Abstract
The worrisome increase in Gram-negative bacteria with borderline susceptibility to carbapenems and of carbapenemase-producing Enterobacteriaceae has significantly undermined their efficacy. Continuous infusion may be the best way to maximize the time-dependent activity of meropenem. The aim of this study was to create dosing nomograms in relation to different creatinine clearance (CLCr) estimates for use in daily clinical practice to target the steady-state concentrations (Csss) of meropenem during continuous infusion at 8 to 16 mg/liter (after the administration of an initial loading dose of 1 to 2 g over 30 min). The correlation between meropenem clearance (CLm) and CLCr was retrospectively assessed in a cohort of critically ill patients (group 1, n = 67) to create a formula for dosage calculation to target Css. The performance of this formula was validated in a similar cohort (group 2, n = 56) by comparison of the observed and the predicted Csss. A significant relationship between CLm and CLCr was observed in group 1 (r = 0.72, P < 0.001). The application of the formula to meropenem dosing in group 2, infusion rate (g/24 h) = [0.078 × CLCr (ml/min) + 2.85] × target Css × (24/1,000), led to a significant correlation between the observed and the predicted Csss (r = 0.92, P < 0.001). Dosing nomograms based on CLCr were created to target the meropenem Css at 8, 12, and 16 mg/liter in critically ill patients. These nomograms could be helpful in improving the treatment of severe Gram-negative infections with meropenem, especially in the presence of borderline susceptible pathogens or even of carbapenemase producers and/or of pathophysiological conditions which may enhance meropenem clearance.
INTRODUCTION
The increasing prevalence of resistance to beta-lactams (11, 36, 39) has prompted carbapenems as one of the cornerstone antibiotic classes retained for the treatment of patients with the most severe infections due to multidrug-resistant (MDR) Gram-negative bacteria (3, 19, 28).
However, in recent years, the tremendous increase in the number of MDR Gram-negative bacteria with borderline susceptibility to carbapenems (3, 28), as well as the increase of carbapenemase-producing strains (8), has significantly undermined their efficacy (19).
These facts have recently prompted both the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) to redefine the microbiological breakpoints of carbapenems against several Gram-negative bacteria, by taking into account also some relevant pharmacological and clinical aspects (27, 37).
The application of the pharmacokinetic/pharmacodynamic (PK/PD) principles has progressively gained major relevance by tailoring the dosing regimens of these antimicrobials with the intent of either maximizing their efficacy or of preventing the emergence of resistant strains (14, 24, 42, 44).
For carbapenems, being time-dependent agents, the maintenance of concentrations for about 40% of the dosing interval above the MIC of the pathogen (t > MIC) was found to be the pharmacodynamic target for maximal bactericidal activity in experimental animal models of infection (29, 30). Although this threshold could suffice in immunocompetent hosts, this could not be the case for optimal cure in the critically ill patients with severe sepsis or septic shock, so that maintenance of trough level above the MIC for the entire dosing interval (Cmin > MIC) was advocated in these cases (33).
Of note, it has also been suggested that trough levels severalfold above the MIC could be useful for carbapenems in some settings (23, 44, 45) and that this approach could be worthwhile even against low-MIC carbapenemase producers (6, 7, 13, 38, 43).
This phenomenon recently raised the question of whether carbapenems can still be used in the treatment of severe infections caused by carbapenemase-producing strains (12). The most recent experimental and clinical data seem to support carbapenem use, but with some fundamental conditions that must be met, such as low carbapenem MIC for the infecting organism (≤4 mg/liter), optimal pharmacodynamic exposure to carbapenem, and combination with another active compound (12).
Importantly, the achievement and maintenance of these thresholds may be extremely difficult when administering intermittent intravenous infusion of standard dosages of meropenem in the critically ill patients, considering that in these patients the elimination rate for most beta-lactams is often significantly increased (18, 34). To obviate this problem, alternative administration regimens in order to attain higher and more stable trough levels with carbapenems, namely, extended infusion and/or continuous infusion, were advocated (18, 40, 47).
At our university teaching hospital, for several years we have been administering meropenem by continuous infusion, with the intent of maximizing its pharmacodynamics for the treatment of documented or suspected Gram-negative infections in the critically ill patients. The predefined target meropenem steady-state plasma concentration (Css) is achieved by means of therapeutic drug monitoring (TDM).
The aims of this study were to extrapolate in a retrospective cohort of patients treated with meropenem by continuous infusion the existing relationship between drug clearance and creatinine clearance (CLCr) in order to create a formula for the calculation of the meropenem daily dosage needed to target Css at predefined levels on the basis of CLCr estimates, to validate the performance of this formula within a similar cohort of patients, and to create user-friendly dosing nomograms helpful for clinicians to target meropenem Css at predefined levels in relation to different degrees of CLCr estimates.
