Abstract
The vancomycin dose necessary for the achievement of target serum trough concentrations during continuous venovenous hemofiltration (CVVH) remains to be elucidated. This was a retrospective cohort study of critically ill adults at a tertiary medical center on concurrent CVVH and vancomycin between 2006 and 2010 with a steady-state vancomycin trough concentration. The 87 included patients were grouped according to low (≤30 ml/kg/h; n = 10) or high (>30 ml/kg/h; n = 77) CVVH hemofiltration rate (HFR) for analysis. Vancomycin goal trough achievement occurred in only 32 (37%) patients. The primary endpoint of trough attainment significantly differed between HFR subgroups: 90% versus 30% in low- and high-HFR individuals, respectively (P < 0.001). Patients with subtherapeutic trough concentrations had a median (interquartile range) HFR of 40 ml/kg/h (range, 37 to 47 ml/kg/h) compared to 36 ml/kg/h (range, 30 to 39 ml/kg/h) in those who achieved the trough goal. Irrespective of goal trough, an inverse correlation existed between HFR and serum vancomycin concentration (r = −0.423; P < 0.001). In the subgroup of 14 methicillin-resistant Staphylococcus aureus (MRSA) patients, trough achievement was similar to the aggregate cohort (36%). Mortality at 28 days was unrelated to trough achievement in both the overall sample (P = 0.516) and in culture-positive MRSA patients (P = 0.396). Critically ill patients undergoing CVVH therapy may experience clinically significant reductions in goal vancomycin troughs. The results of the present study justify prospective evaluations in this population to determine the optimal vancomycin dosing strategy for attainment of goal trough concentrations.
INTRODUCTION
Severe sepsis contributes to 10 to 20% of intensive care unit (ICU) admissions and is associated with an estimated 30% mortality rate (2, 24, 25). Sepsis-related sequelae precipitate nearly 50% of all acute kidney injury cases, which often progress to require renal replacement therapy (1, 3, 31). In a 2003 multicenter practice survey, 86.2% of providers reported utilization of continuous renal replacement therapy (CRRT) as one of their management strategies for acute kidney injury (18). Provision of CRRT accomplishes steady correction of metabolic abnormalities and fluid balance without exacerbating unstable hemodynamics (17, 22). Use of continuous venovenous hemofiltration (CVVH), a convective clearance CRRT modality, has become the preferred modality likely due to the poor solute removal and arterial cannulation complications associated with arteriovenous circuits (17, 18, 20, 28).
Hemofiltration rate impacts solute removal during CVVH (8). Early CVVH literature demonstrated an improvement in mortality with hemofiltration rates of 35 to 45 ml/kg/h compared to rates of 20 ml/kg/h (22). The conflicting results of subsequent studies on the appropriate dose of CRRT have led to varied applications in clinical practice (4, 6, 19). Despite this variability, it is clear that an upward titration of hemofiltration rates occurred in the last decade (4, 6, 19, 22, 26).
The importance of rate-dependent solute removal is magnified when the solute is an essential pharmacotherapeutic intervention. Evolution of hemofiltration rate practices may impact antimicrobial agent adequacy resulting in adverse clinical outcomes in the septic patient (27). The Surviving Sepsis guideline recommendations stress not only activity of anti-infective agents against all likely pathogens but also pharmacokinetic/pharmacodynamic optimization to ensure maximal efficacy (10). Continuous venovenous hemofiltration is known to impact the clearance of vancomycin, an antimicrobial often recommended for empirical coverage in sepsis (5, 10, 12, 14, 23). Published literature on vancomycin clearance during CVVH is limited to small sample sizes, limited patient populations, and lower hemofiltration rates than those currently used (5, 9, 12, 21). With the change in CVVH practices, the adequacy of existing vancomycin dosing strategies has not been investigated. The purpose of the present study was to determine the success rate of an institutional vancomycin dosing recommendation in achieving target trough concentrations in patients on CVVH. The investigators hypothesized that use of high hemofiltration rates would portend failure to achieve goal troughs more often than low hemofiltration rates.
MATERIALS AND METHODS
Subject identification and data collection.
