Abstract
This study evaluated the pharmacological activity of PTX3, administered in combination with voriconazole, in a rat model of pulmonary aspergillosis. The data indicated additive therapeutic activities of these compounds, as demonstrated by the amelioration of respiratory function changes, reduction of lung fungal burden, and increased survival. Overall, we provide clear evidence that the combination of PTX3 with a suboptimal dose of voriconazole might represent a therapeutic option under those clinical conditions where the use of voriconazole alone is not warranted for efficacy and tolerability reasons.
TEXT
Aspergillus-acquired azole resistance and azole side effects represent relevant clinical limitations to the treatment of fungal infections by these organisms (1, 2). The use of combinations of azoles with different classes of antifungal agents is currently under preclinical and clinical evaluation to overcome these problems (3).
Voriconazole (VRC) is the first line of treatment for invasive aspergillosis (4). Recent evidence indicated that VRC administration was associated with a profile of gene expression in monocytes that included cytokines and members of the Toll-like receptor (TLR) family (5, 6). Consistently, an enhanced antifungal activity was shown when VRC was administered in combination with monocytes and neutrophils in mice infected with Aspergillus fumigatus (7).
PTX3 is a multimeric glycoprotein expressed by a variety of somatic cell types and mainly characterized in monocytes as inducible by primary inflammatory stimuli, such as those mediated by interleukin-1β, tumor necrosis factor alpha, and agonists of the TLR family (8, 9). The protein has been shown to be a nonredundant factor in host resistance to A. fumigatus, and it has been successfully used, as a recombinant protein, in several animal models of invasive pulmonary aspergillosis (IPA) (10, 11, 12, 13).
The aim of this study was to determine whether coadministration of PTX3 and VRC, in rats immunosuppressed with cortisone acetate (CA) and infected with A. fumigatus conidia, resulted in additive or synergistic activities of these compounds against the fungus.
Two experiments were performed in randomized block designs on rats immunosuppressed with CA and infected with A. fumigatus, as described previously (13). In the first experiment, we evaluated the activities of PTX3, VRC, and their combination on respiratory parameters, lung weight, and lung fungal burden. In the second experiment, we evaluated the effects of these compounds or their combination on survival. Control animals were administered saline. PTX3 was administered intraperitoneally (i.p.) at doses of 0.15 and 1.5 mg/kg of body weight from day 3 before lung infection until day 3 after lung infection. VRC was administered orally at doses of 20 or 30 mg/kg on the day of infection and then once daily for another 4 days in the first experiment and for another 8 days in the second experiment. The dose of VRC was chosen based on an earlier experiment in which a dose of 30 mg/kg was able to counteract the infection, whereas a dose of 20 mg/kg had little effect. Combination treatments of 20 mg/kg VRC with 0.15 or 1.5 mg/kg PTX3 were performed following the schedule and the administration route described above for each compound. Kaplan-Meier survival analysis and analysis of variance (ANOVA) was used to compare groups, followed by Dunnett's t test.
Respiratory parameters were evaluated on day 4 after infection. Animals were individually placed in a whole-body single plethysmography chamber (EMKA Technologies), and respiratory function parameters were recorded (Table 1). Enhanced pause (Penh) was chosen as the index of respiratory distress and was calculated according to the following formula: Penh = [(TE − RT)/RT](PEF/PIF), where TE is the expiratory time (in seconds), RT is the relaxation time (time to pressure decay to 36% of total box pressure at expiration, in seconds), PEF is the peak expiratory pressure (in ml/s), and PIF is the peak inspiratory pressure (in ml/s). In rats infected by Aspergillus and treated with saline, the tidal volume (TV) was reduced, while respiratory frequency (F; number of inhalations/min) and Penh increased compared to uninfected rats (Table 1). PTX3 and VRC, given alone or in combination, returned the respiratory parameters close to the values recorded in uninfected rats, with variable efficacy depending on dose. Penh was significantly reduced in the group that received PTX3 at 1.5 mg/kg and in the group that received the combination of PTX3 at 1.5 mg/kg and VRC at 20 mg/kg (Table 1).
