Abstract
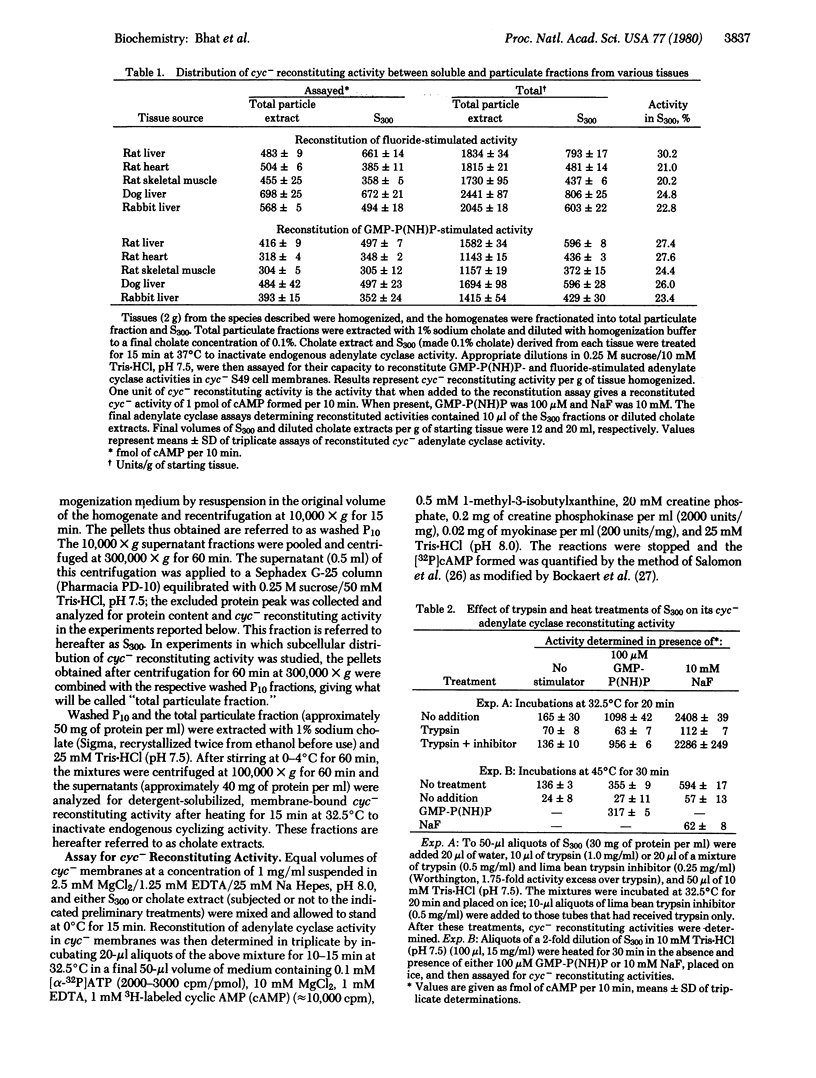
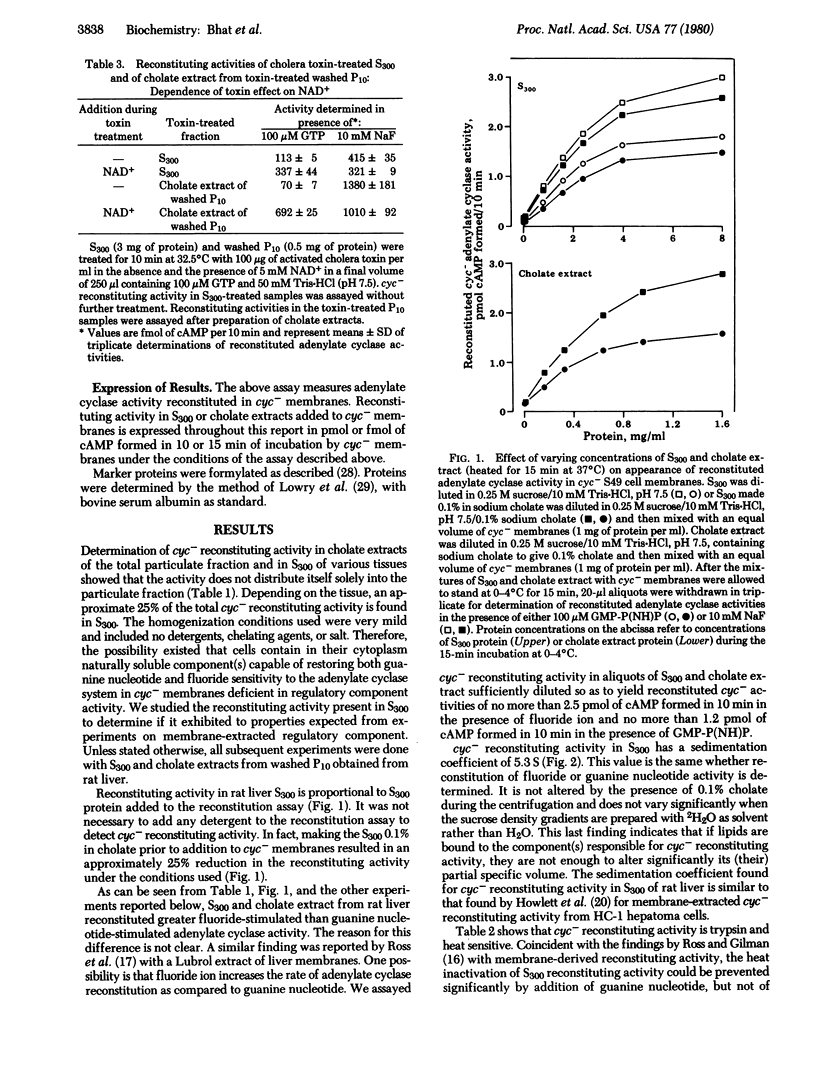
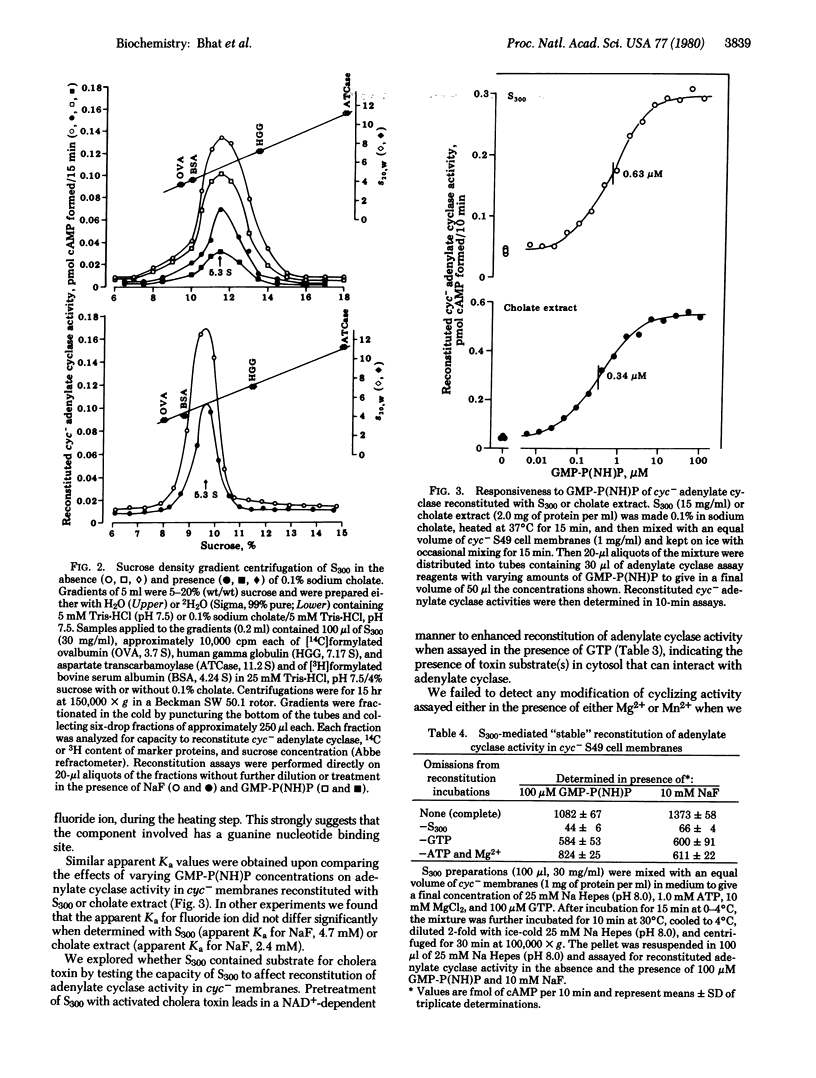
Supernatant fractions (300,000 x g, 60 min) from homogenates of rat liver, heart, and skeletal muscle, dog liver, and rabbit liver prepared without detergent in the homogenization medium (referred to as S300) are shown to contain an activity that restores Mg2+-dependent fluoride- and guanine nucleotide-stimulated cyclizing activity to the adenylate cyclase system [ATP pyrophosphate-lyase (cyclizing); EC 4.6.1.1] in cyc- S49 murine lymphoma cell membranes. Approximately 25% of the total cyc- reconstituting activity in the above tissues is present in S300. Reconstituting activity is proportional to S300, is sensitive to trypsin, is protected against heat inactivation by guanine nucleotide, and has a sedimentation coefficient of 5.3 in both H2O and 2H2O linear sucrose density gradients. Treatment with cholera toxin and NAD+ results in reconstitution of cyc- adenylate cyclase with enhanced activity in the presence of GTP. Reconstituion with S300 is stable, as seen in cyc- membranes after washing. All of these properties of S300 are similar to those of membrane-derived cyc- reconstituting activity. It is concluded that cell cytoplasm contains a naturally soluble protein or mixture of proteins having guanine nucleotide regulatory component activity of adenylate cyclase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramowitz J., Iyengar R., Birnbaumer L. Guanyl nucleotide regulation of hormonally-responsive adenylyl cyclases. Mol Cell Endocrinol. 1979 Dec;16(3):129–146. doi: 10.1016/0303-7207(79)90022-4. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Bearer C. F., Iyengar R. A two-state model of an enzyme with an allosteric regulatory site capable of metabolizing the regulatory ligand. Simplified mathematical treatments of transient and steady state kinetics of an activator and its competitive inhibition as applied to adenylyl cyclases. J Biol Chem. 1980 Apr 25;255(8):3552–3557. [PubMed] [Google Scholar]
