Abstract
Recent reports have revealed the existence of widespread extensively drug-resistant (XDR) P. aeruginosa high-risk clones in health care settings, but there is still scarce information on their specific chromosomal (mutational) and acquired resistance mechanisms. Up to 20 (10.5%) of 190 bloodstream isolates collected from 10 Spanish hospitals met the XDR criteria. A representative number (15 per group) of isolates classified as multidrug-resistant (MDR) (22.6%), resistant to 1 to 2 classes (moderately resistant [modR]) (23.7%), or susceptible to all antibiotics (multiS) (43.2%) were investigated in parallel. Multilocus sequence typing (MLST) analysis revealed that all XDR isolates belonged to sequence type 175 (ST175) (n = 19) or ST111 (n = 1), both recognized as international high-risk clones. Clonal diversity was higher among the 15 MDR isolates (4 ST175, 2 ST111, and 8 additional STs) and especially high among the 15 modR (13 different STs) and multiS (14 STs) isolates. The XDR/MDR pattern in ST111 isolates correlated with the production of VIM-2, but none of the ST175 isolates produced acquired β-lactamases. In contrast, the analysis of resistance markers in 12 representative isolates (from 7 hospitals) of ST175 revealed that the XDR pattern was driven by the combination of AmpC hyperproduction, OprD inactivation (Q142X), 3 mutations conferring high-level fluoroquinolone resistance (GyrA T83I and D87N and ParC S87W), a G195E mutation in MexZ (involved in MexXY-OprM overexpression), and the production of a class 1 integron harboring the aadB gene (gentamicin and tobramycin resistance). Of particular interest, in nearly all the ST175 isolates, AmpC hyperproduction was driven by a novel AmpR-activating mutation (G154R), as demonstrated by complementation studies using an ampR mutant of PAO1. This work is the first to describe the specific resistance markers of widespread P. aeruginosa XDR high-risk clones producing invasive infections.
INTRODUCTION
The increasing prevalence of nosocomial infections produced by multidrug-resistant (MDR) or extensively drug-resistant (XDR) Pseudomonas aeruginosa strains severely compromises the selection of appropriate treatments and is therefore associated with significant morbidity and mortality (29, 36, 44). This growing threat results from the extraordinary capacity of this pathogen for developing resistance to nearly all available antibiotics by the selection of mutations in chromosomal genes and from the increasing prevalence of transferable resistance determinants, particularly those encoding class B carbapenemases (metallo-β-lactamases [MBLs]) or extended-spectrum β-lactamases (ESBLs), frequently cotransferred with genes encoding aminoglycoside-modifying enzymes (31, 32, 45).
Among the mutation-mediated resistance mechanisms, particularly noteworthy are those leading to the repression or inactivation of the carbapenem porin OprD, the hyperproduction of the chromosomal cephalosporinase AmpC, or the upregulation of one of the several efflux pumps encoded in the P. aeruginosa genome (20, 38, 47, 48, 49). Furthermore, the accumulation of several of these chromosomal mutations can lead to the emergence of XDR/MDR strains which may eventually be responsible for notable outbreaks in the hospital setting (12, 54). Likewise, multiple reports over the last decade have warned of the epidemic dissemination of XDR/MDR strains producing acquired resistance mechanisms (particularly integrons bearing MBL and aminoglycoside-modifying-enzyme genes) in multiple hospitals (42, 43, 46, 57, 59, 62).
Even more worrisome are recent reports which have provided evidence of the existence of MDR/XDR clones disseminated in several hospitals worldwide that have been denominated high-risk clones (60). Among them, ST235, ST111, and ST175 are likely those more widespread (9, 13, 14, 15, 30, 34, 51, 58). Nevertheless, there is still scarce information on the chromosomal (mutational) and acquired resistance mechanisms specific to these high-risk clones. Much less information is available on whether these resistance markers are conserved across the different lineages of a given high-risk clone detected in different hospitals (indicating interhospital dissemination of MDR/XDR high-risk clones) or whether we are, in contrast, facing an independent parallel evolution of XDR/MDR profiles in frequent (originally susceptible) high-risk clones in different settings. In order to gain insights into these major epidemiological and clinical questions, we performed a detailed genetic analysis of the epidemiology and resistance markers of XDR/MDR P. aeruginosa clones causing bloodstream infections in 10 different Spanish hospitals.
MATERIALS AND METHODS
Bacterial strains, susceptibility testing, and definition of resistance profiles.
A total of 190 P. aeruginosa isolates recovered from bloodstream infections in a 2008-2009 multicenter study (10 hospitals from different geographic locations) in Spain were studied. The MICs of ceftazidime, cefepime, piperacillin, piperacillin plus tazobactam (fixed concentration of 4 μg/ml), aztreonam, imipenem, meropenem, ciprofloxacin, gentamicin, tobramycin, amikacin, and colistin were determined by broth microdilution following CLSI guidelines and breakpoints (10) in a previous work (7). The expression of ampC and efflux pump-encoding genes (mexB, mexD, mexF, and mexY), as well as the presence of acquired β-lactamases, was also determined in the preceding study. Recent consensus recommendations (35) were used to define MDR (nonsusceptible to ≥3 classes of drugs) and XDR (nonsusceptible to all but 1 or 2 classes of drugs) strains, considering the following 7 antimicrobial classes: cephalosporins (ceftazidime or cefepime), penicillin-β-lactamase inhibitor combinations (piperacillin-tazobactam), monobactams (aztreonam), carbapenems (imipenem, or meropenem), fluoroquinolones (ciprofloxacin), aminoglycosides (gentamicin, tobramycin, or amikacin), and polymyxins (colistin). Strains susceptible to all tested antipseudomonal agents were included in the multisusceptible (multiS) category, and those nonsusceptible to at least one agent in 1 or 2 classes were included in the moderately resistant (modR) category. All XDR isolates (n = 20) and a representative number of isolates showing the MDR, modR, and multiS profiles (15 isolates per group) were studied in detail in this work.
Molecular typing.
Clonal relatedness was evaluated by pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST), each performed blindly in independent laboratories. For PFGE, bacterial DNA embedded in agarose plugs prepared as described previously (26) was digested with SpeI. DNA separation was performed in a contour-clamped homogeneous-electric-field DRIII apparatus (Bio-Rad, La Jolla, CA) under the following conditions: 6 V/cm2 for 26 h with pulse times of 5 to 40 s. DNA macrorestriction patterns were interpreted according to the criteria established by Tenover et al. (55). For MLST analysis, previously described schemes and protocols (11) and available databases and tools (http://pubmlst.org/paeruginosa) (22) were used.
Amplification and sequencing of resistance determinants.
All primers used for amplification of resistance genes are listed in Table 1. ampC, ampR, ampD, and dacB (PBP4) genes were PCR amplified and sequenced, following previously described protocols (38), in strains presenting ampC overexpression. Likewise, genes involved in the regulation of MexAB-OprM (nalB, nalC, and nalD) or MexXY-OprM (mexZ and PA5471) were amplified and sequenced as previously described in the strains that overexpressed these efflux pumps (18, 33, 52, 53). Additionally, the quinolone resistance-determining regions (QRDR) of gyrA, gyrB, parC, and parE were sequenced in ciprofloxacin-nonsusceptible strains (41), while oprD (17) was sequenced in those strains nonsusceptible to imipenem (partial oprD sequence data were obtained from previous work [7, 40]). Finally, the presence of class 1 integrons and the associated resistance determinants was also evaluated by PCR amplification followed by sequencing using previously described protocols (17). After duplicate PCR amplification, sequencing reactions were performed with the BigDye Terminator kit (PE Applied Biosystems, Foster City, CA), and sequences were analyzed on an ABI Prism 3100 DNA sequencer (PE Applied Biosystems). The resulting sequences were then compared with that of wild-type PAO1 and those available at GenBank (www.ncbi.nih.gov/BLAST).