This retrospective cohort study examined adult ICU patients at Mayo Clinic in Rochester, Minnesota, who received concurrent CVVH and vancomycin between January 2006 and December 2010. The institutional review board approved the present study and waived the need for informed consent. An existing CVVH database identified eligible patients who were then grouped according to low (≤30 ml/kg/h) or high (>30 ml/kg/h) hemofiltration rate (6, 7, 19, 21, 22, 26, 28).
The study included adults aged ≥18 years who received 15 to 20 mg/kg (actual body weight [ABW]) vancomycin as a single daily dose consistent with institutional recommendations (5, 12, 27). Goal vancomycin trough concentrations were in accordance with Infectious Diseases Society of America guideline recommendations, 10 to 15 mg/liter or 15 to 20 mg/liter, based on suspected or documented source(s) of infection (23). Trough concentrations were drawn no earlier than before the fourth consistent vancomycin dose or the third if given a loading dose to assure steady-state conditions (5, 11). Vancomycin concentration analysis utilized an Olympus AU680 (Olympus America, Inc., Melville, NY) with the Syva Emit 2000 vancomycin assay (Siemens Healthcare Diagnostics, Inc., Newark, DE). The assay has an analytical range between 5.0 and 50.0 mg/liter, and the between-run coefficient of variation was <10% throughout the analytical range. All CVVH treatments applied the Prismaflex System and the HF 1400 polyarylethersulfone filter (Gambro, Stockholm, Sweden). Blood flow rate was standard at 200 ml/min and anticoagulation achieved with sodium citrate. Prismasate (Gambro, Inc., Lakewood, CO) replaced the hemofiltration fluid, 50% prefilter and 50% postfilter. The attending nephrologist prescribed an individualized hemofiltration rate for each patient. Excluded patients did not authorize their medical chart for review, received <85% of their prescribed hemofiltration rate, had CVVH held for more than eight consecutive hours during vancomycin therapy, required group crossover due to hemofiltration rate prescription changes, had a ≥0.5-ml/kg/h average urine output for six consecutive hours during the study, or received concurrent extracorporeal membrane oxygenation (10, 19, 22, 30). Outcome analysis included only the first ICU admission for each patient during the study time frame.
Demographic data (age, sex, ABW, ideal body weight, body mass index [BMI], baseline renal function, and comorbid conditions), severity of illness scores (Acute Physiology and Chronic Health Evaluation II [APACHE II], Sequential Organ Failure Assessment [SOFA]), and laboratory parameters (serum albumin, blood urea nitrogen) were retrospectively collected for each patient in the study. Other gathered data included admitting diagnosis, primary ICU service, source of infection, renal parameters (daily CVVH hemofiltration rate, dialyzer used, fluid balance, and urine output), weight-based vancomycin dose, and serum trough concentration.
Endpoints.
The primary outcome measured the success rate of vancomycin goal trough achievement between low and high hemofiltration rate groups. Secondary outcomes included trough concentrations in the aggregate cohort, factors associated with hemofiltration rate prescription, and 28-day all-cause mortality.
Data analysis.
No formal sample size calculation was able to be performed and all eligible patients who met inclusion/exclusion criteria were analyzed. Descriptive data were summarized by medians with interquartile ranges (IQR) and percentages. The chi-square test (or the Fisher exact test as appropriate) studied categorical variables and the Wilcoxon rank sum test analyzed continuous variables. The Spearman rank correlation coefficient tested the association between two continuous variables. Patient-specific characteristics were imputed in a multivariate least-squares regression model to analyze factors associated with continuous variables. Survival rates were estimated and compared between subgroups using the Kaplan-Meier method with the log-rank test. All analyses were carried out using the JMP statistical software package (version 8; SAS Institute, Inc., Cary, NC). A P value of <0.05 was considered statistically significant.
RESULTS
Patients.
