Abstract
Biofilms that develop on indwelling devices are a major concern in clinical settings. While removal of colonized devices remains the most frequent strategy for avoiding device-related complications, antibiotic lock therapy constitutes an adjunct therapy for catheter-related infection. However, currently used antibiotic lock solutions are not fully effective against biofilms, thus warranting a search for new antibiotic locks. Metal-binding chelators have emerged as potential adjuvants due to their dual anticoagulant/antibiofilm activities, but studies investigating their efficiency were mainly in vitro or else focused on their effects in prevention of infection. To assess the ability of such chelators to eradicate mature biofilms, we used an in vivo model of a totally implantable venous access port inserted in rats and colonized by either Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, or Pseudomonas aeruginosa. We demonstrate that use of tetrasodium EDTA (30 mg/ml) as a supplement to the gentamicin (5 mg/ml) antibiotic lock solution associated with systemic antibiotics completely eradicated Gram-positive and Gram-negative bacterial biofilms developed in totally implantable venous access ports. Gentamicin-EDTA lock was able to eliminate biofilms with a single instillation, thus reducing length of treatment. Moreover, we show that this combination was effective for immunosuppressed rats. Lastly, we demonstrate that a gentamicin-EDTA lock is able to eradicate the biofilm formed by a gentamicin-resistant strain of methicillin-resistant S. aureus. This in vivo study demonstrates the potential of EDTA as an efficient antibiotic adjuvant to eradicate catheter-associated biofilms of major bacterial pathogens and thus provides a promising new lock solution.
INTRODUCTION
Central venous catheters are routinely used to administer medication or fluids to patients admitted to oncology, nephrology, and intensive care units (2, 47, 52). Although these devices greatly improve patient health, their use is often associated with medical complications due to colonization by pathogenic microorganisms (38). This leads to development of complex bacterial or fungal biofilm communities that display strong tolerance toward antimicrobials (15, 37, 45). Biofilms are difficult to eradicate; moreover, they constitute a potential source of bloodstream infections, a leading cause of health care-associated infections in critically ill patients (29). Currently, there is no fully efficient method for treating catheter-related biofilms aside from traumatic and costly removal of colonized devices (5, 11, 42, 49). However, recent clinical practice guidelines recommended the use of antibiotic lock therapy (ALT) for treatment of uncomplicated long-term catheter-related infections (31). ALT relies on the instillation of highly concentrated antibiotic solutions (up to 1,000 times the MIC) left to dwell in the catheter for 12 to 24 h in order to prevent or eradicate biofilm formation. Although ALT shows a high success rate for coagulase-negative staphylococci and Gram-negative bacterial catheter-related infections (14, 18, 21), catheter removal is still recommended for pathogens such as Staphylococcus aureus and Pseudomonas aeruginosa due to a lack of an efficient antibiotic lock or frequent hematogenous complications (20, 31).
Growing concern over drug-resistant pathogens (32), combined with increasing use of central venous catheters, has led to evaluation of novel lock solutions. While some studies showed that combinations of different antibiotics are more efficient than single-antibiotic lock solutions (1, 9, 34), the efficacy of nonantibiotic compounds is currently being investigated to improve lock solutions and reduce the use of antibiotics.
The anticoagulant heparin is the most widely used ALT adjuvant for reducing catheter colonization and related infection (7, 30). However, heparin activity is also reported to be impaired in gentamicin solutions (16, 48). Other compounds having both anticoagulant and chelating properties, such as sodium citrate and EDTA, have been proposed as antibiofilm ALT adjuvants (36, 44, 51). For instance, association of EDTA and gentamicin demonstrated a potent activity against in vitro biofilms formed by S. aureus, Staphylococcus epidermidis, and P. aeruginosa (6). In addition, preventive EDTA-minocycline locks were shown to reduce the incidence of long-term catheter-related infections in clinical studies (4, 6, 35, 39).
However, despite encouraging results in preventive approaches, only limited in vivo data have validated the use of these nonantibiotic ALT adjuvants. In this study, we evaluated the curative efficacy of a tetrasodium EDTA-gentamicin ALT solution against bacterial biofilms formed in an in vivo model of totally implantable venous access ports (TIVAP) (9). While gentamicin alone, EDTA alone, and a 70% ethanol lock solution were not completely effective against TIVAP-associated biofilms, we showed that single-dose treatment with a gentamicin-EDTA lock solution fully eradicated both Gram-positive and Gram-negative bacterial catheter biofilms. Novel antibiofilm strategies are urgently needed to improve treatment of catheter-related infections and patient outcome with reduced length of antibiotic exposure. Gentamicin-EDTA may lead to salvaging colonized catheters over a short duration, thus directly impacting length of hospital stay, morbidity, and health care costs.
MATERIALS AND METHODS
Bacterial strains.
Luminescent variants of four clinically relevant pathogens, i.e., S. aureus, S. epidermidis, P. aeruginosa, and Escherichia coli, were either purchased (methicillin-susceptible S. aureus [MSSA] Xen36, methicillin-resistant S. aureus [MRSA] Xen31, and S. epidermidis Xen43 from Caliper) or donated (P. aeruginosa Lm1, a bioluminescent derivative of the PAK clinical strain [40], and E. coli EAEC 55989 transformed with stable plasmid pAT881 [19]). S. epidermidis Xen43 is derived from S. epidermidis 1457 (53), a methicillin-susceptible strain (28). S. aureus Xen36 and Xen31 and S. epidermidis Xen43 were cultured in tryptic soy broth (TSB) supplemented with 0.25% glucose, while E. coli and P. aeruginosa strains were grown in lysogeny broth (LB) at 37°C.