Table 1.
Primers used in this work for amplification or cloning of resistance determinants
| Primer | Sequence (5′→3′)a | PCR product size (bp) | Target | Reference or source |
|---|---|---|---|---|
| ampCF | GCGCGCAGGGCGTTCAG | 1,467 | ampC | 37 |
| ampCR2 | CGGAGGGGCGGGGAAGC | This work | ||
| ampDF | GTACGCCTGCTGGACGATG | 910 | ampD | 24 |
| ampDR | GAGGGCAGATCCTCGACCAG | |||
| ampRF | GTCGACCCAGTGCCTTCAGG | 1,391 | ampR | 24 |
| ampRR | CTCGAGAGCGAGATCGTTGC | |||
| AmpRCLF-ERI | TCGAATTCGTCGACCAGTGCCTTCAGG | 1,391 | ampR | This work |
| AmpRCLR-BHI | TCGGATCCCTCGAGAGCGAGATCGTTGC | |||
| dacBF | CGACCATTCGGCGATATGAC | 1,721 | dacB | 38 |
| dacBR | CGCGTAATCCGAAGATCCATC | |||
| oprDF | CGCCGACAAGAAGAACTAGC | 1,412 | oprD | 17 |
| oprDR | GTCGATTACAGGATCGACAG | |||
| gyrA1 | TTATGCCATGAGCGAGCTGGGCAACGACT | 364 | gyrA | 41 |
| gyrA2 | AACCGTTGACCAGCAGGTTGGGAATCTT | |||
| gyrB3 | AGCTCGCAGACCAAGGACAAG | 600 | gyrB | 41 |
| gyrB4 | GGGCTGGGCGATGTAGATGTA | |||
| parC1 | ATGAGCGAACTGGGGCTGGAT | 208 | parC | 41 |
| parC2 | ATGGCGGCGAAGGACTTGGGA | |||
| parE1 | CGGCGTTCGTCTCGGGCGTGGTGAAGGA | 592 | parE | 41 |
| parE2 | TCGAGGGCGTAGTAGATGTCCTTGCCGA | |||
| INT-Ri | CGCAGTGGCGGTTTTCAT | Variable | Class 1 Integrons | 17 |
| qacE-R | CAAGAAAAAGCCAGCCTTTC | |||
| mexRINT | GGATGATGCCGTTCACCTG | 1,016 | nalB | 18 |
| mexR20 | CCAGTAAGCGGATAC | |||
| nalC1 | TCAACCCTAACGAGAAACGCT | 814 | nalC | 33 |
| nalC2 | TCCACCTCACCGAACTGC | |||
| nalD1 | GCGGCTAAAATCGGTACACT | 789 | nalD | 53 |
| nalD2 | ACGTCCAGGTGGATCTTGG | |||
| mexZF | ATTGGATGTGCATGGGTG | 1,000 | mexZ | 52 |
| mexZR | TGGAGATCGAAGGCAGC | |||
| PA5471F | GATCTACCGTTTCAATCACATGGAT | 1,299 | PA5471 | This work |
| PA5471R | GGCCACCTCCTCGATTACCT |
Sites for restriction endonucleases are underlined.
Cloning of ampR genes and complementation studies.
ampR genes from wild-type strain PAO1 and from ST175 XDR clinical isolate PAmb12 (G154R AmpR mutant) were PCR amplified using primers listed in Table 1. PCR products were digested with EcoRI and BamHI and ligated to pUCP24 to obtain pUCPARWT and pUCPARG154R plasmids that were transformed into E. coli XL1-Blue made competent by CaCl2. Transformants were selected in gentamicin (5 μg/ml)–MacConkey agar plates. The cloned genes obtained from three independent experiments were fully sequenced to ascertain the absence of mutations introduced during PCR amplification. Plasmids pUCPARWT and pUCPARG154R were then electroporated into a previously constructed (38) ampR knockout mutant of PAO1 (PAΔR). Transformants were selected on gentamicin (50 μg/ml)–Luria-Bertani (LB) agar plates. The effect of the G154R AmpR mutation in β-lactam resistance and ampC expression and induction was evaluated in transformants and control strains through (i) determination of ceftazidime and imipenem MICs in Mueller-Hinton (MH) agar using Etest strips, (ii) quantification of basal and induced (50 μg/ml cefoxitin) ampC expression through real-time RT-PCR following previously described protocols (24), and (iii) phenotypic determination of AmpC inducibility through the assessment of the presence of antagonism between imipenem and ceftazidime disks (separated by 5 to 30 mm) on MH agar plates, as described previously (24).
RESULTS
Clonal diversity according to susceptibility profiles.
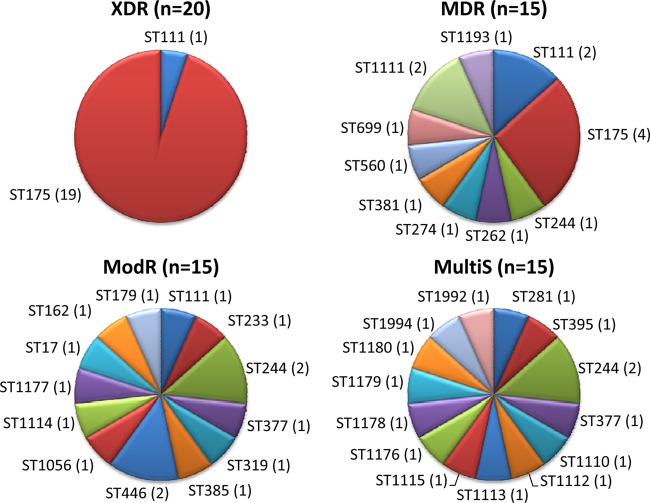
A total of 20 of the 190 isolates (10.5%) met the XDR criteria, whereas 22.6% were categorized as MDR (excluding XDR), 23.7% as modR (resistant to 1 or 2 antimicrobial classes), and 43.2% as multiS (susceptible to all tested antipseudomonal agents). Clonal relatedness was evaluated by MLST in all XDR isolates and in a representative number (15 per group) of MDR, modR, and multiS isolates. As shown in Fig. 1, all XDR isolates belonged to MLST sequence type 175 (ST175) (n = 19) or ST111 (n = 1), both previously identified as internationally spread high-risk clones. Notably, XDR ST175 isolates were detected in 7 of the 10 hospitals, with a wide geographical distribution covering all 4 regions participating in the study. On the other hand, clonal diversity was much higher among the 15 MDR isolates evaluated (4 ST175, 2 ST111, and 8 additional STs) and especially among the 15 modR (13 different STs) and 15 multiS (14 STs) isolates (Fig. 1). Thus, a total of 33 different STs were detected among the 65 isolates evaluated, 16 (49%) of which were contributed to the MLST database as new clones (http://pubmlst.org/paeruginosa). Besides high-risk-clones ST175 (19 XDR and 4 MDR isolates) and ST111 (1 XDR, 2 MDR, and 1 modR), only the clone ST244, not particularly associated with resistance, was detected in more than two isolates (1 MDR, 2 modR, and 2 multiS). Three additional STs were detected in two isolates, whereas the 27 remaining STs were detected in single isolates. Only 3 pairs of STs were identified as single-locus variants (SLVs) through BURST analysis, whereas all 27 other STs (including ST175 and ST111 high-risk clones) were classified as singletons. A similar diversity was observed through PFGE analysis and, notably, 21 of the 23 ST175 isolates were independently identified as the same clone by this approach (not shown).
Fig 1.
Distribution of STs among the XDR, MDR, modR, and multiS isolates studied. The number of isolates of each ST is shown in parentheses.
Genetic markers of widespread XDR/MDR high-risk clones.