During the study period, 435 patients underwent evaluation for eligibility (Fig. 1). The majority of the 348 excluded patients lacked an available steady-state trough concentration (n = 277; 80%) or received vancomycin at a dose different from the institutional recommendation (n = 38; 11%). The 87 included patients had a median age of 62 years (IQR = 55 to 72 years) and were 64% male. The sample was predominantly comprised of mixed-surgical ICU (43%) and medical ICU (26%) patients with critical illness reflected in APACHE II and SOFA scores of 25 (16 to 34) and 12 (7 to 15), respectively. Pulmonary (n = 44) and intra-abdominal (n = 19) sources of infection occurred most commonly. Few differences existed between groups at baseline (Table 1). Hemofiltration rates were 27 ml/kg/h (24 to 30 ml/kg/h) and 39 ml/kg/h (36 to 45 ml/kg/h) in the low (n = 10)- and high (n = 77)-rate groups, respectively (P < 0.0001). The low-hemofiltration-rate group had a significantly higher BMI than high-hemofiltration-rate patients (P < 0.001), and proportionally more intra-abdominal infections occurred in low rate individuals (P = 0.037). First vancomycin dose which met study definition was administered on day −0.1 (days −0.5 to 1.6) from CVVH initiation. Twenty-two patients (25%) received a loading dose, all of which were in the high hemofiltration rate group. The loading doses were 24.0 mg/kg (20.3 to 26.4 mg/kg).
Fig 1.
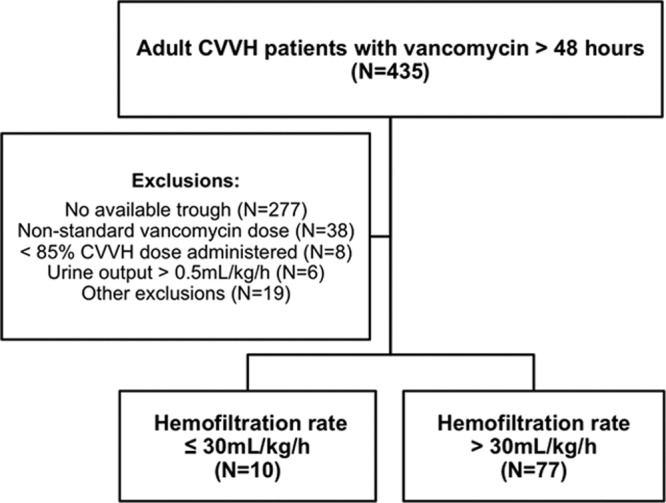
Study flow chart.
Table 1.
Baseline patient characteristics and demographic dataa
| Characteristic | HFR ≤ 30 ml/kg/h (n = 10) | HFR > 30 ml/kg/h (n = 77) | P |
|---|---|---|---|
| Age (yr) | 68 (59–71) | 62 (54–74) | 0.55 |
| No. (%) of male patients | 5 (50) | 51 (66) | 0.32 |
| Score | |||
| APACHE II | 26 (20–34) | 25 (16–35) | 0.99 |
| SOFA | 13 (6–16) | 12 (7–15) | 0.70 |
| Body mass index (kg/m2) | 42 (31–55) | 27 (24–32) | <0.001 |
| Change in body wt (kg)b | 1.2 (−3.9–5.1) | 3.1 (0–9.4) | 0.35 |
| Net fluid balance (liters)b | 9.6 (−0.5–17.0) | 10.3 (3.3–19.0) | 0.59 |
| No. (%) of ICU patients | 0.29 | ||
| Medical ICU | 3 (30) | 20 (26) | |
| Surgical ICU | 6 (60) | 31 (40) | |
| Other ICU | 1 (10) | 26 (34) | |
| No. (%) of patients with admitting diagnosis | 1.00 | ||
| Nonoperative | 5 (50) | 41 (53) | |
| Operative | 5 (50) | 36 (47) | |
| No. (%) of patients with sepsis | 9 (90) | 69 (90) | 1.00 |
| No. (%) of patients with various infection sourcesc | |||
| Respiratory | 4 (40) | 40 (52) | 0.52 |
| Intra-abdominal | 5 (50) | 14 (18) | 0.037 |
| Bacteremia | 3 (30) | 19 (25) | 0.71 |
| Cardiovascular | 0 (0) | 4 (5) | 1.00 |
| Musculoskeletal | 0 (0) | 5 (6) | 1.00 |
| Other/unknown | 1 (10) | 18 (23) | 0.68 |
| Vancomycin dose (mg/kg) | 16.9 (14.8–20.0) | 16.3 (15.3–19.7) | 0.76 |
| No. (%) of patients with target trough | 0.28 | ||
| 10–15 mg/liter | 5 (50) | 23 (30) | |
| 15–20 mg/liter | 5 (50) | 54 (70) |
Abbreviations: HFR, hemofiltration rate; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; ICU, intensive care unit. Values expressed as medians (interquartile ranges) unless noted otherwise in column 1.