MATERIALS AND METHODS
Study design.
Critically ill patients treated with meropenem administered by continuous infusion, both empirically and for documented Gram-negative infections, and who underwent TDM for optimization of meropenem Css between January and December 2009 were included in the retrospective cohort (group 1). The Css data included in this analysis were obtained from patients treated with continuous infusion of meropenem at unmodified dosages for at least 2 days.
At our teaching hospital, continuous infusion of meropenem is appropriately granted through the reconstitution of the solution every 6 h (maximum), in light of the short-term stability of this molecule in aqueous solution at room temperature (4). The desired range of Css for continuous infusion of meropenem was arbitrarily set at 8 to 12 mg/liter. The rationale behind this choice derives from the notions that the EUCAST clinical breakpoint for meropenem against the Enterobacteriaceae is 2 mg/liter and that a Cmin/MIC ratio of 4 to 6 was found to be helpful both in maximizing clinical efficacy and in minimizing the spread of resistance (23, 44).
Data collected from the TDM program were used to estimate meropenem clearance (CLm) in each single case, according to the formula CLm (liter/h) = IR (mg/h)/Css (mg/liter), where IR is the continuous infusion rate of meropenem. Taking into account that meropenem is eliminated mainly by glomerular filtration (29), a linear regression between the individual CLm and the CLCr estimated by means of the Cockcroft and Gault formula was fitted (10). In order to avoid inaccuracy of CLCr estimates, patients bedridden for a long time (>21 days) and/or undergoing any kind of renal replacement therapies were excluded from this analysis. The resulting formula linking CLm with CLCr was used to calculate the IR of meropenem as a function of the CLCr estimates needed to target the desired Css at 8 to 12 mg/liter and to create user-friendly dosing nomograms.
Validation of the formula was then carried out in a similar cohort of critically ill patients who were treated with meropenem by continuous infusion and who had TDM of meropenem Csss between January and December 2010 (group 2). The concordance between observed and predicted meropenem Csss was assessed by linear regression analysis and by the Bland-Altman test. The protocol of the study was submitted to the Ethical Committee of the Azienda Ospedaliero-Universitaria Santa Maria della Misericordia of Udine, which deemed ethical approval unnecessary.
Meropenem assay.
Meropenem plasma concentrations were analyzed by means of a validated high-performance liquid chromatography (HPLC) method with UV detection (26) with some modifications, as previously described (32).
Briefly, for meropenem extraction, 25 μl of a 1 μg/μl cefepime solution was added as an internal standard to 1 ml of calibration, quality control, or patient sample, which was then mixed and transferred into an extraction cartridge conditioned with 2 ml of methanol and then with 2 ml of 0.05 M phosphate buffer at pH 4. After the extraction cartridge was washed with 2 ml of 0.05 M phosphate buffer at pH 4, the sample was eluted with 800 μl of a solution of 0.05 M phosphate buffer (pH 6)-methanol (9:1, vol/vol), and then 20 μl of the eluate was injected into the HPLC system (125S Beckman HPLC system coupled with Beckman 166 UV detector; Beckman Instruments, Berkeley, CA). Separation was carried out through an Ultrasphere C18 column (octyldecyl saline [ODS], 250 mm by 4.6 mm by 5 μm; Beckman, Berkeley, CA) with a solution of phosphate buffer-acetonitrile (91:9, vol/vol) at a flow-rate of 1.2 ml/min in isocratic conditions (cefepime and meropenem retention times were 3.8 and 9.1 min, respectively).
Precision and accuracy were assessed by performing replicate analyses of quality control samples against calibration standards. Intra- and interassay coefficients of variation were always less than 10%. The low limit of detection was 0.5 mg/liter.
Statistical analysis.
Descriptive data inside each group were expressed as means ± standard deviations (SD). Categorical variables were compared by the χ2 tests with Yate's correction or Fisher's exact test, when needed, whereas continuous variables were compared by means of the Student t test. A P value of <0.05 was required to achieve statistical significance. The statistical analysis was carried out with Sigma-Stat version 3.1.
