxicity). For hepatitis B virus (HBV), HCV, and HIV-1, the cholesterol-lowering effects of PERLs in vitro provide antiviral activity (31). As recent reports have implicated cholesterol as an important molecule for DENV infection (6, 23, 32, 35), enhancement of iminosugar anti-DENV activity via PERL delivery may be a function of lowering cholesterol levels. Whereas NB-DNJ-containing PERLs administered to mice mediated enhancement of iminosugar efficacy, PBS-containing-PERLs themselves had no direct anti-DENV effects (apart from statistically insignificant lowering of viral load in tissues). Taken together, these results indicate that PERL-mediated enhancement of anti-DENV activity is likely an outcome of more complex processes than simply lowering cholesterol levels. To advance this work, we intend to perform further toxicity studies in mice to elucidate the dose of liposomes that manifests adverse effects in vivo. This will enable us to test higher liposome doses than those examined in the present study, to look more specifically at a correlation between PERL treatment and cholesterol lowering, and to assess the mechanism of antiviral enhancement.
The need for antiviral drugs effective against dengue virus is clear and ever increasing. Despite unanswered mechanistic questions, the in vivo mouse and in vitro human cell data presented here provide a strong indication that PERL-encapsulated iminosugars possess considerable potential as anti-DENV therapeutics. As such, this dual approach could be pursued for development of DENV antivirals for clinical use. Future studies on liposomes will aim to retain all of the necessary characteristics of liposomes tested here while incorporating both (i) alternate drug loading protocols to enhance loading capacity and (ii) lipid compositions with sufficient affordability of production, stability (shelf life and in vivo), and drug retention compatible with development for clinical use.
Supplementary Material
ACKNOWLEDGMENTS
We thank Urban Ramstedt, Brennan Klose, and Peter Laing at United Therapeutics, Inc., for compounds and access to unpublished data, Stephanie Pollock for advice, experimental assistance with liposomes and access to unpublished data, Emelie Wahlstedt for preliminary cytotoxicity data, Kate Williams for help with preliminary mouse experiments, Susana Orozco for assistance with figures, and Terry Butters for [14C]NB-DNJ.
A.C.S. was also funded by a Clarendon Fund Scholarship and a Santander Graduate Scholarship from Pembroke College, Oxford.
Footnotes
Published ahead of print 15 October 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Ardes-Guisot N, et al. 2011. Selection of the biological activity of DNJ neoglycoconjugates through click length variation of the side chain. Org. Biomol. Chem. 9:5373–5388 [DOI] [PubMed] [Google Scholar]
- 2. Balsitis SJ, et al. 2009. Tropism of dengue virus in mice and humans defined by viral nonstructural protein 3-specific immunostaining. Am. J. Trop. Med. Hyg. 80:416–424 [PubMed] [Google Scholar]
- 3. Balsitis SJ, et al. 2010. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 6:e1000790 doi:10.1371/journal.ppat.1000790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bosch I, et al. 2002. Increased production of interleukin-8 in primary human monocytes and in human epithelial and endothelial cell lines after dengue virus challenge. J. Virol. 76:5588–5597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carr JM, et al. 2003. Supernatants from dengue virus type-2 infected macrophages induce permeability changes in endothelial cell monolayers. J. Med. Virol. 69:521–528 [DOI] [PubMed] [Google Scholar]
- 6. Ceballos-Olvera I, Chavez-Salinas S, Medina F, Ludert JE, del Angel RM. 2010. JNK phosphorylation, induced during dengue virus infection, is important for viral infection and requires the presence of cholesterol. Virology 396:30–36 [DOI] [PubMed] [Google Scholar]