From ICU admission to trough concentration.
Some total percentages may equal >100 due to a patient having multiple infectious sources.
Microbiologic confirmation of infection was present in 43 (49%) cases. Fourteen patients (16%) developed culture-positive methicillin-resistant Staphylococcus aureus (MRSA) infections, of which 7 (50%) experienced an MRSA bacteremia. The severity of illness at ICU admission in this subgroup compared well to the aggregate cohort with the median (IQR) APACHE II score of 25 (18 to 37) and SOFA score of 11 (7 to 13). The baseline BMI for these patients was 28 kg/m2 (23 to 34 kg/m2), and they were prescribed a hemofiltration rate of 41 ml/kg/h (37 to 49 ml/kg/h).
Endpoints.
Trough concentrations were obtained a median (IQR) of 3.0 (3.0 to 4.0) days after vancomycin therapy commenced. Overall, goal trough attainment occurred in 32 (37%) of the patients. For the primary endpoint of trough achievement according to hemofiltration rate subgroup, low-rate patients achieved goal trough concentrations 90% of the time, while only 30% of high-hemofiltration-rate cases reached their goal (P < 0.001). Patients with subtherapeutic trough concentrations had hemofiltration rates of 40 ml/kg/h (37 to 47 ml/kg/h), in contrast to hemofiltration rates of 36 ml/kg/h (30 to 39 ml/kg/h) in patients who reached goal trough levels (P < 0.001). Irrespective of trough goal, a significant inverse correlation exists between hemofiltration rate and vancomycin serum concentration (Fig. 2; P < 0.001). Mortality at 28 days did not differ according to trough achievement (Fig. 3; P = 0.516).
Fig 2.
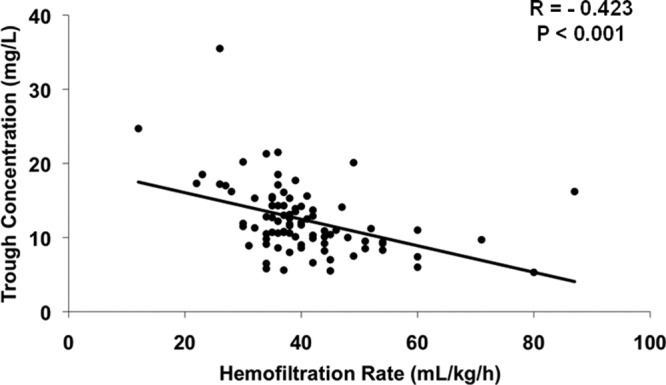
Vancomycin serum trough concentrations compared to hemofiltration rate in the aggregate cohort.
Fig 3.
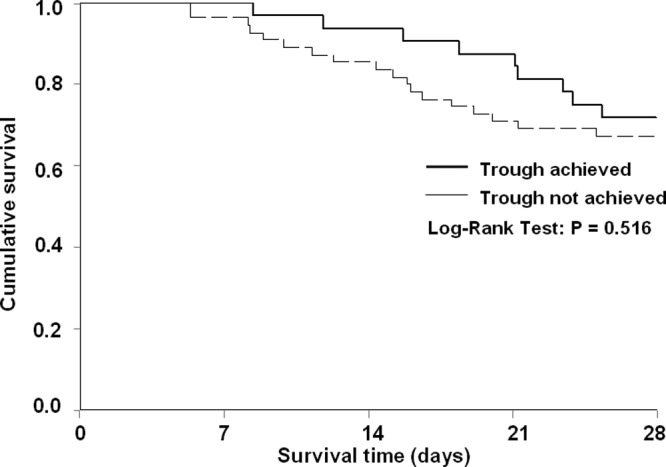
Kaplan-Meier survival curve for trough achievers versus nonachievers.
In the subgroup of 14 patients with a documented MRSA infection, 2 (14%) patients were prescribed a ≤30-ml/kg/h hemofiltration rate, whereas 12 (86%) received >30 ml/kg/h. Nine MRSA patients (64%) had subtherapeutic trough concentrations. Both low-hemofiltration-rate patients were within goal range, whereas 9 (75%) high-hemofiltration-rate patients manifested subtherapeutic troughs (P = 0.110). Similar to the aggregate sample, no difference in 28-day mortality was noted between trough achievers and nonachievers (P = 0.396).