RESULTS
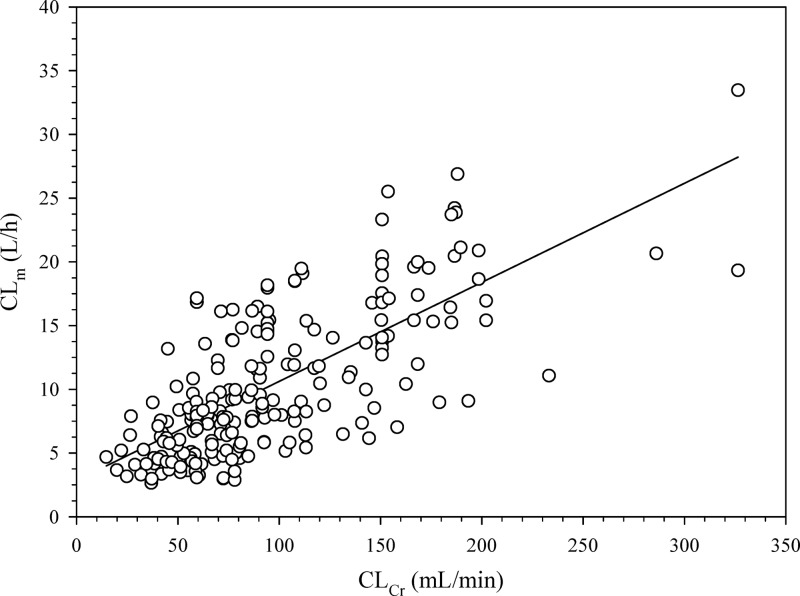
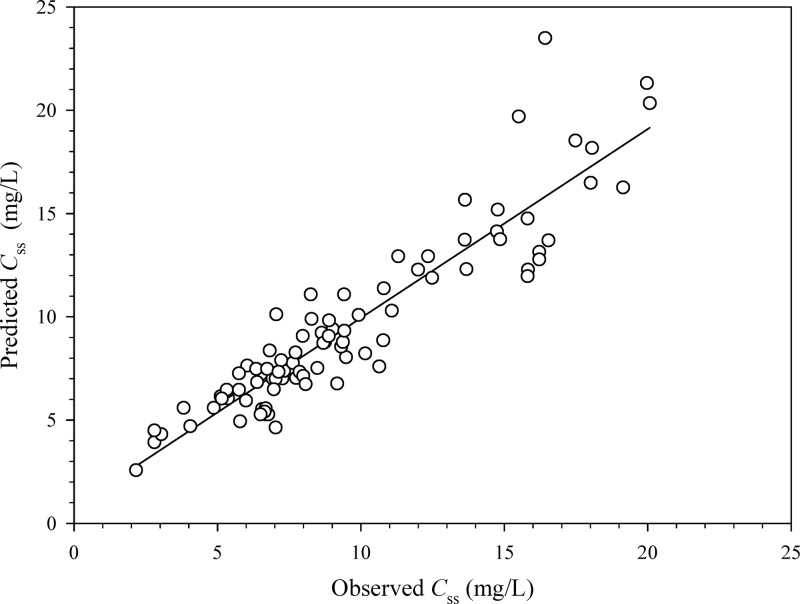
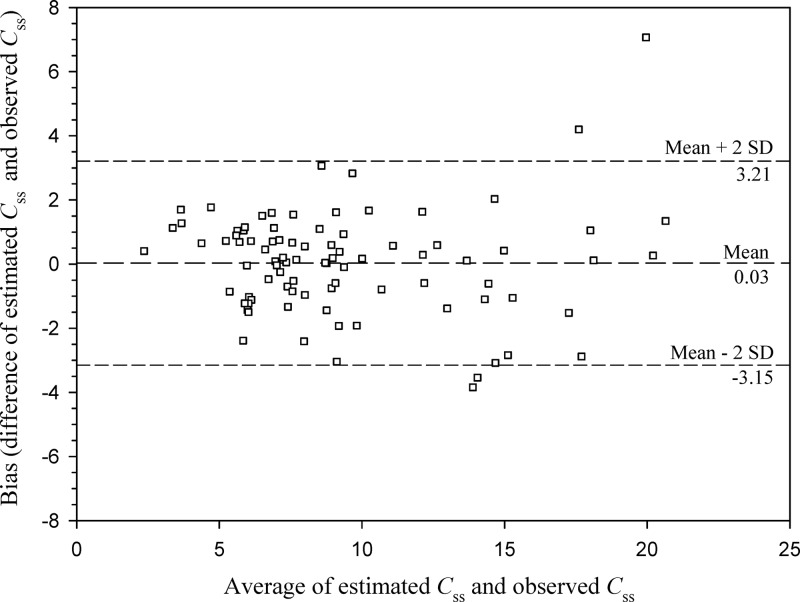
Sixty-seven patients were included in group 1, whereas 56 were included in group 2. Table 1 outlines their characteristics and shows that no bias in terms of demographic and clinical features existed between the two groups. The main reasons for meropenem use were hospital-acquired pneumonia and bloodstream infections. Figure 1 depicts the highly significant linear relationship existing between CLm and CLCr in group 1 (r = 0.72, P < 0.001), which is described by the following formula: CLm (liters/h) = 0.078 × CLCr (ml/min) + 2.85. By expressing CLm as a function of CLCr estimates, the IR of meropenem needed by continuous infusion to achieve a predefined target Css was estimated by means of the following equation: IR (g/24 h) = [0.078 × CLCr (ml/min) + 2.85] × target Css × (24/1,000). Validation of this equation in group 2 showed a highly significant correlation between the observed and the predicted meropenem Csss (r = 0.92, P < 0.001; Fig. 2). The Bland-Altman analysis of validity, by revealing that 95% of the data points lied within the ±2 standard deviations of the mean difference, confirmed that no significant bias existed (Fig. 3).