Table 1.
Respiratory parameters and lung fungal burden in immunosuppressed rats treated with PTX3, VRC, and combinations of the two agents
| Treatment | n | Dose (mg/kg) | Schedulea (route) | Respiratory parametersb (mean ± SEM) | Mean ± SEM lung wt (g) | Fungal burden, as log10 CFU or GMIc |
|---|---|---|---|---|---|---|
| Uninfectedd | 14 | TV, 1.03 ± 0.04; F, 88 ± 2; Penh, 0.53 ± 0.03 | 1.0 ± 0.06 | |||
| Saline | 4 | −3, +3 (i.p.) | TV, 0.66 ± 0.01***; F, 125 ± 18; Penh, 1.07 ± 0.11** | 2.0 ± 0.3* | Lung CFU, 4.7 ± 0.3; lung GMI, 6.2 ± 1.2; serum GMI, 4.5 ± 0.5 | |
| PTX3 | 5 | 0.15 | −3, +3 (i.p.) | TV, 0.74 ± 0.06; F, 132 ± 16; Penh, 0.97 ± 0.21 | 1.6 ± 0.1 | Lung CFU, 3.3 ± 1.0; lung GMI, 5.0 ± 1.7; serum GMI, 4.4 ± 2.0 |
| PTX3 | 5 | 1.5 | −3, +3 (i.p.) | TV, 0.75 ± 0.08; F, 107 ± 21; Penh, 0.57 ± 0.07f | 1.3 ± 0.1*f | Lung CFU, 1.7 ± 0.9; lung GMI, 2.7 ± 2.2; serum GMI, 2.7 ± 1.8 |
| VRC | 5 | 20 | 0, +4 (os) | TV, 0.81 ± 0.04; F, 119 ± 12; Penh, 1.10 ± 0.33 | 1.7 ± 0.2 | Lung CFU, 3.4 ± 0.8; lung GMI, 5.0 ± 1.4; serum GMI, 5.4 ± 1.6 |
| VRC | 6 | 30 | 0, +4 (os) | TV, 0.73 ± 0.09; F, 118 ± 15; Penh, 0.65 ± 0.07 | 1.1 ± 0.1*f | Lung CFU, 1.3 ± 0.8*f; lung GMI, 3.0 ± 1.9; serum GMI, 2.2 ± 1.4 |
| PTX3 + VRCe | 4 | 0.15/20 | −3, +3 (i.p.)/0, +4 (os) | TV, 0.66 ± 0.05; F, 133 ± 15; Penh, 1.04 ± 0.06 | 1.4 ± 0.1 | Lung CFU: 3.8 ± 0.3*f; lung GMI, 8.5 ± 0.3; serum GMI, 7.5 ± 1.7 |
| PTX3 + VRC | 6 | 1.5/20 | −3, +3 (i.p.)/ 0, +4 (os) | TV, 0.75 ± 0.07; F, 108 ± 7; Penh, 0.60 ± 0.05***f | 1.1 ± 0.1*f | Lung CFU: 0.1 ± 0.1***g; lung GMI, 0.4 ± 0.2***g; serum GMI, 0.4 ± 0.4***f |
The numbers identify start and finish of administration days with the day of infection as day zero.
The evaluated respiratory parameters (means ± SEM) refer to results for rats sacrificed 4 days after infection with A. fumigatus, which was administered intratracheally with 5 ×107 conidia in 0.2 ml of saline. TV, tidal volume, the air displaced between normal inspiration and expiration (in ml); F, frequency of breathing (number of inspirations/min). The calculation of Penh is described in the text. These data were evaluated with a one-way ANOVA for each of the three main treatment groups, PTX3, VRC, and the combination of the two, compared to the group of rats that received only saline. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (based on ANOVA). For the saline-treated group, the one-way ANOVA compared this group with the uninfected group.