Nephrologists individualized CVVH hemofiltration rate prescriptions for each patient and variability in prescribing practices existed among practitioners. Hemofiltration rates in the aggregate cohort ranged from 12 to 87 ml/kg/h. The prescribed hemofiltration rate (in ml/kg/h) was independently associated with ABW (P < 0.001), sex (P = 0.002), BMI (P = 0.006), and baseline serum creatinine (P = 0.03) in a multivariate analysis.
DISCUSSION
In this retrospective cohort analysis of CVVH patients, vancomycin goal trough concentration achievement occurred significantly more often in individuals receiving low hemofiltration rates compared to patients receiving high hemofiltration rates. Increases in hemofiltration rates regardless of vancomycin goal range significantly correlated with reductions in trough concentrations. Similar to the aggregate cohort, in the subset of patients with culture-positive MRSA infections, the majority of patients in the high-hemofiltration-rate group demonstrated subtherapeutic trough concentrations. Hemofiltration rate prescribing practices varied among providers and factors shown to be independently associated with the prescribed rate (in ml/kg/h) included ABW, sex, BMI, and baseline serum creatinine.
These findings are clinically relevant due to the paucity of available vancomycin pharmacokinetic data during CVVH. Joy et al. characterized the clearance of vancomycin during CVVH in eight non-critically ill end-stage renal disease patients. Each patient received a 500-mg dose and CVVH clearance of vancomycin depended on hemofiltration rates when assessed at 500 and 1,000 ml/h (12). Boereboom et al. described vancomycin clearance during CVVH in two cases of septic shock and multiple organ dysfunction syndrome. Determination of vancomycin pharmacokinetics occurred after a single dose in one individual and on the sixth day of therapy in the other study subject. Apparent volumes of distribution (Vd) were 0.66 and 0.52 liter/kg, respectively, and the terminal half-lives were 15.4 and 20.3 h for vancomycin. The CVVH clearance of vancomycin was 1,400 ml/h at a hemofiltration rate of 1,500 ml/h (19 and 20 ml/kg/h) in the two patients (5). New evidence tested these findings in seven patients given a single 12.5- to 20-mg/kg vancomycin dose during CVVH at hemofiltration rates of 800 to 1,200 ml/h (9). The calculated sieving coefficient of 0.71 ± 0.13 approximated existing literature (0.70 ± 0.15 to 0.89 ± 0.03) (5, 9, 12). Chaijamorn et al. identified vancomycin CVVH clearance of 730 ml/h, a rate lower than that documented in prior studies, and 50% of total clearance attributable to nonrenal mechanisms, similar to the findings of Macias and coworkers (9, 15). A possible reason for the difference in the CVVH clearance of vancomycin between studies may pertain to differences in the applied hemofiltration rates. Also, existing literature varies in the distribution of predilution and postdilution replacement fluid administration, which is known to influence CVVH clearance of vancomycin (29). The present study elaborates on the unique attributes of critically ill patients exposed to concurrent vancomycin and CVVH. Distinct similarities exist with prior literature, particularly with respect to dialyzer selection and blood flow rate. In addition, we document similarly poor trough achievement at 37% compared to recently released literature on this topic (30 to 50%) (21, 33). The current analysis included a heterogeneous group of critically ill individuals exposed to a consistent weight-based dosing strategy with serum trough concentrations drawn under steady-state conditions. Also, hemofiltration rates were generally higher than in previous studies, and predilution consistently comprised 50% of replacement fluid administration.
Several studies note that increased doses of CRRT are not associated with improvement in patient outcomes (4, 6, 19, 32). The mechanism by which this treatment modality fails to provide benefit has yet to be fully understood. It is well known that early appropriate antibiotics are essential to the management of individuals with severe sepsis or septic shock and that delays in therapy are associated with dramatic increases in mortality (13). The appropriateness of antimicrobials encompasses not only the spectrum of activity but also the adequacy of dosing with pharmacokinetic and pharmacodynamic optimization (10, 16). Given the present findings, it is possible that the absence of benefit associated with increasing hemofiltration rates during CRRT may be, in part, attributable to a reduced ability to achieve therapeutic goals for antimicrobials; however, further research is necessary to investigate this hypothesis.