- 7. Chang J, et al. 2011. Combination of alpha-glucosidase inhibitor and ribavirin for the treatment of dengue virus infection in vitro and in vivo. Antiviral Res. 89:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang J, et al. 2011. Competitive inhibitor of cellular alpha-glucosidases protects mice from lethal dengue virus infection. Antiviral Res. 92:369–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang J, et al. 2009. Novel imino sugar derivatives demonstrate potent antiviral activity against flaviviruses. Antimicrob. Agents Chemother. 53:1501–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cook CS, Karabatsos PJ, Schoenhard GL, Karim A. 1995. Species dependent esterase activities for hydrolysis of an anti-HIV prodrug glycovir and bioavailability of active SC-48334. Pharm. Res. 12:1158–1164 [DOI] [PubMed] [Google Scholar]
- 11. Dejnirattisai W, et al. 2010. Cross-reacting antibodies enhance dengue virus infection in humans. Science 328:745–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Durbin AP, et al. 2008. Phenotyping of peripheral blood mononuclear cells during acute dengue illness demonstrates infection and increased activation of monocytes in severe cases compared to classic dengue fever. Virology 376:429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fink K, et al. 2009. Depletion of macrophages in mice results in higher dengue virus titers and highlights the role of macrophages for virus control. Eur. J. Immunol. 39:2809–2821 [DOI] [PubMed] [Google Scholar]
- 14. Fischl MA, et al. 1994. The safety and efficacy of combination N-butyl-deoxynojirimycin (SC-48334) and zidovudine in patients with HIV-1 infection and 200–500 CD4 cells/mm3. J. Acquir. Immune Defic. Syndr. 7:139–147 [PubMed] [Google Scholar]
- 15. Fox A, et al. 2011. Immunological and viral determinants of dengue severity in hospitalized adults in Ha Noi, Viet Nam. PLoS Negl. Trop. Dis. 5:e967 doi:10.1371/journal.pntd.0000967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gu B, et al. 2007. Antiviral profiles of novel iminocyclitol compounds against bovine viral diarrhea virus, West Nile virus, dengue virus and hepatitis B virus. Antivir. Chem. Chemother. 18:49–59 [DOI] [PubMed] [Google Scholar]
- 17. Halstead SB. 1989. Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Rev. Infect. Dis. 11(Suppl 4):S830–S839 [DOI] [PubMed] [Google Scholar]
- 18. Halstead SB. 1980. Dengue haemorrhagic fever—a public health problem and a field for research. Bull. World Health Organ. 58:1–21 [PMC free article] [PubMed] [Google Scholar]
- 19. James S, Simmons CP, James AA. 2011. Ecology. Mosquito trials. Science 334:771–772 [DOI] [PubMed] [Google Scholar]
- 20. Klibanov AL, Maruyama K, Torchilin VP, Huang L. 1990. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 268:235–237 [DOI] [PubMed] [Google Scholar]
- 21. Kou Z, et al. 2008. Monocytes, but not T or B cells, are the principal target cells for dengue virus (DV) infection among human peripheral blood mononuclear cells. J. Med. Virol. 80:134–146 [DOI] [PubMed] [Google Scholar]
- 22. Kyle JL, Harris E. 2008. Global spread and persistence of dengue. Annu. Rev. Microbiol. 62:71–92 [DOI] [PubMed] [Google Scholar]
- 23. Lee CJ, Lin HR, Liao CL, Lin YL. 2008. Cholesterol effectively blocks entry of flavivirus. J. Virol. 82:6470–6480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liang PH, et al. 2006. Novel five-membered iminocyclitol derivatives as selective and potent glycosidase inhibitors: new structures for antivirals and osteoarthritis. Chembiochem 7:165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Libraty DH, et al. 2002. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J. Infect. Dis. 185:1213–1221 [DOI] [PubMed] [Google Scholar]
- 26. Libraty DH, et al. 2002. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 186:1165–1168 [DOI] [PubMed] [Google Scholar]
- 27. Miller JL, et al. 2008. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 4:e17 doi:10.1371/journal.ppat.0040017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peiris JS, Porterfield JS. 1979. Antibody-mediated enhancement of Flavivirus replication in macrophage-like cell lines. Nature 282:509–511 [DOI] [PubMed] [Google Scholar]