The GMI values were calculated according to the following formula: optical density (OD) of the sample/mean cutoff control OD.
Uninfected rats received only CA.
For combination treatments, the doses and schedules are reported as PTX3/VRC data.
Significantly different (P < 0.05) from comparison group according to Dunnett's t test.
Significantly different (P < 0.01) from comparison group according to Dunnett's t test.
After recording the respiratory function values, rats were anesthetized (50 mg/kg pentobarbital, i.p.) and sacrificed, and lungs were excised and weighed. Fungal burden was evaluated both in lung samples and in blood, as described previously (13) (Table 1). Pulmonary infection with Aspergillus conidia induced an increase of lung weight in saline-treated rats, likely due to edema. Lung weight was progressively reduced as a function of dose in both the VRC- and PTX3-treated rats (Table 1). The combinations of PTX3 at 0.15 or 1.5 mg/kg with VRC at 20 mg/kg proved to be more effective than either single compound administered alone, with lung weight reduction showing an additive effect. Lung fungal burden was evaluated on serially diluted (1:10, 1:100, and 1:1,000) lung homogenates either as CFU or as the galactomannan index (GMI; Platelia Aspergillus; Bio-Rad Laboratories), as described previously (13) (Table 1). The GMI was also evaluated in blood (Table 1). Both VRC and PTX3, at the highest doses, reduced the lung CFU (about 70% and 60%, respectively), while VRC at 20 mg/kg and PTX3 at 0.15 mg/kg reduced the lung CFU to 30% of that of rats that received saline. Comparable results were obtained only for the highest doses when fungal burden was evaluated based on the GMI in lung and serum (Table 1), while VRC at 20 mg/kg and PTX3 at 0.15 mg/kg were less active (20% GMI reduction compared to saline). A significant reduction was observed in the lung CFU of rats treated with the combination of PTX3 at 0.15 mg/kg and VRC at 20 mg/kg. The combination of PTX3 at 1.5 mg/kg with VRC at 20 mg/kg resulted in a synergistic effect on both the lung CFU and GMI (more than 90% reduction) (Table 1).
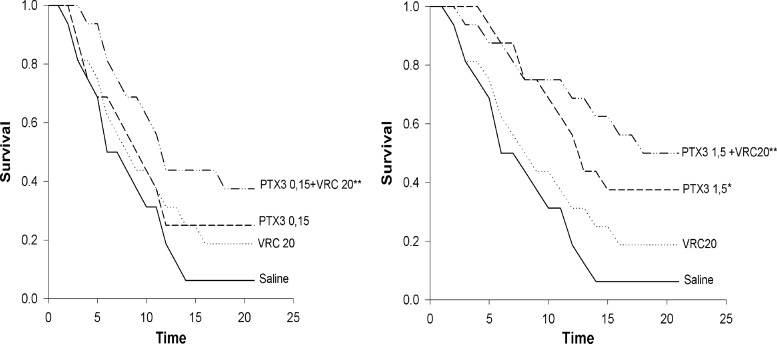
After IPA induction, the median survival time of saline-treated rats was 6 days (interquartile range [IR], 4 to 12). Rats treated with 30 mg/kg VRC had a significant reduction in mortality rate (median survival time, >21 days) (data not shown). The treatments with 20 mg/kg of VRC or with 0.15 mg/kg of PTX3 had only a slight effect on mortality compared to saline-treated rats, while the combination treatment significantly reduced mortality (median survival time, 12 days; IR, 7 to >21 days; P < 0.01) (Fig. 1, left graph). PTX3 at the highest dose of 1.5 mg/kg significantly reduced mortality (median survival time, 13 days; IR, 8 to >21 days; P < 0.05). Again, the combination treatment exerted a more significant protective effect (median survival time, 18 days; IR, 8 to >21 days; P < 0.01) (Fig. 1, right graph), indicating the additive activities of the compounds.
Fig 1.