The susceptibility profiles and resistance mechanisms of the XDR, MDR, and modR isolates studied are shown in Table 2. The presence of acquired β-lactamases was only detected in the 4 isolates belonging to the ST111 high-risk clone. Namely, the 3 XDR/MDR ST111 isolates produced a VIM-2 MBL and/or an OXA-46 oxacillinase located in different class 1 integrons, whereas the modR isolate produced the narrow-spectrum penicillinase PSE-1, also encoded in a class 1 integron. Interestingly, the 3 XDR/MDR ST111 isolates (recovered from 2 distantly located hospitals) additionally showed the same specific inactivating mutation in OprD (W339X), as well as two QRDR mutations (GyrA T83I and ParC S87L) conferring high-level fluoroquinolone resistance.
Table 2.
Susceptibility profiles and resistance mechanisms of the studied isolates
| Profile | PFGE | ST | IDa | MIC (μg/ml) and clinical category (CLSI breakpoint)b |
Resistance mechanism(s)c |
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATM | CAZ | FEP | PTZ | IMP | MER | GEN | TOB | AMK | CIP | COL | ampCd | oprD | gyrA | parC | Integrons | |||||||||||||||
| XDR | 3 | 111 | 238 | 64 | R | 128 | R | 128 | R | 32 | S | 64 | R | 64 | R | 64 | R | 64 | R | 128 | R | 32 | R | 2 | S | N | W339Xe | T83I | S87L | (1) aacA7-blaVIM-2-aac(6′)-II (2) aac(6′)-Ib-blaOXA46 |
| 1 | 175 | 12 | 16 | I | 16 | I | 16 | I | 32 | S | 16 | R | 16 | R | 64 | R | 16 | R | 4 | S | 32 | R | 0.5 | S | P | Q142Xf | T83I, D87N | S87W | ||
| 1 | 175 | 27 | 32 | R | 64 | R | 128 | R | 64 | S | 8 | I | 64 | R | 64 | R | 64 | R | 2 | S | 32 | R | 2 | S | P | Q142X | T83I, D87N | S87W | ||
| 2 | 175 | 43 | 16 | I | 64 | R | 32 | R | 128 | R | 32 | R | 16 | R | 64 | R | 64 | R | 8 | S | 32 | R | 1 | S | P | Q142X | T83I, D87N | S87W | ||
| 1 | 175 | 93 | 32 | R | 64 | R | 32 | R | 128 | R | 16 | R | 16 | R | 64 | R | 64 | R | 4 | S | 32 | R | 0.25 | S | P | Q142X | T83I, D87N | S87W | ||
| 1 | 175 | 123 | 32 | R | 32 | R | 32 | R | 128 | R | 16 | R | 32 | R | 64 | R | 32 | R | 4 | S | 32 | R | 0.12 | S | P | Q142X | T83I, D87N | S87W | ||
| 1 | 175 | 147 | 16 | I | 32 | R | 32 | R | 64 | S | 32 | R | 16 | R | 64 | R | 64 | R | 4 | S | 32 | R | 4 | I | P | Q142X | T83I, D87N | S87W | ||
| 1 | 175 | 179 | 16 | I | 32 | R | 32 | R | 128 | R | 32 | R | 16 | R | 64 | R | 32 | R | 4 | S | 32 | R | 1 | S | P | Q142X | T83I, D87N | S87W | ||
| 1 | 175 | 207 | 16 | I | 32 | R | 16 | I | 64 | S | 16 | R | 16 | R | 64 | R | 8 | I | 0.5 | S | 32 | R | 0.12 | S | P | Q142X | T83I, D87N | S87W | ||
| MDR | 3 | 111 | 19 | 4 | S | 64 | R | 16 | I | 32 | S | 64 | R | 64 | R | 64 | R | 16 | R | 8 | S | 16 | R | 0.5 | S | N | W339X | T83I | S87L | aacA7-blaVIM-2-aac(6′)-II |
| 3 | 111 | 81 | 8 | S | 4 | S | 8 | S | 8 | S | 64 | R | 16 | R | 64 | R | 64 | R | 16 | S | 32 | R | 1 | S | N | W339X | T83I | S87L | aac(6′)-Ib-blaOXA46 | |
| 1 | 175 | 67 | 8 | S | 8 | S | 8 | S | 16 | S | 8 | I | 4 | S | 64 | R | 16 | R | 8 | S | 32 | R | 0.25 | S | P | Q142X | T83I, D87N | S87W | ||
| 2 | 175 | 75 | 16 | I | 8 | S | 16 | I | 16 | S | 2 | S | 1 | S | 64 | R | 32 | R | 4 | S | 32 | R | 0.06 | S | N | WT | T83I, D87N | S87W | ||
| 1 | 175 | 245 | 8 | S | 8 | S | 4 | S | 64 | S | 32 | R | 8 | I | 32 | R | 2 | S | 0.25 | S | 32 | R | 0.125 | S | P | Q142X | T83I, D87N | S87W | ||
| 1 | 175 | 258 | 2 | S | 8 | S | 16 | I | 8 | S | 32 | R | 2 | S | 64 | R | 64 | R | 8 | S | 32 | R | 0.5 | S | P | Q142X | T83I, D87N | S87W | ||
| 13 | 244 | 205 | 16 | I | 8 | S | 16 | I | 16 | S | 2 | S | 8 | I | 4 | S | 1 | S | 4 | S | 32 | R | 0.5 | S | N | T83I | S87L | |||
| 14 | 262 | 60 | 32 | R | 8 | S | 32 | R | 32 | S | 64 | R | 64 | R | 4 | S | 1 | S | 16 | S | 1 | S | 1 | S | N | nt673Ins(T) | ||||
| 15 | 274 | 148 | 64 | R | 128 | R | 128 | R | 256 | R | 2 | S | 1 | S | 16 | R | 4 | S | 16 | S | 0.25 | S | 1 | S | P | |||||
| 20 | 381 | 187 | 16 | I | 128 | R | 64 | R | 32 | S | 1 | S | 0.25 | S | 2 | S | 0.5 | S | 2 | S | 32 | R | 0.03 | S | P | T83I, D87N | S87L | |||
| 25 | 560 | 89 | 16 | I | 4 | S | 8 | S | 16 | S | 8 | I | 0.25 | S | 2 | S | 1 | S | 16 | S | 0.5 | S | 1 | S | N | nt1113Ins(AT) | ||||
| 26 | 699 | 37 | 4 | S | 2 | S | 32 | R | 4 | S | 8 | I | 0.5 | S | 4 | S | 1 | S | 8 | S | 4 | R | 2 | S | N | WT | T83I | WT | ||
| 29 | 1111 | 59 | 16 | I | 64 | R | 32 | R | 128 | R | 32 | R | 8 | I | 4 | S | 0.5 | S | 4 | S | 0.12 | S | 0.5 | S | P | MPg | ||||
| 29 | 1111 | 62 | 16 | I | 16 | I | 16 | I | 64 | S | 16 | R | 32 | R | 2 | S | 0.25 | S | 2 | S | 8 | R | 1 | S | N | nt457Ins(A) | T83I | WT | ||
| 39 | 1193 | 20 | 128 | R | 128 | R | 64 | R | 256 | R | 2 | S | 2 | S | 1 | S | 1 | S | 8 | S | 0.06 | S | 0.5 | S | P | |||||
| ModR | 4 | 17 | 191 | 4 | S | 2 | S | 16 | I | 8 | S | 0.5 | S | 0.5 | S | 4 | S | 1 | S | 8 | S | 32 | R | 0.25 | S | N | T83I | S87L | ||
| 5 | 111 | 131 | 0,25 | S | 1 | S | 4 | S | 1 | S | 0.12 | S | 0.06 | S | 8 | I | 16 | R | 32 | I | 8 | R | 0.12 | S | N | T83I | S87W | aacA4-blaPSE-1-aadA2 | ||
| 6 | 162 | 17 | 8 | S | 4 | S | 4 | S | 8 | S | 32 | R | 8 | I | 1 | S | 0.25 | S | 2 | S | 0.12 | S | 0.25 | S | N | nt482Δ17 | ||||
| 7 | 179 | 54 | 4 | S | 2 | S | 16 | I | 8 | S | 0.5 | S | 0.25 | S | 1 | S | 1 | S | 4 | S | 0.12 | S | 2 | S | N | |||||
| 8 | 233 | 97 | 16 | I | 2 | S | 4 | S | 16 | S | 16 | R | 8 | I | 2 | S | 0.5 | S | 4 | S | 0.06 | S | 0.25 | S | N | nt1163Δ2 | ||||
