Abstract
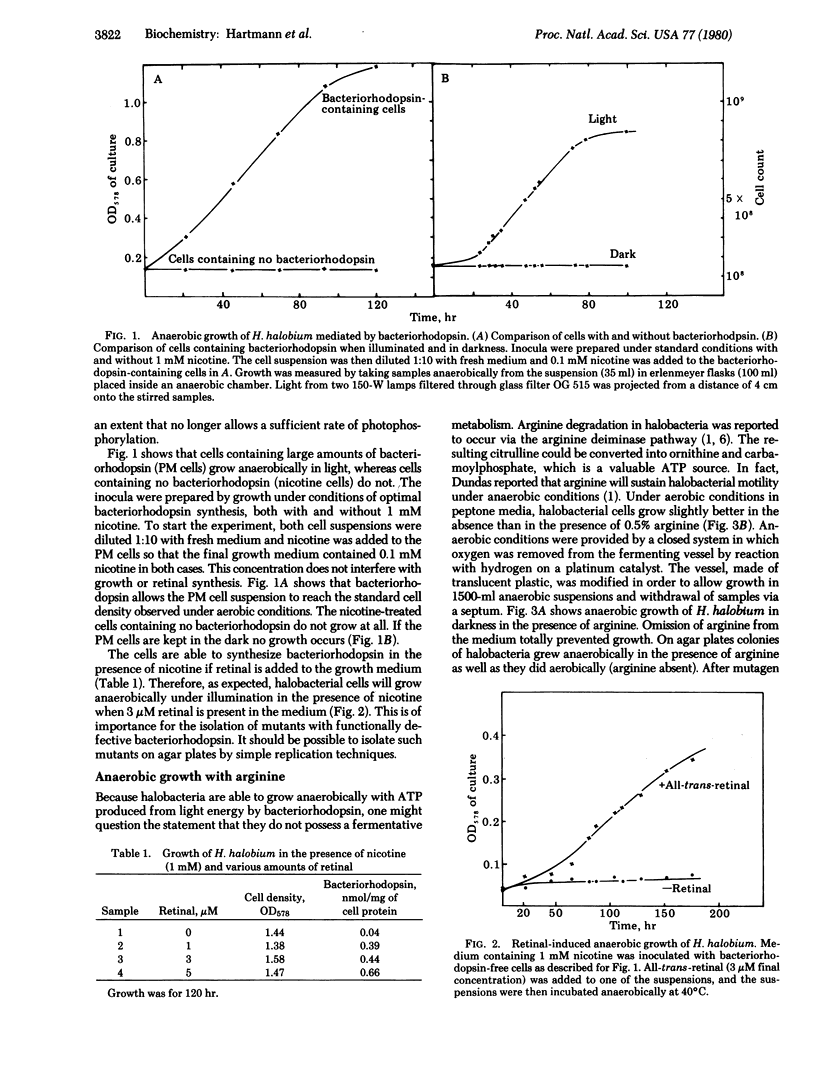
An energy-transducing pathway in halobacteria is described. Arginine mediates substrate level phosphorylation and allows the cells to grow anaerobically. Bacteriorhodopsin plus light can function as an alternative energy source under these conditions, provided the cells contain the pigment when transferred to the anaerobic environment. Therefore the selection of mutants functionally defective in ATP synthase or bacteriorhodopsin becomes possible.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Danon A., Stoeckenius W. Photophosphorylation in Halobacterium halobium. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1234–1238. doi: 10.1073/pnas.71.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundas I. E., Halvorson H. O. Arginine metabolism in Halobacterium salinarium, an obligately halophilic bacterium. J Bacteriol. 1966 Jan;91(1):113–119. doi: 10.1128/jb.91.1.113-119.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundas I. E. Ornithine carbamoyltransferase from Halobacterium salinarium. Eur J Biochem. 1972 May 23;27(2):376–380. doi: 10.1111/j.1432-1033.1972.tb01847.x. [DOI] [PubMed] [Google Scholar]
- Dundas I. E. Physiology of halobacteriaceae. Adv Microb Physiol. 1977;15:85–120. doi: 10.1016/s0065-2911(08)60315-x. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Oesterhelt D. Bacteriorhodopsin-mediated photophosphorylation in Halobacterium halobium. Eur J Biochem. 1977 Jul 15;77(2):325–335. doi: 10.1111/j.1432-1033.1977.tb11671.x. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Sickinger H. D., Oesterhelt D. Quantitative aspects of energy conversion in halobacteria. FEBS Lett. 1977 Oct 1;82(1):1–6. doi: 10.1016/0014-5793(77)80873-9. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Krippahl G. Light inhibition of respiration in Halobacterium halobium. FEBS Lett. 1973 Oct 1;36(1):72–76. doi: 10.1016/0014-5793(73)80339-4. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Sumper M., Reitmeier H., Oesterhelt D. Biosynthesis of the purple membrane of halobacteria. Angew Chem Int Ed Engl. 1976 Apr;15(4):187–194. doi: 10.1002/anie.197601871. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