Several important limitations exist for this type of study. The retrospective nature limits the ability to perform formal pharmacokinetic modeling and establish causality. A potential selection bias exists due to the large portion of patients excluded for the absence of an available trough concentration. Although the strict study definition for trough concentrations led to reductions in the sample size, it facilitated assessment of concentrations under steady-state conditions which strengthens the analysis. Patient size, sex, and baseline serum creatinine were found to be independently associated with prescribed hemofiltration rate. To our knowledge, this is the first time patient-specific factors that may influence dose prescribing practices have been characterized (28). Unfortunately, the only way to minimize confounding factors such as these would be to perform a randomized controlled trial that prospectively accounts for these attributes among hemofiltration rate dose groups. Vancomycin dose variability existed; however, the relative contribution of this factor to the outcome is likely minimal given the lack of a significant difference between groups at baseline. Finally, the external validity of the results may be affected by the utilization of different CRRT modalities and institutional CVVH practices, including, but not limited to, hemofiltration rate prescription patterns, blood flow rate, and dialyzer selection.
These study results suggest that critically ill patients exposed to current CVVH hemofiltration rates during vancomycin therapy may experience a significant reduction in the ability to achieve goal trough concentrations. Prospective studies are necessary in critically ill patients exposed to CVVH to determine the optimal vancomycin dosing strategy for the attainment of goal trough concentrations.
ACKNOWLEDGMENTS
Support for this project was provided by NIH/NCRR CTSA grant number UL1 RR024150. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Published ahead of print 17 September 2012
REFERENCES
- 1. Ali T, et al. 2007. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J. Am. Soc. Nephrol. 18:1292–1298 [DOI] [PubMed] [Google Scholar]
- 2. Angus DC, et al. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303–1310 [DOI] [PubMed] [Google Scholar]
- 3. Bagshaw SM, George C, Bellomo R. 2008. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol. Dial. Transplant. 23:1569–1574 [DOI] [PubMed] [Google Scholar]
- 4. Bellomo R, et al. 2009. Intensity of continuous renal-replacement therapy in critically ill patients. N. Engl. J. Med. 361:1627–1638 [DOI] [PubMed] [Google Scholar]
- 5. Boereboom FT, Ververs FF, Blankestijn PJ, Savelkoul TJ, van Dijk A. 1999. Vancomycin clearance during continuous venovenous haemofiltration in critically ill patients. Intensive Care Med. 25:1100–1104 [DOI] [PubMed] [Google Scholar]
- 6. Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, Zandstra DF, Kesecioglu J. 2002. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit. Care Med. 30:2205–2211 [DOI] [PubMed] [Google Scholar]
- 7. Boussekey N, et al. 2008. A pilot randomized study comparing high and low volume hemofiltration on vasopressor use in septic shock. Intensive Care Med. 34:1646–1653 [DOI] [PubMed] [Google Scholar]
- 8. Bressolle F, et al. 1994. Clinical pharmacokinetics during continuous haemofiltration. Clin. Pharmacokinet. 26:457–471 [DOI] [PubMed] [Google Scholar]
- 9. Chaijamorn W, Jitsurong A, Wiwattanawongsa K, Wanakamanee U, Dandecha P. 2011. Vancomycin clearance during continuous venovenous haemofiltration in critically ill patients. Int. J. Antimicrob. Agents 38:152–156 [DOI] [PubMed] [Google Scholar]
- 10. Dellinger RP, et al. 2008. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Med. 36:296–327 [DOI] [PubMed] [Google Scholar]
- 11. Jeffres MN, et al. 2006. Predictors of mortality for methicillin-resistant Staphylococcus aureus health-care-associated pneumonia: specific evaluation of vancomycin pharmacokinetic indices. Chest 130:947–955 [DOI] [PubMed] [Google Scholar]