| 10 | 244 | 16 | 2 | S | 2 | S | 4 | S | 8 | S | 2 | S | 4 | S | 0.25 | S | 0.12 | S | 2 | S | 32 | R | 0.25 | S | N | T83I | S87L | |||
| 12 | 244 | 125 | 16 | I | 4 | S | 8 | S | 16 | S | 1 | S | 2 | S | 1 | S | 0.5 | S | 2 | S | 0.5 | S | 1 | S | N | |||||
| 17 | 319 | 48 | 4 | S | 4 | S | 2 | S | 4 | S | 4 | S | 1 | S | 0.5 | S | 0.25 | S | 4 | S | 0.25 | S | 8 | I | N | |||||
| 18 | 377 | 25 | 8 | S | 4 | S | 4 | S | 16 | S | 32 | R | 8 | I | 1 | S | 1 | S | 2 | S | 0.5 | S | 4 | I | N | nt433Δ14 | ||||
| N/D | 385 | 181 | 4 | S | 4 | S | 16 | I | 4 | S | 2 | S | 0.5 | S | 2 | S | 0.5 | S | 4 | S | 0.12 | S | 0.25 | S | N | |||||
| 22 | 446 | 63 | 8 | S | 16 | I | 8 | S | 64 | S | 16 | R | 4 | S | 2 | S | 0.5 | S | 4 | S | 0.06 | S | 1 | S | P | MP | ||||
| 23 | 446 | 199 | 16 | I | 32 | R | 16 | I | 64 | S | 4 | S | 0.5 | S | 2 | S | 0.5 | S | 2 | S | 0.06 | S | 0.5 | S | P | |||||
| 27 | 1056 | 186 | 8 | S | 2 | S | 16 | I | 4 | S | 4 | S | 0.12 | S | 4 | S | 1 | S | 8 | S | 2 | I | 0,5 | S | N | T83I | WT | |||
| 31 | 1114 | 120 | 4 | S | 4 | S | 2 | S | 4 | S | 64 | R | 2 | S | 1 | S | 0.5 | S | 2 | S | 0.12 | S | 0.125 | S | P | MP | ||||
| 34 | 1177 | 132 | 32 | R | 32 | R | 16 | I | 32 | S | 0,5 | S | 0.5 | S | 2 | S | 0.5 | S | 4 | S | 0.25 | S | 0.125 | S | P | |||||
ID, strain identification number.
ATM, aztreonam; CAZ, ceftazidime; FEP, cefepime; PTZ, piperacillin-tazobactam; IMP, imipenem; MER, meropenem; GEN, gentamicin; TOB, tobramycin; AMK, amikacin; CIP, ciprofloxacin; COL, colistin.
Sequencing results are in comparison to those of reference wild-type strain PAO1 (www.pseudomonas.com). All isolates showed wild-type gyrB sequence, and a single isolate (ST381) presented a mutation in parE (E459K). WT, wild type; nt, nucleotide.
Strains were considered positive for ampC overexpression when the corresponding mRNA level was at least 10-fold higher than that of PAO1, negative if lower than 5-fold, and borderline if between 5- and 10-fold (7). P, positive; N, negative.
TGG→TAG (premature stop codon) (boldface indicates the mutated nucleotide).
CAA→TAA (premature stop codon) (boldface indicates the mutated nucleotide).
MP, multiple polymorphisms of unknown effect compared to PAO1 sequence.
On the other hand, all but 1 of the 23 (19 XDR and 4 MDR) high-risk-clone ST175 isolates had a similar resistance profile, which included cephalosporins, penicillin-β-lactamase inhibitor combinations, monobactams, carbapenems, fluoroquinolones, and aminoglycosides (gentamicin and tobramycin). Borderline susceptibility to cephalosporins, penicillin-β-lactamase inhibitor combinations, and/or monobactams determined that 3 of the ST175 isolates were classified as MDR instead of XDR. The discordant isolate was susceptible to all β-lactams but resistant to ciprofloxacin, gentamicin, and tobramycin. Moreover, in contrast to ST111 isolates, all but this single isolate were documented to overexpress the chromosomal cephalosporinase AmpC instead of producing acquired β-lactamases. Table 2 shows a detailed analysis of the resistance mechanisms detected in 12 representative (from 7 different hospitals) ST175 isolates, including 8 of the XDR isolates and the 4 MDR isolates. As can be observed, in addition to showing AmpC overexpression (see next section for detailed analysis of the genetic mechanisms), all but the single carbapenem-susceptible isolate presented the same specific inactivating mutation in OprD (Q142X), which was not detected in any of the other carbapenem-resistant clones (Table 2). Likewise, high-level ciprofloxacin resistance in ST175 isolates was driven in all cases by a specific combination of 3 QRDR mutations (GyrA T83I and D87N and ParC S87W) not detected in any of the other fluoroquinolone-resistant clones studied (Table 2). Finally, in all ST175 isolates, gentamicin and tobramycin resistance resulted from the production of a class 1 integron harboring aadB as a single gene cassette, coding for Ant(2″)-Ia.
The prevalence and genetic markers of efflux pump (MexAB and MexXY) overexpression in XDR/MDR high-risk clones was also investigated, and the results are shown in Table 3. None of the ST111 isolates overexpressed mexB or mexY. In contrast, 11 of the 23 (48%) XDR/MDR ST175 isolates overexpressed mexY. Nevertheless, despite the fact that over 50% of ST175 isolates did not exceed the previously defined mexY overexpression threshold of 10-fold (compared to its expression in wild-type PAO1) (7), all of them showed the same mexZ sequence, which specifically contained the G195E substitution, previously demonstrated to determine MexXY-OprM overexpression (19). Thus, despite a wide range (from <5- to 37-fold) of mexY expression levels being documented for our collection of 23 isolates, the mexZ G195E mutation is an additional conserved genetic marker of resistance in XDR/MDR ST175 isolates. In addition to the mexZ mutation, all ST175 isolates studied showed several substitutions in gene PA5471 (also involved in MexXY-OprM regulation) compared to its sequence in PAO1 (Table 3). However, all the substitutions detected were found to be evenly distributed among reference wild-type P. aeruginosa genomes (www.pseudomonas.com), suggesting that they are just polymorphisms not involved in MexXY-OprM overexpression. As shown in Table 3, mexY overexpression was also frequent in sporadic MDR/resistant clones but, in contrast to ST175, mostly resulted from partial deletions of the mexZ coding sequence.
Table 3.
