Abstract
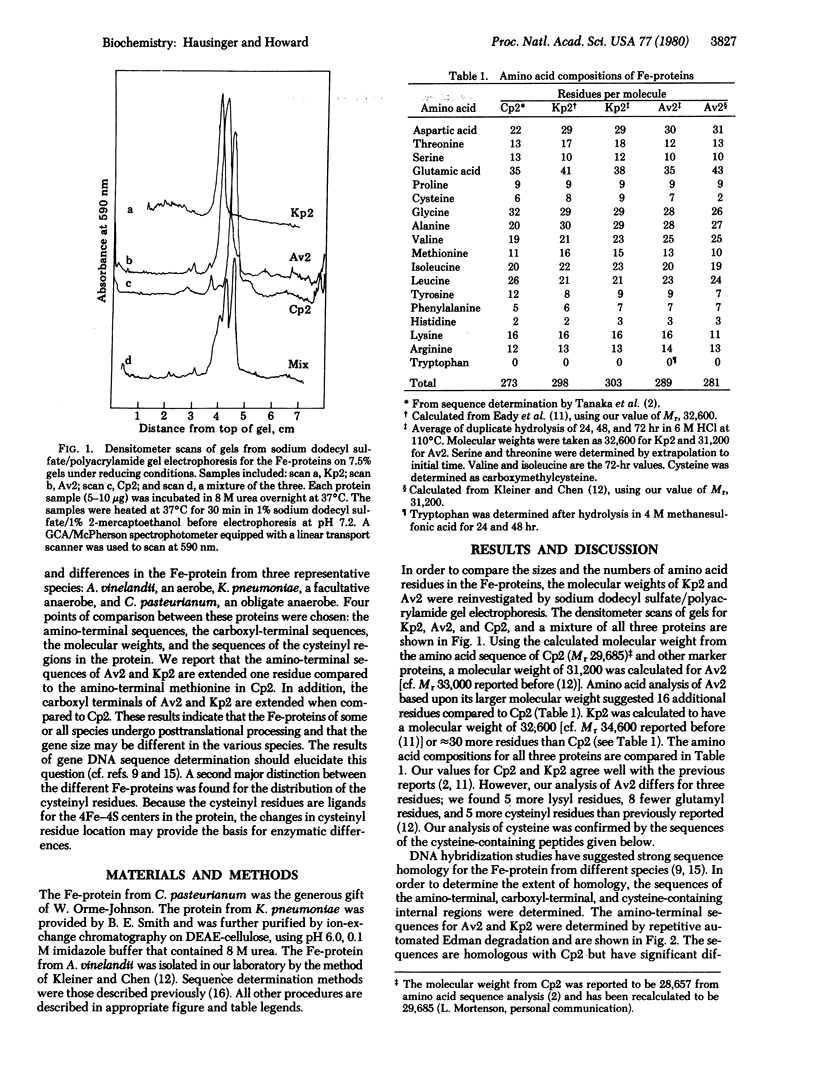
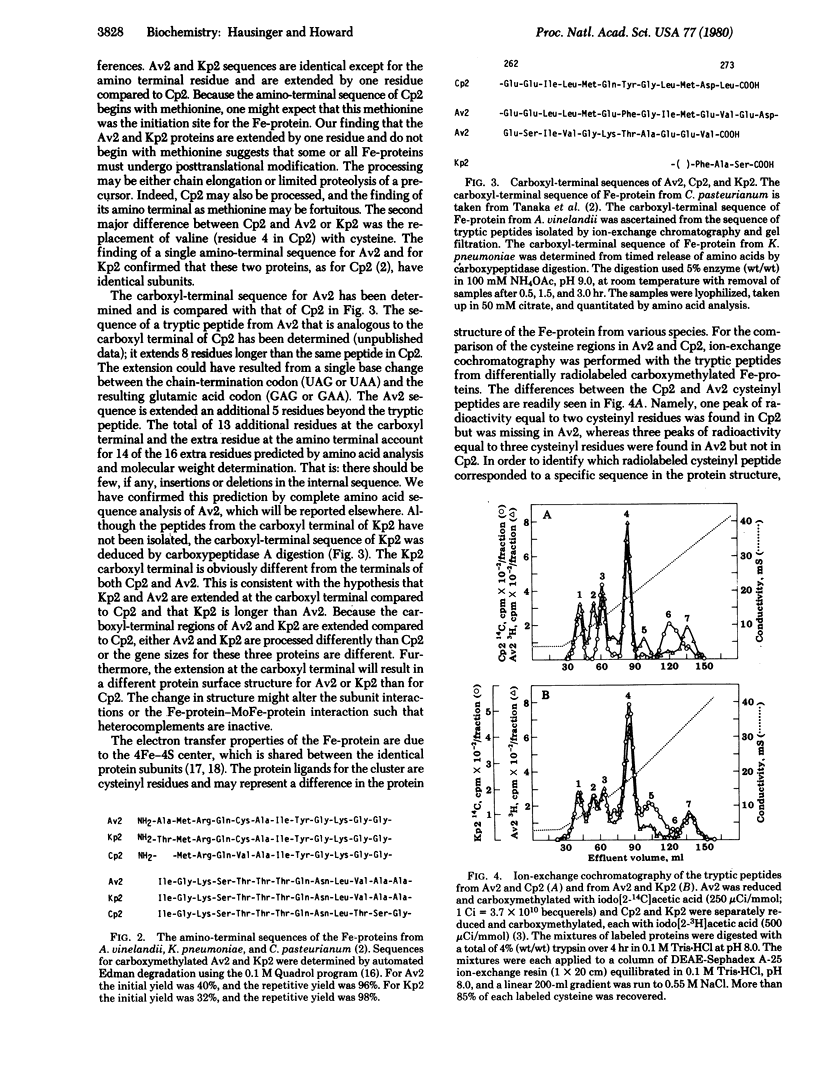
The molecular weights, amino acid compositions, amino- and carboxyl-terminal sequences, and ion-exchange peptide maps of the cysteine-containing tryptic peptides were determined for the iron proteins from the nitrogen fixation complexes of Azotobacter vinelandii (Av2) and Klebsiella pneumoniae (Kp2). Our results are compared to the known amino acid sequence of the iron protein from Clostridium pasteurianum (Cp2) [Tanaka, M., Haniu, M., Yasunobu, K. & Mortenson, L. E. (1977) J. Biol. Chem. 252, 7093-7100]. Previous studies have shown the iron proteins to have similar enzymatic functions and spectroscopic properties. Furthermore, the DNAs coding for the iron protein from many different species cross-hybridize [Ruvkun, G. B. & Ausubel, F. M. (1980) Proc. Natl. Acad. Sci. USA 77, 191-195]. Our results indicate that the protein structures are similar yet have significant differences. The amino-terminal sequences of Av2 and Kp2 are extended compared to the amino-terminal methionine of Cp2 and may indicate a different initiation site in these proteins. The aminoterminal sequences for Av2 and Kp2 are more homologous with each other than either of these are with Cp2. The carboxyl-terminal sequences are extended in Av2(14 residues) and Kp2 (≈30 residues) compared to Cp2. The amino- and carboxyl-terminal sequences establish that either the structural gene sizes are different in the three organisms or extensive posttranslational modification must occur in some species. Because cysteinyl residues are involved at the active site of the iron protein, a sensitive peptide mapping technique was used to compare cysteinyl peptides of the iron protein from the three species. Av2 and Kp2 have a redistribution of cysteinyl residues when compared to Cp2. Three important differences in the cysteine distributions were found, namely, residue 4 is valine and residue 148 is alanine in Cp2, but cysteinyl residues occupy these positions in Av2, whereas residue 231 is cysteine in Cp2 but alanine in Av2. The peptide mapping technique provides a method for the investigation of selective chemical modification of cysteinyl residues.