- Birnbaumer L. Hormone-sensitive adenylyl cyclases. Useful models for studying hormone receptor functions in cell-free systems. Biochim Biophys Acta. 1973 Sep 10;300(2):129–158. doi: 10.1016/0304-4157(73)90002-6. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Swartz T. L., Abramowitz J., Mintz P. W., Iyengar R. Transient and steady state kinetics of the interaction of guanyl nucleotides with the adenylyl cyclase system from rat liver plasma membranes. Interpretation in terms of a simple two-state model. J Biol Chem. 1980 Apr 25;255(8):3542–3551. [PubMed] [Google Scholar]
- Bockaert J., Hunzicker-Dunn M., Birnbaumer L. Hormone-stimulated desensitization of hormone-dependent adenylyl cyclase. Dual action of luteninizing hormone on pig graafian follicle membranes. J Biol Chem. 1976 May 10;251(9):2653–2663. [PubMed] [Google Scholar]
- Bourne H. R., Coffino P., Tomkins G. M. Selection of a variant lymphoma cell deficient in adenylate cyclase. Science. 1975 Feb 28;187(4178):750–752. doi: 10.1126/science.163487. [DOI] [PubMed] [Google Scholar]
- Braun T., Dods R. F. Development of a Mn-2+-sensitive, "soluble" adenylate cyclase in rat testis. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1097–1101. doi: 10.1073/pnas.72.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett A. C., Sternweis P. C., Macik B. A., Van Arsdale P. M., Gilman A. G. Reconstitution of catecholamine-sensitive adenylate cyclase. Association of a regulatory component of the enzyme with membranes containing the catalytic protein and beta-adrenergic receptors. J Biol Chem. 1979 Apr 10;254(7):2287–2295. [PubMed] [Google Scholar]
- Iyengar R., Abramowitz J., Bordelon-Riser M., Birnbaumer L. Hormone receptor-mediated stimulation of adenylyl cyclase systems. Nucleotide effects and analysis in terms of a simple two-state model for the basic receptor-affected enzyme. J Biol Chem. 1980 Apr 25;255(8):3558–3564. [PubMed] [Google Scholar]