Survival rates for rats (n = 16) immunosuppressed with cortisone acetate and infected intratracheally with A. fumigatus conidia (5 ×107 conidia in 0.2 ml of saline) and then treated with saline or PTX3, VRC, or a combination of the two, as indicated. (Left) Survival of rats receiving the combination of PTX3 at 0.15 mg/kg and VRC at 20 mg/kg and the corresponding single treatments. (Right) Effects of the combination of PTX3 at 1.5 mg/kg and VRC at 20 mg/kg and the corresponding single treatments. The PTX3 administration schedule was begun on day 3 before lung infection until day 3 after lung infection. Voriconazole was administered on the day of infection and then once daily for the next 8 days. The same administration schedule for each compound was maintained for the drug combinations. *, P < 0.05, and **, P < 0.001, based on Kaplan-Meier analysis.
Our data provide evidence that coadministration of PTX3 and VRC is effective in ameliorating respiratory function changes and in reducing lung fungal burden. Combinations of VRC and PTX3 led to IPA resolution in rats, enhancing both survival rate and median survival time. The mechanism underlying such an activity remains to be elucidated. However, it is reasonable to speculate that PTX3 further enhances the already-described immune-modulating activity of VRC against this fungus (5, 6). This study suggests that coadministration of PTX3 and VRC, in a clinical setting of therapy or prophylaxis, might represent a valid strategy to counteract infection by A. fumigatus.
Footnotes
Published ahead of print 24 September 2012
REFERENCES
- 1. D'Angelo C, et al. 2009. Exogenous pentraxin 3 restores antifungal resistance and restrains inflammation in murine chronic granulomatous disease. J. Immunol. 183:4609–4618 [DOI] [PubMed] [Google Scholar]
- 2. Garlanda C, et al. 2002. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 420:182–186 [DOI] [PubMed] [Google Scholar]
- 3. Gaziano R, et al. 2004. Anti-Aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals. Antimicrob. Agents Chemother. 48:4414–4421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Howard SJ, Arendrup MC. 2011. Acquired antifungal drug resistance in Aspergillus fumigatus: epidemiology and detection. Med. Mycol. 49:S90–S95 [DOI] [PubMed] [Google Scholar]
- 5. Inforzato A, et al. 2008. Structural characterization of PTX3 disulfide bond network and its multimeric status in cumulus matrix organization. J. Biol. Chem. 283:10147–10161 [DOI] [PubMed] [Google Scholar]
- 6. Inforzato A, et al. 2006. Structure and function of the long pentraxin PTX3 glycosidic moiety: fine-tuning of the interaction with C1q and complement activation. Biochemistry 45:11540–11551 [DOI] [PubMed] [Google Scholar]
- 7. Johnson MD, MacDougall C, Ostrosky-Zeichner L, Perfect JR, Rex JH. 2004. Combination antifungal therapy. Antimicrob. Agents Chemother. 48:693–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lat A, Thompson GR., III 2011. Update on the optimal use of voriconazole for invasive infections. Infect. Drug Resist. 4:43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lo Giudice P, et al. 2010. Efficacy of PTX3 in a rat model of invasive aspergillosis. Antimicrob. Agents Chemother. 54:4513–4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simitsopoulou M, et al. 2007. Expression of immunomodulatory genes in human monocytes induced by voriconazole in the presence of Aspergillus fumigatus. Antimicrob. Agents Chemother. 51:1048–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simitsopoulou M, et al. 2008. Immunomodulatory effects of voriconazole on monocytes challenged with Aspergillus fumigatus: different role of Toll-like receptors. Antimicrob. Agents Chemother. 52:3301–3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vora S, Chauhan S, Brummer E, Stevens DA. 1998. Activity of voriconazole with neutrophils or monocytes against Aspergillus fumigatus: effects of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. Antimicrob. Agents Chemother. 12:2299–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walsh TJ, et al. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infection Diseases Society of America. Clin. Infect. Dis. 46:327–360 [DOI] [PubMed] [Google Scholar]