Sequence analysis of genes involved in MexAB-OprM and MexXY-OprM regulation
| ST (resistance profile) | IDa | Expression levelb |
Presence of polymorphism(s) in sequencec |
|||||
|---|---|---|---|---|---|---|---|---|
| MexAB-OprM regulator |
MexXY-OprM regulator |
|||||||
| mexB | mexY | nalB | nalC | nalD | mexZ | PA5471 | ||
| 175 (XDR/MDR) | Severald | V | V | WT | (G71E), A186T | WT | G195E | (L88P), D161G, H182Q, (V243A), V266 M |
| 244 (MDR) | 205 | P | P | WT | WT | T11N | WT | (L88P), G157D, D161G, H182Q, (V243A) |
| 262 (MDR) | 60 | P | P | T69P | (G71E), (S209R) | WT | WT | (L88P), D161G, H182Q, (V243A) |
| 560 (MDR) | 89 | N | P | nt283Δ11 | (C40R), (L88P), (S112N), (D119E), (I237V), (V243A), P244L | |||
| 699 (MDR) | 37 | N | P | nt207Δ10 | WT | |||
| 1111 (MDR) | 62 | P | N | I68T, (V126E), V132A | (G71E), (S209R) | WT | ||
| 179 (modR) | 54 | N | P | nt630Δ21 | (L88P), D161G, H182Q, (V243A) | |||
| 244 (modR) | 125 | P | P | WT | WT | WT | nt292Δ10 | (L88P), G157D, D161G, H182Q, (V243A) |
| 377 (modR) | 25 | P | N | (V126E) | nt239Δ10 | WT | ||
| 1056 (modR) | 186 | P | P | WT | (G71E), (S209R) | WT | nt278Δ2 | (C40R), (L88P), (S112N), (D119E), (I237V), (V243A) |
ID, strain identification number.
Previously defined breakpoints were used (7). Strains were considered positive for mexY overexpression when the corresponding mRNA level was at least 10-fold higher than that of PAO1, negative if lower than 5-fold, and borderline if between 5- and 10-fold. Strains were considered positive for mexB overexpression when the corresponding mRNA level was at least 3-fold higher than that of PAO1, negative if lower than 2-fold, and borderline if between 2- and 3-fold. V, variable; P, positive; N, negative.
Sequencing results are in comparison (www.pseudomonas.com) to those of reference wild-type strain PAO1; polymorphisms present in reference wild-type strain PA14 are shown in parentheses. WT, wild type; nt, nucleotide.
Regulatory genes were sequenced in all ST175 isolates overexpressing mexB (n = 7) or mexY (n = 11), as well as 2 additional isolates not showing overexpression of either of the efflux pumps. The same sequences were documented in all cases, and therefore, they are shown only once.
A number of ST175 (7 of 23, 30.4%) isolates were also found to overexpress mexB (expression level ≥3-fold compared to that of wild-type PAO1) (7). Nevertheless, as occurred for mexY, despite a relatively wide range of mexB expression levels (<2- to 6.6-fold), all ST175 isolates studied showed the same sequence for the regulators nalB (mexR), nalC, and nalD; nalB and nalD sequences were identical to those of PAO1, whereas nalC showed in all cases two substitutions (G71E and A186T) (Table 3). The G71E substitution is a very frequent polymorphism among wild-type P. aeruginosa reference genomes, and it is therefore not thought to be involved in MexAB-OprM overexpression (33). On the other hand, the A186T substitution is not common among sequenced genomes but has been detected in a few clinical strains (8, 56). However, its role in MexAB-OprM overexpression, if any, still needs to be experimentally demonstrated.
Characterization of a novel AmpR-activating mutation driving AmpC hyperproduction in XDR/MDR P. aeruginosa ST175 high-risk clone.
In order to determine the genetic markers of AmpC hyperproduction in XDR/MDR ST175 isolates, ampC and the genes involved in its regulation (ampD, dacB [PBP4], and ampR) were sequenced in a representative number of isolates from this high-risk clone (8 of the XDR isolates and the 4 MDR isolates, including the single isolate not showing ampC overexpression), as well as in all other sporadic MDR/modR clones with documented AmpC hyperproduction (Table 4).
Table 4.
Sequencing of genes involved in AmpC hyperproduction
| Profile | ST | IDa | ampCb | Presence of polymorphism(s) in sequencec |
|||
|---|---|---|---|---|---|---|---|
| ampC | dacB | ampD | ampR | ||||
| XDR | 175 | 12 | P | WT | WT | G148A, D183Y | G154R |
| 175 | 27 | P | WT | WT | G148A, D183Y | G154R | |
| 175 | 43 | P | WT | WT | T139 M, G148A, D183Y | WT | |
| 175 | 93 | P | WT | WT | G148A, D183Y | G154R | |
| 175 | 123 | P | WT | WT | G148A, D183Y | G154R | |
| 175 | 147 | P | WT | P59S | G148A, D183Y | G154R | |
| 175 | 179 | P | WT | WT | G148A, D183Y | G154R | |
| 175 | 207 | P | WT | WT | G148A, D183Y | G154R | |
| MDR | 175 | 67 | P | WT | WT | G148A, D183Y | G154R |
| 175 | 75 | N | WT | WT | G148A, D183Y | WT | |
| 175 | 245 | P | WT | WT | G148A, D183Y | G154R | |
| 175 | 258 | P | WT | WT | G148A, D183Y | G154R | |
| 274 | 148 | P | (T105A), (G391A) | A394P | R11L, P41L, G148A | WT | |
| 381 | 187 | P | (T105A), G229S, G248S | WT | G148A, D183Y | (G283E), (M288R) | |
| 1111 | 59 | P | (T105A) | T239S | G156S | WT | |
| 1193 | 20 | P | (T105A), L176R, D233E | Deletion from nt 853 | A134V | WT | |
| ModR | 446 | 63 | P | (T105A), (V205L),(G391A) | WT | WT | (E114A), (G283E), (M288R) |
| 446 | 199 | P | (T105A), (V205L), (G391A) | WT | WT | (E114A), (G283E), (M288R) | |
| 1114 | 120 | P | (T105A), (V205L),V356I, (G391A) | Q33H, Q37H, L469 M | WT | A51T, (E114A) | |
| 1177 | 132 | P | (T105A), L176R | Q212Xd | G148, D183Y | WT | |
ID, strain identification number.
Strains were considered positive for ampC overexpression when the corresponding mRNA level was at least 10-fold higher than that of PAO1, negative if lower than 5-fold, and borderline if between 5 and 10-fold (7). P, positive; N, negative.
Sequencing results are in comparison (www.pseudomonas.com) to those of reference wild-type strain PAO1; polymorphisms present in reference wild-type strain PA14 are shown in parentheses. WT, wild type; nt, nucleotide.
CAG→TAG (premature stop codon) (boldface indicates the mutated nucleotide).
Regarding ampC, all ST175 isolates showed a wild-type sequence identical to that of PAO1. Curiously, all other clones showed several AmpC polymorphisms, including the T105A substitution, which has been correlated with more-efficient carbapenem and cefepime hydrolysis (50), although there is still controversy on the real contribution of these polymorphisms, found in wild-type reference strains such as PA14, to the resistance profiles (61).
The selection of AmpC-hyperproducing mutants during antimicrobial therapy is well known to be driven mainly by ampD and/or dacB mutations (23, 38, 39). This was evident in sporadic MDR/modR clones, frequently showing inactivating mutations in dacB (deletions or stop codons) or mutations in key conserved residues of ampD (P41L, G156S, or H157R) (16, 24). The DacB A394P substitution found in one of the isolates has also been previously detected in spontaneous in vitro mutants of PAO1 overexpressing ampC (39), but the effect of the other dacB substitutions, despite affecting conserved residues, still needs to be experimentally explored (Table 4).