Table 1.
Patient characteristics
| Characteristic | Result |
P valuea | |
|---|---|---|---|
| Group 1 (n = 67 patients) | Group 2 (n = 56 patients) | ||
| Mean age (yr) ± SD | 64.2 ± 14.3 | 59.2 ± 18.5 | 0.09 |
| Gender (male/female) | 46/21 | 44/12 | 0.29b |
| Mean body wt (kg) ± SD | 79.4 ± 17.2 | 82.2 ± 20.2 | 0.39 |
| Mean ht (cm) ± SD | 172.3 ± 8.4 | 173.7 ± 9.3 | 0.36 |
| Mean body mass index (kg/m2) ± SD | 26.7 ± 5.2 | 27.1 ± 5.3 | 0.67 |
| Mean CLCr (ml/min)c,d ± SD | 93.9 ± 54.1 | 106.9 ± 77.8 | 0.24 |
| Mean meropenem Cssc (mg/liter) ± SD | 12.9 ± 6.2 | 10.2 ± 5.1 | 0.08 |
| No. (%) of patients admitted to the hospital | |||
| Intensive care unit ward | 33 (49.3) | 22 (39.3) | 0.36b |
| Surgical ward | 19 (28.3) | 13 (23.2) | 0.66b |
| Medical ward | 15 (22.4) | 21 (37.5) | 0.10b |
| No. (%) of patients by reason for meropenem | |||
| Hospital-acquired pneumonia | 25 (37.3) | 12 (21.4) | 0.08b |
| Bloodstream infections | 14 (20.9) | 13 (23.2) | 0.93b |
| Empirical use for severe sepsis | 11 (16.4) | 14 (25.0) | 0.34b |
| Intraabdominal infections | 9 (13.4) | 3 (5.4) | 0.23b |
| Meningitis | 6 (9.0) | 3 (5.4) | 0.68b |
| Skin and soft tissue infections | 2 (3.0) | 7 (12.5) | 0.10b |
| Urinary tract infections | 0 (0) | 4 (7.1) | 0.09b |
Statistical significance was assessed by means of unpaired t test, unless otherwise specified.
Test χ2.
At first TDM.
Estimated by the Cockcroft and Gault formula (10).
Fig 1.
Relationship between individual CLm and CLCr values estimated by means of the Cockcroft and Gault formula (8) in group 1 (n = 67 patients and 213 samples): CLm (liter/h) = 0.078 × CLCr (ml/min) + 2.85 (r = 0.72, P < 0.001).
Fig 2.
Relationship between the predicted and the observed meropenem Csss in group 2 (n = 56 patients and 99 samples) (r = 0.92, P < 0.001).
Fig 3.
Bland-Altman test assessing agreement between estimated and observed Csss in group 2 (n = 56 patients and 99 samples).
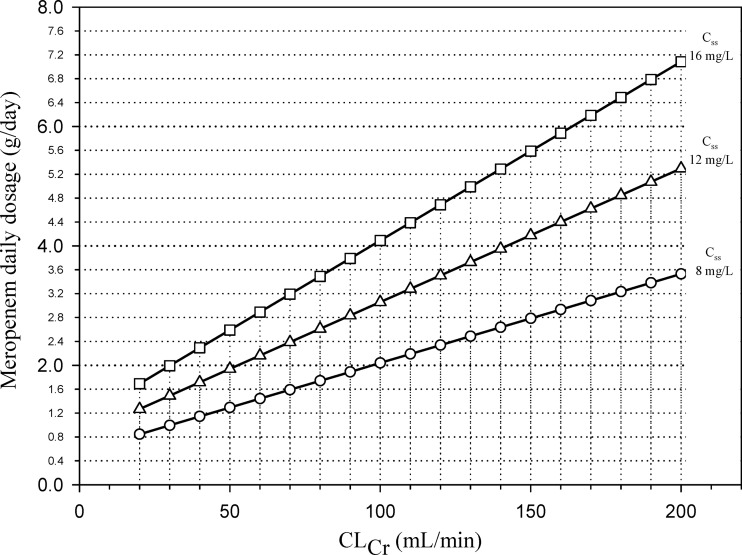
User-friendly nomograms based on the former equation were created to help clinicians in the calculation of the meropenem daily dosage, which has to be administered by continuous infusion for the achievement of target Csss of 8, 12, or 16 mg/liter in the critically ill patients as a function of their CLCr estimates (Fig. 4).
Fig 4.