- 29. Pollock S, et al. 2010. Uptake and trafficking of liposomes to the endoplasmic reticulum. FASEB J. 24:1866–1878 [DOI] [PubMed] [Google Scholar]
- 30. Pollock S, Dwek RA, Burton DR, Zitzmann N. 2008. N-Butyldeoxynojirimycin is a broadly effective anti-HIV therapy significantly enhanced by targeted liposome delivery. AIDS 22:1961–1969 [DOI] [PubMed] [Google Scholar]
- 31. Pollock S, et al. 2010. Polyunsaturated liposomes are antiviral against hepatitis B and C viruses and HIV by decreasing cholesterol levels in infected cells. Proc. Natl. Acad. Sci. U. S. A. 107:17176–17181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puerta-Guardo H, et al. 2010. Antibody-dependent enhancement of dengue virus infection in U937 cells requires cholesterol-rich membrane microdomains. J. Gen. Virol. 91:394–403 [DOI] [PubMed] [Google Scholar]
- 33. Qu X, et al. 2011. Inhibitors of endoplasmic reticulum alpha-glucosidases potently suppress hepatitis C virus virion assembly and release. Antimicrob. Agents Chemother. 55:1036–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rawlings AJ, et al. 2009. Synthesis and biological characterisation of novel N-alkyl-deoxynojirimycin alpha-glucosidase inhibitors. Chembiochem 10:1101–1105 [DOI] [PubMed] [Google Scholar]
- 35. Rothwell C, et al. 2009. Cholesterol biosynthesis modulation regulates dengue viral replication. Virology 389:8–19 [DOI] [PubMed] [Google Scholar]
- 36. Sayce AC, Miller JL, Zitzmann N. 2010. Targeting a host process as an antiviral approach against dengue virus. Trends Microbiol. 18:323–330 [DOI] [PubMed] [Google Scholar]
- 37. Schieffelin JS, et al. 2010. Neutralizing and non-neutralizing monoclonal antibodies against dengue virus E protein derived from a naturally infected patient. Virol. J. 7:28 doi:10.1186/1743-422X-7-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schul W, Liu W, Xu HY, Flamand M, Vasudevan SG. 2007. A dengue fever viremia model in mice shows reduction in viral replication and suppression of the inflammatory response after treatment with antiviral drugs. J. Infect. Dis. 195:665–674 [DOI] [PubMed] [Google Scholar]
- 39. Schwendener RA. 2007. Liposomes in biology and medicine. Adv. Exp. Med. Biol. 620:117–128 [DOI] [PubMed] [Google Scholar]
- 40. Shresta S, Sharar KL, Prigozhin DM, Beatty PR, Harris E. 2006. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J. Virol. 80:10208–10217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Standish K, Kuan G, Aviles W, Balmaseda A, Harris E. 2010. High dengue case capture rate in four years of a cohort study in Nicaragua compared to national surveillance data. PLoS Negl. Trop. Dis. 4:e633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stewart JC. 1980. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 104:10–14 [DOI] [PubMed] [Google Scholar]
- 43. Tricou V, Minh NN, Farrar J, Tran HT, Simmons CP. 2011. Kinetics of viremia and NS1 antigenemia are shaped by immune status and virus serotype in adults with dengue. PLoS Negl. Trop. Dis. 5:e1309 doi:10.1371/journal.pntd.0001309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vaughn DW, et al. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 181:2–9 [DOI] [PubMed] [Google Scholar]
- 45. Whitby K, et al. 2005. Castanospermine, a potent inhibitor of dengue virus infection in vitro and in vivo. J. Virol. 79:8698–8706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wichmann O, et al. 2011. Dengue in Thailand and Cambodia: an assessment of the degree of underrecognized disease burden based on reported cases. PLoS Negl. Trop. Dis. 5:e996 doi:10.1371/journal.pntd.0000996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zellweger RM, Prestwood TR, Shresta S. 2010. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe 7:128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.