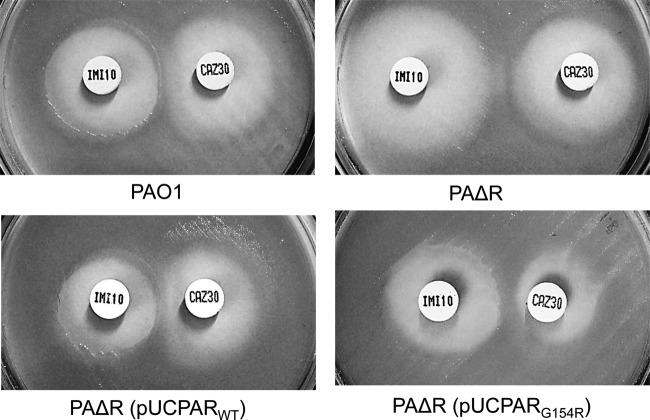
The analysis of ST175 high-risk-clone isolates, however, provided quite different and unexpected results (Table 4). All of the isolates (including the one negative for ampC overexpression) presented two polymorphisms (G148A and D183Y) in AmpD which are very frequent in wild-type strains and are therefore not involved in ampC overexpression, but only two of the 13 isolates studied that overexpressed ampC showed mutations in conserved residues of AmpD (T139M) or DacB (P59S). In contrast, all ST175 isolates, except for the one that did not overexpress ampC and the AmpD T139M mutant, showed a specific amino acid replacement (G154R) in a highly conserved residue of AmpR. Although other substitutions (A51T, E114A, G283E, or M288R) were detected in some sporadic MDR/modR clones, they were all common polymorphisms found in the genomes of wild-type reference strains, such as PA14 (www.pseudomonas.com). Indeed, specific amino acid substitutions, namely, R86C, G102E, and D135N, characterized in enterobacterial species such as Citrobacter freundii, are known to determine a conformational change in the LysR-type AmpR regulator, which alters its interaction with the DNA operator to convert the protein into a transcriptional activator (4, 28). Of them, only the D135N mutation has been detected so far in a single P. aeruginosa isolate (1). Thus, in order to explore the effect of the G154R substitution, wild-type and mutant ampR genes were cloned in parallel and transformed into the ampR knockout mutant of PAO1 (PAΔR). The documented effects of the G154R AmpR mutation in β-lactam resistance and ampC expression and induction are shown in Table 5 and Fig. 2 (double-disk AmpC induction test). As expected, the introduction of wild-type AmpR in PAΔR restored ampC inducibility and basal imipenem (potent AmpC inducer) susceptibility. On the other hand, the introduction of the G154R mutant not only restored inducibility but also determined a drastic increase in basal ampC expression and in the MIC of ceftazidime (weak AmpC inducer). Thus, our results clearly indicate that the G154R mutation converts AmpR into a transcriptional activator.
Table 5.
Effect of the G154R AmpR mutation in β-lactam resistance and ampC expression
| Strain | MIC (μg/ml)a |
Mean ampC expressionb ± SD |
||
|---|---|---|---|---|
| CAZ | IMP | Basal | Inducedc | |
| PAO1 | 1 | 1.5 | 1 | 52.1 ± 17.9 |
| PAΔR | 2 | 0.25 | 3.3 ± 1.7 | 3.2 ± 1.8 |
| PAΔR(pUCPΔRWT) | 1.5 | 2 | 3.0 ± 1.3 | 42.5 ± 19.2 |
| PAΔR(pUCPΔRG154R) | 8 | 2 | 74.7 ± 25.2 | 258.8 ± 37.8 |
CAZ, ceftazidime; IMP, imipenem.
Amount of ampC mRNA relative to PAO1 basal levels.
Induction experiments were carried out with 50 μg/ml of cefoxitin.
Fig 2.
Results for double-disk (imipenem-ceftazidime) AmpC induction test in wild-type PAO1, the ampR knockout mutant PAΔR, and PAΔR complemented with wild-type ampR (pUCPARWT) or with the G154R mutant (pUCPARG154R).
DISCUSSION
Over the last years, several reports have provided strong evidence for the existence of XDR/MDR P. aeruginosa high-risk clones disseminated in several hospitals worldwide. In this work, we report for the first time a detailed analysis of the genetic markers of antibiotic resistance in such lineages in comparison to those in sporadic clones. Among P. aeruginosa XDR/MDR high-risk clones, ST235, ST111, and ST175 are those likely to be more widespread (9, 13, 14, 15, 30, 34, 51, 58). Of them, ST235 isolates were not detected in our multicenter study of bloodstream infections, but other recent reports have detected this high-risk clone in Spain, linked either to a large outbreak of GES-5 class A carbapenemase-producing P. aeruginosa in a hospital in Madrid (58) or to the class B carbapenemase VIM-13, autochthonous from the Balearic Islands (25). A few of the XDR/MDR isolates from our study belonged to ST111, linked to the MBL VIM-2, but the vast majority (19 of 20) of XDR isolates belonged to ST175, detected in 7 of the 10 hospitals participating in the multicenter study. Likewise, other recent works have revealed that ST175 is also the high-risk clone more widespread in French hospitals (9).
Through a detailed analysis, we determined the genetic markers of antibiotic resistance of the highly disseminated ST175 clone. These markers included an inactivating mutation in OprD (Q142X) that determines carbapenem resistance, a mutation in AmpR (G154R) that drives AmpC hyperproduction (conferring resistance to penicillins, cephalosporins, and monobactams), 3 QRDR mutations leading to high-level fluoroquinolone resistance (GyrA T83I and D87N and ParC S87W), and the production of a class 1 integron harboring the aadB gene (gentamicin and tobramycin resistance). All ST175 isolates additionally showed several substitutions in genes involved in the regulation of efflux pumps. Of them, the G195E mutation in MexZ has been clearly demonstrated to be involved in the overexpression of the efflux pump MexXY-OprM (19), which further increases resistance to multiple antipseudomonal agents, including fluoroquinolones, aminoglycosides, and cefepime. Notably, this complex set of resistance markers was conserved in nearly all ST175 isolates studied, which were recovered from 7 different hospitals with a wide geographical distribution covering all 4 regions participating in the study. Nevertheless, we recently reported two large outbreaks of ST175 P. aeruginosa producing MBL VIM-2 (59) or VIM-20 (15, 48) in two hospitals in different Spanish cities. Thus, we explored whether the genetic markers were conserved among the ST175 strains from those outbreaks and found that the OprD and AmpR mutations were replaced by the corresponding MBL but that all of them contained the same set of 3 QRDR mutations, suggesting that fluoroquinolone resistance is at the bottom line of the evolution of the XDR/MDR ST175 high-risk clone. Indeed, this adds to growing and worrisome examples of strong linkage of fluoroquinolone resistance to XDR/MDR phenotypes, particularly noteworthy being the extended-spectrum β-lactamases in Enterobacteriaceae or methicillin resistance in Staphylococcus aureus. Nevertheless, it still needs to be experimentally addressed whether this link is favored by the mutagenic effects of fluoroquinolones (6) and/or genetic capitalism (5). Likewise, whether there are specific features of certain high-risk clones that promote the development of mutation-driven resistance and/or the acquisition of resistance determinants through horizontal gene transfer still needs to be explored.
Among the resistance mechanisms detected in the widespread ST175 clone, a specific mutation (G154R) in the transcriptional regulator AmpR is particularly noteworthy. AmpR is a LysR-type transcriptional regulator required for AmpC induction. Upon exposure to certain β-lactams, such as imipenem or cefoxitin (AmpC inducers), certain cell wall metabolites generated interact with AmpR, determining a conformational change which alters its interaction with the DNA operator to convert the protein from a repressor into a transcriptional activator of ampC expression (21). A similar effect (AmpR-mediated activation of ampC expression) is obtained through the mutational inactivation of several enzymes involved in peptidoglycan recycling, such as AmpD and/or DacB (PBP4). Indeed, the selection of AmpC-hyperproducing mutants, frequently leading to the failure of antimicrobial therapy with antipseudomonal penicillins or cephalosporins, is known to be driven mainly by mutations that inactivate AmpD and/or DacB, leading to the derepression of the chromosomal cephalosporinase (23, 38, 39). Consistent with this, the sporadic MDR/modR clones that hyperproduced AmpC frequently showed inactivating mutations in dacB or ampD. There is, however, a third possibility for the activation of ampC expression, the acquisition of specific mutations in AmpR that directly provoke the required conformational change. Indeed, specific amino acid substitutions, namely, R86C, G102E, and D135N, characterized in enterobacterial species such as C. freundii, are known to produce such an activating effect (4, 28). Here, we demonstrate that the G154R mutation in P. aeruginosa also activates AmpR, leading to a drastic increase in basal ampC expression and conferring resistance to the weak AmpC-inducer β-lactams, such as ceftazidime. The MIC of ceftazidime for PAO1 increased from 1 to 8 μg/ml when the G154R AmpR mutation was introduced, similar to the effect produced by the inactivation of AmpD (24). The MICs of ceftazidime for ST175 clinical isolates ranged from 8 to 64 μg/ml, perhaps suggesting the presence of additional factors, such as variations in the outer membrane permeability or the expression of penicillin-binding proteins, which modulate the level of resistance (39). Moreover, the implication of such AmpR-activating mutations could extend far beyond the overexpression of ampC and β-lactam resistance, since recent works demonstrate that AmpR is a global transcriptional regulator connected to quorum sensing, alginate production, biofilm formation, and the expression of several other virulence factors (2, 3, 27). Thus, ongoing research in our laboratory will explore whether the AmpR-activating mutations, such as G154R, could have an effect on the expression of these relevant pathogenicity traits, perhaps playing a role in the success of the widespread ST175 high-risk clone.