Nomograms based on CLCr estimates by means of the Cockcroft and Gault formula (8) for the calculation of the meropenem daily dosage administered by continuous infusion which is necessary for the achievement of a target Css of 8 mg/liter (circles), 12 mg/liter (triangles), and 16 mg/liter (squares) in critically ill patients.
DISCUSSION
Our study allowed the successful implementation and validation of dosing nomograms based on CLCr estimates for targeting steady-state concentrations of meropenem administered by continuous infusion in the critically ill patients. These may be especially useful in routine clinical practice considering that, differently from other antibiotic classes, like glycopeptides or aminoglycosides, which may rely on therapeutic drug monitoring for dose individualization, this practice, although recently advocated, is still infrequently applied for beta-lactams (41).
The worldwide increasing prevalence of resistance among Gram-negative pathogens is of great concern for the clinical success of antimicrobial treatment of severe infections in the critically ill patients. Marketed antibiotics are progressively losing their efficacy, and unfortunately new antimicrobials are still lacking (5). From this worrisome scenario, it appears that the only viable strategy which nowadays can help to improve the clinical outcome in patients with severe Gram-negative infections may be the optimization of use of the currently available drugs.
The pharmacokinetic/pharmacodynamic properties of beta-lactams call for dosing strategies devoted to the rapid attainment and maintenance of plasma concentrations exceeding the pathogen MIC for a significant proportion of the dosing interval (24, 42). Meropenem exhibits time-dependent antimicrobial activity (29, 48), and it is widely recognized that in experimental animal models, a t > MIC for about 40% of the dosing interval may ensure bactericidal activity (15).
However, some clinical studies suggest that higher percentages may be useful for optimal treatment of Gram-negative-related infections in the critically ill patients, especially if immunocompromised. Among 60 febrile neutropenic patients with bacteremia, a t > MIC for meropenem exceeding 75% of the dosing interval allowed a clinical response rate as high as 80% (1). Interestingly, the authors suggested that when using standard intermittent intravenous infusions of meropenem over 30 min with the intent of achieving this pharmacodynamic threshold, low single doses administered more frequently (500 mg every 6 h) may be comparable to higher single doses administered at longer intervals (1 g every 8 h).
It has been recently advocated that in critically ill patients with severe sepsis or septic shock, maintenance of trough level above the MIC for the entire dosing interval (Cmin > MIC) might be helpful to improve beta-lactam efficacy (33).
Additionally, recent studies suggest, both from the clinical and from the epidemiological perspective, that meropenem Cmin some-fold higher than the pathogen MIC could maximize its efficacy, especially in deep-seated infections. In a study carried out among 101 adult patients treated with meropenem for lower respiratory tract infections, the only significant predictor of clinical response among the various pharmacodynamic indexes tested was found to be a Cmin/MIC ratio of >5 (23). Likewise, in experimental models, a Cmin/MIC ratio of 6 was found to be superior to a ratio of 2 for meropenem in suppressing bacterial resistance development against Pseudomonas aeruginosa (44, 45).
On these bases, it could be reasonably supposed that maintenance of a Cmin/MIC ratio of 4 to 6 could maximize the effectiveness of meropenem, either in terms of clinical outcome or in terms of prevention of resistance spread.
Unfortunately, these thresholds may be very difficult to achieve when administering standard dosages of meropenem by intermittent infusion over 30 min due to its short elimination half-life, and this could be especially true against pathogens with borderline susceptibility.
In agreement with the time-dependent antimicrobial activity exhibited by the beta-lactams, continuous infusion should be considered, under the same daily dosage, the most useful mode of administration to increase the Cmin/MIC ratio. This approach has been recently assessed by several authors for meropenem in the clinical setting (9, 22, 25, 35, 40).
Considering that the current EUCAST clinical breakpoint for meropenem against the Enterobacteriaceae is of 2 mg/liter (16), this could mean that targeting Csss for continuous infusion meropenem at 8 to 12 mg/liter could maximize the empirical treatment with this carbapenem against severe infections caused by meropenem-susceptible Gram-negative organisms in routine clinical practice, regardless of the degree of susceptibility of the pathogen. This choice could be extremely helpful, especially against borderline susceptible pathogens, even if lower Css could be sufficient for more susceptible ones.
Our study allowed validating user-friendly nomograms helpful for clinicians in dosing meropenem by continuous infusion for targeting Csss at these values as a function of the patient's CLCr estimate.
Interestingly, the range of CLCr estimates for which this validation was performed is between 20 and 200 ml/min. This renders the nomograms suitable even for patients with augmented renal clearance, a pathophysiological condition which was recently shown to be rather frequent among critically ill patients (17), especially in those with brain trauma, acute leukemia, or extended burn injuries (46). Of note, according to our nomograms, the meropenem daily dosage needed to achieve Css of 12 mg/liter by continuous infusion is lower than the maximum currently licensed dosage for this carbapenem, namely, 6 g per day, even for patients with CLCr as high as 200 ml/min. Accordingly, no increase in adverse events due to significant drug overexposure may be reasonably expected when applying this approach.