- Iyengar R., Birnbaumer L. Coupling of the glucagon receptor to adenylyl cyclase by GDP: evidence for two levels of regulation of adenylyl cyclase. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3189–3193. doi: 10.1073/pnas.76.7.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar R., Swartz T. L., Birnbaumer L. Coupling of glucagon receptor to adenylyl cyclase. Requirement of a receptor-related guanyl nucleotide binding site for coupling of receptor to the enzyme. J Biol Chem. 1979 Feb 25;254(4):1119–1123. [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Bourne H. R. Genetic evidence that cholera toxin substrates are regulatory components of adenylate cyclase. J Biol Chem. 1978 Oct 25;253(20):7120–7123. [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Bourne H. R. Reconstitution of cholera toxin-activated adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3113–3117. doi: 10.1073/pnas.75.7.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Londos C., Salomon Y., Lin M. C., Harwood J. P., Schramm M., Wolff J., Rodbell M. 5'-Guanylylimidodiphosphate, a potent activator of adenylate cyclase systems in eukaryotic cells. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3087–3090. doi: 10.1073/pnas.71.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Mechanism of action of choleragen. Evidence for ADP-ribosyltransferase activity with arginine as an acceptor. J Biol Chem. 1977 Apr 10;252(7):2455–2457. [PubMed] [Google Scholar]
- Pecker F., Hanoune J. Activation of epinephrine-sensitive adenylate cyclase in rat liver by cytosolic protein-nucleotide complex. J Biol Chem. 1977 Apr 25;252(8):2784–2786. [PubMed] [Google Scholar]
- Pfeuffer T. GTP-binding proteins in membranes and the control of adenylate cyclase activity. J Biol Chem. 1977 Oct 25;252(20):7224–7234. [PubMed] [Google Scholar]