ACKNOWLEDGMENTS
We are grateful to all clinical microbiologists and infectious disease specialists involved in this project from the Hospital Universitario de Bellvitge (Barcelona), Consorci Sanitari Parc Tauli (Barcelona), Hospital Universitario Virgen de la Macarena (Sevilla), Hospital Vall de Hebrón (Barcelona), Hospital Universitari Son Espases (Palma de Mallorca), Hospital de Sant Pau (Barcelona), Hospital Universitario Marqués de Valdecilla (Santander), Hospital Virgen del Rocío (Sevilla), Hospital Reina Sofía (Córdoba), and Hospital Mutua de Tarrasa (Barcelona).
This work was supported by the Ministerio de Ciencia e Innovación of Spain and the Instituto de Salud Carlos III, through the Spanish Network for Research in Infectious Diseases (grants REIPI C03/14 and RD06/0008) and grants PI08/0276, PS09/00033, and PI12/00103.
Footnotes
Published ahead of print 8 October 2012
REFERENCES
- 1. Bagge N, et al. 2002. Constitutive high expression of chromosomal beta-lactamase in Pseudomonas aeruginosa caused by a new insertion sequence (IS1669) located in ampD. Antimicrob. Agents Chemother. 46:3406–3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balasubramanian D, et al. 2011. Co-regulation of b-lactam resistance, alginate production and quorum sensing in Pseudomonas aeruginosa. J. Med. Microbiol. 60:147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balasubramanian D, et al. 2012. The regulatory Repertoire of Pseudomonas aeruginosa AmpC β-lactamase regulator AmpR includes virulence genes. PLoS One 7:e34067 doi:10.1371/journal.pone.0034067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balcewich MD, et al. 2010. Crystal structure of the AmpR effector binding domain provides insight into the molecular regulation of inducible AmpC beta-lactamase. J. Mol. Biol. 400:998–1010 [DOI] [PubMed] [Google Scholar]
- 5. Baquero F. 2004. From pieces to patterns: evolutionary engineering in bacterial pathogens. Nat. Rev. Microbiol. 2:510–518 [DOI] [PubMed] [Google Scholar]
- 6. Blázquez J, Oliver A, Gómez-Gómez JM. 2002. Mutation and evolution of antibiotic resistance: antibiotics as promoters of antibiotic resistance? Curr. Drug Targets 3:345–349 [DOI] [PubMed] [Google Scholar]
- 7. Cabot G, et al. 2011. Overexpression of AmpC and efflux pumps in Pseudomonas aeruginosa isolates from bloodstream infections: prevalence and impact on resistance in a Spanish multicenter study. Antimicrob. Agents Chemother. 55:1906–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campo Esquisabel AB, Rodríguez MC, Ocampo-Sosa A, Rodríguez C, Martínez-Martínez L. 2011. Mechanisms of resistance in clinical isolates of Pseudomonas aeruginosa less susceptible to cefepime than to ceftazidime. Clin. Microbiol. Infect. 17:1817–1822 [DOI] [PubMed] [Google Scholar]
- 9. Cholley P, et al. 2011. Most multidrug-resistant Pseudomonas aeruginosa isolates from hospitals in eastern France belong to a few clonal types. J. Clin. Microbiol. 49:2578–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial susceptibility testing, vol 28, no 3, 18th informational supplement. CLSI document M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 11. Curran B, Jonas D, Grundmann H, Pitt T, Dowson CG. 2004. Development of a multilocus sequence typing scheme for the opportunistic pathogen Pseudomonas aeruginosa. J. Clin. Microbiol. 42:5644–5649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deplano A, et al. 2005. Molecular characterization of an epidemic clone of panantibiotic-resistant Pseudomonas aeruginosa. J. Clin. Microbiol. 43:1198–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edalucci E, et al. 2008. Acquisition of different carbapenem resistance mechanisms by an epidemic clonal lineage of Pseudomonas aeruginosa. Clin. Microbiol. Infect. 14:88–90 [DOI] [PubMed] [Google Scholar]
- 14. Empel J, et al. 2007. Outbreak of Pseudomonas aeruginosa infections with PER-1 extended-spectrum beta-lactamase in Warsaw, Poland: further evidence for an international clonal complex. J. Clin. Microbiol. 45:2829–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. García-Castillo M, et al. 2011. Wide dispersion of ST175 clone despite high genetic diversity of carbapenem-nonsusceptible Pseudomonas aeruginosa clinical strains in 16 Spanish hospitals. J. Clin. Microbiol. 49:2905–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Généreux C, et al. 2004. Mutational analysis of the catalytic centre of the Citrobacter freundii AmpD N-acetylmuramyl-l-alanine amidase. Biochem. J. 377:111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gutiérrez O, et al. 2007. Molecular epidemiology and mechanisms of carbapenem resistance in Pseudomonas aeruginosa isolates from Spanish hospitals. Antimicrob. Agents Chemother. 51:4329–4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hocquet D, Bertrand X, Köhler T, Talon DP, Plésiat 2003. Genetic and phenotypic variations of a resistant Pseudomonas aeruginosa epidemic clone. Antimicrob. Agents Chemother. 47:1887–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hocquet D, Nordmann P, El Garch F, Cabanne L, Plésiat P. 2006. Involvement of the MexXY-OprM efflux system in emergence of cefepime resistance in clinical strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:1347–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hocquet D, et al. 2007. Pseudomonas aeruginosa may accumulate drug resistance mechanisms without losing its ability to cause bloodstream infections. Antimicrob. Agents Chemother. 51:3531–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jacobs C, Frere JM, Normark S. 1997. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible beta-lactam resistance in Gramnegative bacteria. Cell 88:823–832 [DOI] [PubMed] [Google Scholar]
- 22. Jolley KA, Chan MS, Maiden MC. 2004. mlstdbNet—distributed multi-locus sequence typing (MLST) databases. BMC Bioinformatics 5:86 doi:10.1186/1471-2105-5-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Juan C, et al. 2005. Molecular mechanisms of beta-lactam resistance mediated by AmpC hyperproduction in Pseudomonas aeruginosa clinical strains. Antimicrob. Agents Chemother. 49:4733–4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Juan C, MoyÁ B, Pérez JL, Oliver A. 2006. Stepwise upregulation of the Pseudomonas aeruginosa chromosomal cephalosporinase conferring high level beta-lactam resistance involves three AmpD homologues. Antimicrob. Agents Chemother. 50:1780–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Juan C, et al. 2010. Metallo-beta-lactamase-producing Pseudomonas putida as a reservoir of multidrug resistance elements that can be transferred to successful Pseudomonas aeruginosa clones. J. Antimicrob. Chemother. 65:474–478 [DOI] [PubMed] [Google Scholar]
- 26. Kaufmann ME. 1998. Pulsed-field gel electrophoresis. Methods Mol. Med. 15:33–50 [DOI] [PubMed] [Google Scholar]
- 27. Kong KF, et al. 2005. Pseudomonas aeruginosa AmpR is a global transcriptional factor that regulates expression of AmpC and PoxB β-lactamases, proteases, quorum sensing, and other virulence factors. Antimicrob. Agents Chemother. 49:4567–4575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuga A, Okamoto R, Inoue M. 2000. ampR gene mutations that greatly increase class C β-lactamase activity in Enterobacter cloacae. Antimicrob. Agents Chemother. 44:561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leibovici L, et al. 1998. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J. Intern. Med. 244:379–386 [DOI] [PubMed] [Google Scholar]