Although no definitive evidence exists that target Csss of 8 to 12 mg/liter are really needed for optimal meropenem efficacy in critically ill patients, another important reason for applying this strategy in clinical practice is represented by the worrisome rapid spread of carbapenemase producers among Gram-negative bacteria in Europe (8).
Several studies provided evidence that carbapenems might have an effect on carbapenemase-producing Enterobacteriaceae (7, 13, 43). Additionally, it has been recently shown by in vitro time-kill assay that meropenem at concentrations 4× the MIC was bactericidal (>3 log10 reduction CFU/ml) even when tested alone against various strains of Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae with an MIC of 2 to 4 mg/liter (38).
Likewise, the first case of a meropenem-nonsusceptible carbapenemase-positive K. pneumoniae bloodstream infection which was successfully treated with high-dose, continuous-infusion meropenem by maintaining a mean Css (22.45 mg/liter) at a level about 3-fold higher than the MIC of the pathogen (8 mg/liter) (20) was recently reported.
In agreement with these findings, it may be speculated that maintenance of the Css/MIC ratio of 4 may be helpful also in treating carbapenemase-producing Enterobacteriaceae. Our nomogram predicts that continuous infusion administration of the currently maximum licensed dosage for meropenem of 6 g/day may enable an optimal Css/MIC ratio of 4 against carbapenemase-producing Enterobacteriaceae with an MIC of 4 mg/liter, that is, a Css of 16 mg/liter, even for patients with CLCr estimates as high as 160 ml/min.
It should not be overlooked that two major aspects must be kept in mind when applying continuous infusion meropenem. First, treatment must always be started with a 1- to 2-g pulse loading dose of meropenem administered over 30 min in order to promptly achieve therapeutically effective concentrations, with continuous infusion starting immediately afterward. Second, since meropenem is stable in aqueous solution for a maximum of 6 to 8 h at 40°C (4, 21), in order to avoid significant degradation, reconstitution of the solution every 6 to 8 h (maximum) must be recommended for optimal use.
While continuous infusion of meropenem is increasingly becoming the preferred mode of administration, not everyone currently uses this, and so the generalizability of the current data should be interpreted in this light.
This study has two important limitations. First, estimation of CLCr by means of the Cockcroft and Gault formula may be inaccurate in some settings. Although it remains the most reliable for estimating renal function for drug dosing adjustments in patients with renal impairment (31) and it is broadly adopted in clinical practice thanks to its good linear relationship with the 24-h-measured CLCr (10), indeed its use must be avoided for long-term bedridden patients, since it could overestimate the actual renal function as a result of the reduced creatinine output from atrophic muscles. Additionally, it has been recently shown that its precision may be suboptimal in patients with augmented renal clearance (62%) (2), so a measured CLCr should be performed to accurately guide drug dosing in this setting. Second, the nomograms may not be reliable for patients undergoing renal replacement therapy or with very high CLCr estimates greater than 200 ml/min.
In conclusion, the expectation is that these nomograms may be helpful for clinicians in tailoring the most appropriate dosing regimen for meropenem in the empirical treatment of severe Gram-negative-related infections in the critically ill patients, especially when caused by borderline susceptible pathogens or even by carbapenemase producing microorganisms and/or when in the presence of augmented renal clearance.
Hopefully, the routine application of these nomograms, by enabling a more appropriate use of meropenem, could improve the clinical outcome in critically ill patients treated for severe Gram-negative infections, and, when coupled with appropriate policies of carbapenem restriction use, could also contribute to slow down the rapid spread of carbapenemases among Enterobacteriaceae.
ACKNOWLEDGMENTS
F.P. has been on the speakers' bureau of Astra Zeneca. P.V. has been a consultant to, has been on the speakers' bureau of, and has received grant support from Astra Zeneca. None of the other authors has a potential conflict of interest to report.
No financial support was received for this study.