Keywords: iron-sulfur proteins, cysteinyl peptides, nitrogenase
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eady R. R., Smith B. E., Cook K. A., Postgate J. R. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem J. 1972 Jul;128(3):655–675. doi: 10.1042/bj1280655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich D. W., Burris R. H. Complementary functioning of the component proteins of nitrogenase from several bacteria. J Bacteriol. 1978 Jun;134(3):936–943. doi: 10.1128/jb.134.3.936-943.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum W. O., Mortenson L. E., Chen J. S., Holm R. H. Quantitative extrusions of the Fe4S4 cores of the active sites of ferredoxins and the hydrogenase of Clostridium pasteurianum. J Am Chem Soc. 1977 Jan 19;99(2):584–595. doi: 10.1021/ja00444a044. [DOI] [PubMed] [Google Scholar]
- Kleiner D., Chen C. H. Physical and chemical properties of the nitrogenase proteins form Azotobacter vinelandii. Arch Mikrobiol. 1974 Jun 7;98(1):93–100. doi: 10.1007/BF00425272. [DOI] [PubMed] [Google Scholar]
- Kohlmiller N. A., Howard J. B. The primary structure of the alpha subunit of protocatechuate 3,4-dioxygenase. I. Isolation and sequence of the tryptic peptides. J Biol Chem. 1979 Aug 10;254(15):7302–7308. [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Purification and properties of nitrogenase from Rhodospirillum rubrum, and evidence for phosphate, ribose and an adenine-like unit covalently bound to the iron protein. Biochem J. 1978 Oct 1;175(1):251–259. doi: 10.1042/bj1750251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundell D. J., Howard J. B. Isolation and partial characterization of two different subunits from the molybdenum-iron protein of Azotobacter vinelandii nitrogenase. J Biol Chem. 1978 May 25;253(10):3422–3426. [PubMed] [Google Scholar]
- Mazur B. J., Rice D., Haselkorn R. Identification of blue-green algal nitrogen fixation genes by using heterologous DNA hybridization probes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):186–190. doi: 10.1073/pnas.77.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortenson L. E., Thorneley R. N. Structure and function of nitrogenase. Annu Rev Biochem. 1979;48:387–418. doi: 10.1146/annurev.bi.48.070179.002131. [DOI] [PubMed] [Google Scholar]
- Roberts G. P., MacNeil T., MacNeil D., Brill W. J. Regulation and characterization of protein products coded by the nif (nitrogen fixation) genes of Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):267–279. doi: 10.1128/jb.136.1.267-279.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. Interspecies homology of nitrogenase genes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):191–195. doi: 10.1073/pnas.77.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. M., Dayhoff M. O. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. Science. 1978 Jan 27;199(4327):395–403. doi: 10.1126/science.202030. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Yasunobu K. T. The amino acid sequence of Clostridium pasteurianum iron protein, a component of nitrogenase. I. Tryptic peptides. J Biol Chem. 1977 Oct 25;252(20):7081–7088. [PubMed] [Google Scholar]
- Thorneley R. N., Eady R. R. Nitrogenase of Klebsiella pneumoniae: evidence for an adenosine triphosphate-induced association of the iron-sulphur protein. Biochem J. 1973 Jun;133(2):405–408. doi: 10.1042/bj1330405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. A., Mortenson L. E. Effect of magnesium adenosine 5'-triphosphate on the accessibility of the iron of clostridial azoferredoxin, a component of nitrogenase. Biochemistry. 1974 May 21;13(11):2382–2388. doi: 10.1021/bi00708a023. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Mortenson L. E. The nitrogen-fixing complex of bacteria. Biochim Biophys Acta. 1975 Mar 31;416(1):1–52. doi: 10.1016/0304-4173(75)90012-9. [DOI] [PubMed] [Google Scholar]