- 30. Libisch B, Balogh B, Füzi M. 2009. Identification of two multidrug-resistant Pseudomonas aeruginosa clonal lineages with a countrywide distribution in Hungary. Curr. Microbiol. 58:111–116 [DOI] [PubMed] [Google Scholar]
- 31. Lister PD, Wolter DJ, Hanson ND. 2009. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 22:582–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Livermore DM. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis. 34:634–640 [DOI] [PubMed] [Google Scholar]
- 33. Llanes C, et al. 2004. Clinical strains of Pseudomonas aeruginosa overproducing MexAB-OprM and MexXY efflux pumps simultaneously Antimicrob. Agents Chemother. 48:1797–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maatallah M, et al. 2011. Population structure of Pseudomonas aeruginosa from five Mediterranean countries: evidence for frequent recombination and epidemic occurrence of CC235. PLoS One 6:e25617 doi:10.1371/journal.pone.0025617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Magiorakos AP, et al. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18:268–281 [DOI] [PubMed] [Google Scholar]
- 36. Mesaros N, et al. 2007. Pseudomonas aeruginosa: resistance and therapeutics options in the turn of the new millennium. Clin. Microbiol. Infect. 13:560–578 [DOI] [PubMed] [Google Scholar]
- 37. Moya B, Juan C, Albertí S, Pérez JL, Oliver A. 2008. Benefit of having multiple ampD genes for acquiring β-lactam resistance without losing fitness and virulence in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 52:3694–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moya B, et al. 2009. β-Lactam resistance response triggered by inactivation of a nonessential penicillin-binding protein. PLoS Pathog. 5:e1000353 doi:10.1371/journal.ppat.1000353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moya B, et al. 2012. Pan-β-lactam resistance development in Pseudomonas aeruginosa clinical strains: molecular mechanisms, penicillin-binding proteins profiles and binding affinities. Antimicrob. Agents Chemother. 56:4771–4778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ocampo-Sosa AA, et al. 2012. Alterations of OprD in carbapenem-intermediate and -susceptible strains of Pseudomonas aeruginosa isolated from patients with bacteremia in a Spanish multicenter study. Antimicrob. Agents Chemother. 56:1703–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oh H, Stenhoff S, Jalal S, Wretlind B. 2003. Role of efflux pumps and mutations in genes for topoisomerases II and IV in fluoroquinolone-resistant Pseudomonas aeruginosa strains. Microb. Drug Resist. 8:323–328 [DOI] [PubMed] [Google Scholar]
- 42. Pagani L, et al. 2005. Nosocomial outbreak caused by multidrug-resistant Pseudomonas aeruginosa producing IMP-13 metallo-beta-lactamase J. Clin. Microbiol. 43:3824–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peña C, et al. 2007. Nosocomial spread of Pseudomonas aeruginosa producing the metallo-beta-lactamase VIM-2 in a Spanish hospital: clinical and epidemiological implications. Clin. Microbiol. Infect. 13:1026–1029 [DOI] [PubMed] [Google Scholar]
- 44. Peña C, et al. 2012. Prospective multicenter study of the impact of carbapenem resistance on mortality in Pseudomonas aeruginosa bloodstream infections. Antimicrob. Agents Chemother. 56:1265–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Poole K. 2011. Pseudomonas aeruginosa: resistance to the max. Front. Microbiol. 2:65 doi:10.3389/fmicb.2011.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pournaras S, et al. 2003. Hospital outbreak of multiple clones of Pseudomonas aeruginosa carrying the unrelated metallo-beta-lactamase gene variants blaVIM-2 and blaVIM-4. J. Antimicrob. Chemother. 51:1409–1414 [DOI] [PubMed] [Google Scholar]
- 47. Quale J, Bratu S, Gupta J, Landman D. 2006. Interplay of efflux system, ampC, and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 50:1633–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Riera E, et al. 2011. Pseudomonas aeruginosa carbapenem resistance mechanisms in Spain: impact on the activity of imipenem, meropenem and doripenem. J. Antimicrob. Chemother. 66:2022–2027 [DOI] [PubMed] [Google Scholar]
- 49. Rodríguez-Martínez JM, Poirel L, Nordmann P. 2009. Molecular epidemiology and mechanisms of carbapenem resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:4783–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rodríguez-Martínez JM, Poirel L, Nordmann P. 2009. Extended-spectrum cephalosporinases in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:1766–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Samuelsen O, et al. 2010. Molecular epidemiology of metallo-beta-lactamase-producing Pseudomonas aeruginosa isolates from Norway and Sweden shows import of international clones and local clonal expansion. Antimicrob. Agents Chemother. 54:346–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sobel ML, McKay GA, Poole K. 2003. Contribution of the MexXY multidrug transporter to aminoglycoside resistance in Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 47:3202–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sobel ML, Hocquet D, Cao L, Plesiat P, Poole K. 2005. Mutations in PA3574 (nalD) lead to increased MexAB-OprM expression and multidrug resistance in laboratory and clinical isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:1782–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Suarez C, et al. 2011. A large sustained endemic outbreak of multiresistant Pseudomonas aeruginosa: a new epidemiological scenario for nosocomial acquisition. BMC Infect. Dis. 11:272 doi:10.1186/1471-2334-11-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tenover FC, et al. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tomás M, et al. 2010. Efflux pumps, OprD porin, AmpC beta-lactamase, and multiresistance in Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob. Agents Chemother. 54:2219–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Van der Bij AK, et al. 2011. First outbreak of VIM-2 metallo-β-lactamase-producing Pseudomonas aeruginosa in The Netherlands: microbiology, epidemiology and clinical outcomes. Int. J. Antimicrob. Agents 37:513–518 [DOI] [PubMed] [Google Scholar]
- 58. Viedma E, et al. 2009. Nosocomial spread of colistin-only-sensitive sequence type 235 Pseudomonas aeruginosa isolates producing the extended-spectrum beta-lactamases GES-1 and GES-5 in Spain. Antimicrob. Agents Chemother. 53:4930–4933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Viedma E, et al. 2012. VIM-2 producing multidrug-resistant Pseudomonas aeruginosa ST175 clone, Spain. Emerg. Infect. Dis. 18:1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Woodford N, Turton JF, Livermore DM. 2011. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 35:736–755 [DOI] [PubMed] [Google Scholar]
- 61. Zamorano L, MoyÁ B, Juan C, Oliver A. 2010. Differential beta-lactam resistance response driven by ampD or dacB (PBP4) inactivation in genetically diverse Pseudomonas aeruginosa strains. J. Antimicrob. Chemother. 65:1540–1542 [DOI] [PubMed] [Google Scholar]
- 62. Zavascki AP, Gaspareto PB, Martins AF, Gonçalves AL, Barth AL. 2005. Outbreak of carbapenem-resistant Pseudomonas aeruginosa producing SPM-1 metallo-{beta}-lactamase in a teaching hospital in southern Brazil. J. Antimicrob. Chemother. 56:1148–1151 [DOI] [PubMed] [Google Scholar]