Footnotes
Published ahead of print 8 October 2012
REFERENCES
- 1. Ariano RE, et al. 2005. Pharmacokinetics and pharmacodynamics of meropenem in febrile neutropenic patients with bacteremia. Ann. Pharmacother. 39:32–38 [DOI] [PubMed] [Google Scholar]
- 2. Baptista JP, et al. 2011. A comparison of estimates of glomerular filtration in critically ill patients with augmented renal clearance. Crit. Care 15:R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baughman RP. 2009. The use of carbapenems in the treatment of serious infections. J. Intensive Care Med. 24:230–241 [DOI] [PubMed] [Google Scholar]
- 4. Berthoin K, Le Duff CS, Marchand-Brynaert J, Carryn S, Tulkens PM. 2010. Stability of meropenem and doripenem solutions for administration by continuous infusion. J. Antimicrob. Chemother. 65:1073–1075 [DOI] [PubMed] [Google Scholar]
- 5. Boucher HW, et al. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1–12 [DOI] [PubMed] [Google Scholar]
- 6. Bulik CC, et al. 2010. Comparison of the activity of a human simulated, high-dose, prolonged infusion of meropenem against Klebsiella pneumoniae producing the KPC carbapenemase versus that against Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 54:804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bulik CC, Nicolau DP. 2010. In vivo efficacy of simulated human dosing regimens of prolonged-infusion doripenem against carbapenemase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 54:4112–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Canton R, et al. 2012. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 18:413–431 [DOI] [PubMed] [Google Scholar]
- 9. Chytra I, et al. 2012. Clinical and microbiological efficacy of continuous versus intermittent application of meropenem in critically ill patients: a randomized open-label controlled trial. Crit. Care 16:R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41 [DOI] [PubMed] [Google Scholar]
- 11. Coque TM, Baquero F, Canton R. 2008. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill. 13(48):pii=19044. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19044 [PubMed] [Google Scholar]
- 12. Daikos GL, Markogiannakis A. 2011. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin. Microbiol. Infect. 17:1135–1141 [DOI] [PubMed] [Google Scholar]
- 13. Daikos GL, et al. 2007. Activity of imipenem against VIM-1 metallo-beta-lactamase-producing Klebsiella pneumoniae in the murine thigh infection model. Clin. Microbiol. Infect. 13:202–205 [DOI] [PubMed] [Google Scholar]
- 14. DeRyke CA, Lee SY, Kuti JL, Nicolau DP. 2006. Optimising dosing strategies of antibacterials utilising pharmacodynamic principles: impact on the development of resistance. Drugs 66:1–14 [DOI] [PubMed] [Google Scholar]
- 15. Drusano GL. 2004. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug.’ Nat. Rev. Microbiol. 2:289–300 [DOI] [PubMed] [Google Scholar]
- 16. EUCAST 2011. Clinical breakpoints. European Committee on Antimicrobial Susceptibility, Vaxjo, Sweden: http://www.eucast.org/clinical_breakpoints/ [Google Scholar]
- 17. Fuster-Lluch O, Geronimo-Pardo M, Peyro-Garcia R, Lizan-Garcia M. 2008. Glomerular hyperfiltration and albuminuria in critically ill patients. Anaesth. Intensive Care 36:674–680 [DOI] [PubMed] [Google Scholar]
- 18. Goncalves-Pereira J, Povoa P. 2011. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of beta-lactams. Crit. Care 15:R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hawkey PM, Livermore DM. 2012. Carbapenem antibiotics for serious infections. BMJ 344:e3236 doi:10.1136/bmj.e3236 [DOI] [PubMed] [Google Scholar]
- 20. Ho VP, et al. 2011. Use of meropenem by continuous infusion to treat a patient with a Bla(kpc-2)-positive Klebsiella pneumoniae blood stream infection. Surg. Infect. (Larchmt.) 12:325–327 [DOI] [PubMed] [Google Scholar]
- 21. Keel RA, Sutherland CA, Crandon JL, Nicolau DP. 2011. Stability of doripenem, imipenem and meropenem at elevated room temperatures. Int. J. Antimicrob. Agents 37:184–185 [DOI] [PubMed] [Google Scholar]
- 22. Langgartner J, Vasold A, Gluck T, Reng M, Kees F. 2008. Pharmacokinetics of meropenem during intermittent and continuous intravenous application in patients treated by continuous renal replacement therapy. Intensive Care Med. 34:1091–1096 [DOI] [PubMed] [Google Scholar]
- 23. Li C, Du X, Kuti JL, Nicolau DP. 2007. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob. Agents Chemother. 51:1725–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lodise TP, Lomaestro BM, Drusano GL, Society of Infectious Diseases Pharmacists 2006. Application of antimicrobial pharmacodynamic concepts into clinical practice: focus on beta-lactam antibiotics: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 26:1320–1332 [DOI] [PubMed] [Google Scholar]