- Pfeuffer T. Guanine nucleotide-controlled interactions between components of adenylate cyclase. FEBS Lett. 1979 May 1;101(1):85–89. [PubMed] [Google Scholar]
- Pfeuffer T., Helmreich E. J. Activation of pigeon erythrocyte membrane adenylate cyclase by guanylnucleotide analogues and separation of a nucleotide binding protein. J Biol Chem. 1975 Feb 10;250(3):867–876. [PubMed] [Google Scholar]
- RALL T. W., SUTHERLAND E. W. Adenyl cyclase. II. The enzymatically catalyzed formation of adenosine 3',5'-phosphate and inorganic pyrophosphate from adenosine triphosphate. J Biol Chem. 1962 Apr;237:1228–1232. [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Resolution of some components of adenylate cyclase necessary for catalytic activity. J Biol Chem. 1977 Oct 25;252(20):6966–6969. [PubMed] [Google Scholar]
- Ross E. M., Howlett A. C., Ferguson K. M., Gilman A. G. Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J Biol Chem. 1978 Sep 25;253(18):6401–6412. [PubMed] [Google Scholar]
- Ross E. M., Maguire M. E., Sturgill T. W., Biltonen R. L., Gilman A. G. Relationship between the beta-adrenergic receptor and adenylate cyclase. J Biol Chem. 1977 Aug 25;252(16):5761–5775. [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sharp G. W., Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971 Jan 22;229(5282):266–269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- Stancel G. M., Gorski J. Analysis of cytoplasmic and nuclear estrogen-receptor proteins by sucrose density gradient centrifugation. Methods Enzymol. 1975;36:166–176. doi: 10.1016/s0076-6879(75)36018-7. [DOI] [PubMed] [Google Scholar]
- Walseth T. F., Johnson R. A. The enzymatic preparation of [alpha-(32)P]nucleoside triphosphates, cyclic [32P] AMP, and cyclic [32P] GMP. Biochim Biophys Acta. 1979 Mar 28;562(1):11–31. doi: 10.1016/0005-2787(79)90122-9. [DOI] [PubMed] [Google Scholar]