- 12. Joy MS, Matzke GR, Frye RF, Palevsky PM. 1998. Determinants of vancomycin clearance by continuous venovenous hemofiltration and continuous venovenous hemodialysis. Am. J. Kidney Dis. 31:1019–1027 [DOI] [PubMed] [Google Scholar]
- 13. Kumar A, et al. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is a critical determinant of survival in human septic shock. Crit. Care Med. 34:1589–1596 [DOI] [PubMed] [Google Scholar]
- 14. Liu C, et al. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin. Infect. Dis. 52:285–292 [DOI] [PubMed] [Google Scholar]
- 15. Macias WL, Mueller BA, Scarim SK. 1991. Vancomycin pharmacokinetics in acute renal failure: preservation of nonrenal clearance. Clin. Pharmacol. Ther. 50:688–694 [DOI] [PubMed] [Google Scholar]
- 16. Mueller BA, Pasko DA, Sowinski KM. 2003. Higher renal replacement therapy dose delivery influences on drug therapy. Artif. Organs. 27:808–814 [DOI] [PubMed] [Google Scholar]
- 17. O'Reilly P, Tolwani A. 2005. Renal replacement therapy III: IHD, CRRT, SLED. Crit. Care Clin. 21:367–378 [DOI] [PubMed] [Google Scholar]
- 18. Overberger P, Pesacreta M, Palevsky PM. 2007. Management of renal replacement therapy in acute kidney injury: a survey of practitioner prescribing practices. Clin. J. Am. Soc. Nephrol. 2:623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palevsky PM, et al. 2008. Intensity of renal support in critically ill patients with acute kidney injury. N. Engl. J. Med. 359:7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. RENAL Study Investigators 2008. Renal replacement therapy for acute kidney injury in Australian and New Zealand intensive care units: a practice survey. Crit. Care Resusc. 10:225–230 [PubMed] [Google Scholar]
- 21. Roberts DM, et al. 2012. Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy: a multicentre pharmacokinetic study. Crit. Care Med. 40:1523–1528 [DOI] [PubMed] [Google Scholar]
- 22. Ronco C, et al. 2000. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 356:26–30 [DOI] [PubMed] [Google Scholar]
- 23. Rybak MJ, et al. 2009. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 49:325–327 [DOI] [PubMed] [Google Scholar]
- 24. Shen HN, Lu CL, Yang HH. 2010. Epidemiologic trend of severe sepsis in Taiwan from 1997 through 2006. Chest 138:298–304 [DOI] [PubMed] [Google Scholar]
- 25. Shorr AF, Micek ST, Jackson WL, Jr, Kollef MH. 2007. Economic implications of an evidence-based sepsis protocol: can we improve outcomes and lower costs? Crit. Care Med. 35:1257–1262 [DOI] [PubMed] [Google Scholar]
- 26. Tolwani AJ, et al. 2008. Standard versus high-dose CVVHDF for ICU-related acute renal failure. J. Am. Soc. Nephrol. 19:1233–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trotman RL, Williamson JC, Shoemaker DM, Salzer WL. 2005. Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin. Infect. Dis. 41:1159–1166 [DOI] [PubMed] [Google Scholar]
- 28. Uchino S, et al. 2007. Continuous renal replacement therapy: a worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. kidney) investigators. Intensive Care Med. 33:1563–1570 [DOI] [PubMed] [Google Scholar]
- 29. Uchino S, Cole L, Morimatsu H, Goldsmith D, Bellomo R. 2002. Clearance of vancomycin during high-volume haemofiltration: impact of pre-dilution. Intensive Care Med. 28:1664–1667 [DOI] [PubMed] [Google Scholar]
- 30. Uchino S, Fealy N, Baldwin I, Morimatsu H, Bellomo R. 2003. Continuous is not continuous: the incidence and impact of circuit “down-time” on uraemic control during continuous veno-venous haemofiltration. Intensive Care Med. 29:575–578 [DOI] [PubMed] [Google Scholar]
- 31. Uchino S, et al. 2005. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813–818 [DOI] [PubMed] [Google Scholar]
- 32. Van Wert R, Friedrich JO, Scales DC, Wald R, Adhikari NK. 2010. High-dose renal replacement therapy for acute kidney injury: systematic review and meta-analysis. Crit. Care Med. 38:1360–1369 [DOI] [PubMed] [Google Scholar]
- 33. Wilson FP, Berns JS. 2012. Vancomycin levels are frequently subtherapeutic during continuous venovenous hemodialysis (CVVHD). Clin. Nephrol. 77:329–331 [DOI] [PMC free article] [PubMed] [Google Scholar]