- 25. Lorente L, Lorenzo L, Martin MM, Jimenez A, Mora ML. 2006. Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to Gram-negative bacilli. Ann. Pharmacother. 40:219–223 [DOI] [PubMed] [Google Scholar]
- 26. Mendez AS, Steppe M, Schapoval EE. 2003. Validation of HPLC and UV spectrophotometric methods for the determination of meropenem in pharmaceutical dosage form. J. Pharm. Biomed. Anal. 33:947–954 [DOI] [PubMed] [Google Scholar]
- 27. Mouton JW, et al. 2011. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: the EUCAST approach. Clin. Microbiol. Infect. 18:E37–E45 [DOI] [PubMed] [Google Scholar]
- 28. Nicolau DP. 2008. Carbapenems: a potent class of antibiotics. Expert Opin. Pharmacother. 9:23–37 [DOI] [PubMed] [Google Scholar]
- 29. Nicolau DP. 2008. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin. Infect. Dis. 47(Suppl 1):S32–S40 [DOI] [PubMed] [Google Scholar]
- 30. Ong CT, Tessier PR, Li C, Nightingale CH, Nicolau DP. 2007. Comparative in vivo efficacy of meropenem, imipenem, and cefepime against Pseudomonas aeruginosa expressing MexA-MexB-OprM efflux pumps. Diagn. Microbiol. Infect. Dis. 57:153–161 [DOI] [PubMed] [Google Scholar]
- 31. Park EJ, et al. 2012. A systematic comparison of Cockcroft-Gault and modification of diet in renal disease equations for classification of kidney dysfunction and dosage adjustment. Ann. Pharmacother. 46:1174–1187 [DOI] [PubMed] [Google Scholar]
- 32. Pea F, et al. 2011. TDM-guided therapy with daptomycin and meropenem in a morbidly obese, critically ill patient. Ann. Pharmacother. 45:e37. [DOI] [PubMed] [Google Scholar]
- 33. Pea F, Viale P. 2009. Bench-to-bedside review: appropriate antibiotic therapy in severe sepsis and septic shock—does the dose matter? Crit. Care 13:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pea F, Viale P, Furlanut M. 2005. Antimicrobial therapy in critically ill patients: a review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin. Pharmacokinet. 44:1009–1034 [DOI] [PubMed] [Google Scholar]
- 35. Perrott J, Mabasa VH, Ensom MH. 2010. Comparing outcomes of meropenem administration strategies based on pharmacokinetic and pharmacodynamic principles: a qualitative systematic review. Ann. Pharmacother. 44:557–564 [DOI] [PubMed] [Google Scholar]
- 36. Pitout JD. 2010. Infections with extended-spectrum beta-lactamase-producing Enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs 70:313–333 [DOI] [PubMed] [Google Scholar]
- 37. Polsfuss S, Bloemberg GV, Giger J, Meyer V, Hombach M. 2012. Comparison of European Committee on Antimicrobial Susceptibility Testing (EUCAST) and CLSI screening parameters for the detection of extended-spectrum beta-lactamase production in clinical Enterobacteriaceae isolates. J. Antimicrob. Chemother. 67:159–166 [DOI] [PubMed] [Google Scholar]
- 38. Pournaras S, et al. 2011. Activity of tigecycline alone and in combination with colistin and meropenem against Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae strains by time-kill assay. Int. J. Antimicrob. Agents 37:244–247 [DOI] [PubMed] [Google Scholar]
- 39. Rahal JJ. 2009. Antimicrobial resistance among and therapeutic options against Gram-negative pathogens. Clin. Infect. Dis. 49(Suppl 1):S4–S10 [DOI] [PubMed] [Google Scholar]
- 40. Roberts JA, et al. 2009. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J. Antimicrob. Chemother. 64:142–150 [DOI] [PubMed] [Google Scholar]
- 41. Roberts JA, Norris R, Paterson DL, Martin JH. 2012. Therapeutic drug monitoring of antimicrobials. Br. J. Clin. Pharmacol. 73:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schuck EL, Derendorf H. 2005. Pharmacokinetic/pharmacodynamic evaluation of anti-infective agents. Expert Rev. Anti Infect. Ther. 3:361–373 [DOI] [PubMed] [Google Scholar]
- 43. Souli M, et al. 2011. Efficacy of carbapenems against a metallo-beta-lactamase-producing Escherichia coli clinical isolate in a rabbit intra-abdominal abscess model. J. Antimicrob. Chemother. 66:611–617 [DOI] [PubMed] [Google Scholar]
- 44. Tam VH, Nikolaou M. 2011. A novel approach to pharmacodynamic assessment of antimicrobial agents: new insights to dosing regimen design. PLoS Comput. Biol. 7:e1001043 doi:10.1371/journal.pcbi.1001043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tam VH, et al. 2005. Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:4920–4927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Udy AA, Roberts JA, Lipman J. 2011. Implications of augmented renal clearance in critically ill patients. Nat. Rev. Nephrol. 7:539–543 [DOI] [PubMed] [Google Scholar]
- 47. Wang D. 2009. Experience with extended-infusion meropenem in the management of ventilator-associated pneumonia due to multidrug-resistant Acinetobacter baumannii. Int. J. Antimicrob. Agents 33:290–291 [DOI] [PubMed] [Google Scholar]
- 48. Zhanel GG, et al. 2007. Comparative review of the carbapenems. Drugs 67:1027–1052 [DOI] [PubMed] [Google Scholar]