Determination of in vitro MICs.
MICs were determined by broth microdilution per CLSI guidelines (10). Briefly, exponentially growing bacteria were diluted to a final inoculum of 5 × 105 bacteria/ml. Results were read after 16 to 18 h of culture. The MIC was defined as the result for the first well without visible growth. MIC determination was performed in TSB glucose at 0.25% for S. aureus and S. epidermidis and in LB for E. coli and P. aeruginosa (Table 1).
Table 1.
MIC and concentrations used for in vivo ALTa
| Microorganism | MIC |
ALT concn |
|||||
|---|---|---|---|---|---|---|---|
| Gentamicin (μg/ml) | Vancomycin (μg/ml) | EDTA (mg/ml) | Ethanol (%) | Gentamicin (μg/ml) | EDTA (mg/ml) | Ethanol (%) | |
| MSSA | 8 | 1.5 | 0.94 | 6.25 | 5,000 | 30 | 70 |
| MRSA | >1,024 | 1.5 | 0.94 | 12.5 | 5,000 | 30 | ND |
| S. epidermidis | 3 | 3 | 0.94 | 12.5 | 5,000 | 30 | ND |
| E. coli | 8 | ND | 3.75 | 12.5 | 5,000 | 30 | ND |
| P. aeruginosa | 4 | ND | 3.75 | 6.25 | 5,000 | 30 | ND |
ALT, antibiotic lock therapy; MSSA, methicillin-susceptible S. aureus; MRSA, methicillin-resistant S. aureus; ND, not done. MIC results are expressed as means of results of at least 3 experiments.
Antibacterial agents.
Gentamicin sulfate, vancomycin hydrochloride, EDTA-tetrasodium salt, and ethanol (70%) were purchased from Sigma-Aldrich, Inc. Distilled water was purchased from Gibco for preparing antibacterial solutions.
Animal model.
Male CD/SD (IGS:Crl) rats purchased from Charles River weighed 275 to 300 g and were allowed to acclimatize using 12-h day/night cycles for 1 week before use at the Institut Pasteur animal facilities accredited by the French Ministry of Agriculture to perform experiments on live rodents (accreditation number A75-15 27, issued on 12 November 2004, and number A75-15 04, issued on 22 May 2008), in compliance with French and European regulations on the care and protection of laboratory animals (EC Directive 86/609, French Law 2001-486, issued on 6 June 2001). Protocols were approved by the veterinary staff of the Institut Pasteur animal facility and were performed in compliance with NIH Animal Welfare Insurance number A5476-01, issued on 2 July 2007.
Catheter placement.
TIVAP implantation was performed as described previously (9). Briefly, surgical placement of TIVAP in anesthetized rats was carried out as follows: the port was implanted at the dorsal midline toward the lower end of the thoracic vertebrae by creating a subcutaneous pocket. The catheter was tunneled subcutaneously into the ventral side in the clavicle region, inserted into the jugular vein by a microincision, and progressively inserted into the superior vena cava up to the right atrium. Patency of TIVAP was maintained by flushing with 1× sterile phosphate-buffered saline (PBS) followed by a heparin lock (500 IU/ml) every day. Prior to inoculation of clinical strains, all rats were checked for the absence of infection by plating 100 μl blood, as well as monitoring for the absence of any luminescence signals.
Inoculation of TIVAP in immunocompetent rats.
A previously optimized (9) inoculum dose of 106 cells of S. aureus MSSA Xen36, 108 cells of S. aureus MRSA Xen31 or S. epidermidis Xen43, 104 cells of E. coli pAT881, or 106 cells of P. aeruginosa in 100 μl 1× PBS was injected through a silicone septum into the port using a Huber needle. Overnight cultures were diluted in 1× PBS to the optimized inoculum dose. The inoculum size was also confirmed by plating it for CFU/ml on respective antibiotic plates. Control rats received 1× PBS. Colonization of the TIVAP was monitored using the IVIS-100 imaging system (Xenogen Corporation, Alameda, CA).
Immune suppression and infection in catheterized rats.
The immune system of the rats was suppressed using cyclophosphamide (Sigma-Aldrich catalog no. C0768-5G). The optimized dose and regimen of cyclophosphamide delivery, determined by estimating total blood leukocyte count using the animal blood cell counter Vet ABC (SCIL, Germany), was used as described previously (9). A cyclophosphamide dose of 100 mg/kg of body weight was finally selected for giving intraperitoneal injections to rats on day −4 of inoculation. The inoculum dose of 102 MSSA Xen36 cells/100 μl 1× PBS was used for TIVAP inoculation and confirmed by plating for CFU/ml. Control catheterized and immunosuppressed rats received 100 μl 1× PBS only. Prior to inoculation of clinical strains, all rats were checked for the absence of infection, as for immunocompetent rats.
Extraction and quantification of viable bacteria from the biofilm.
TIVAP were carefully wiped with 70% ethanol before extracting intraluminal biofilm bacteria to avoid contaminants. The catheter was cut into small pieces, and a slit was made horizontally to expose the lumen; the port was next transferred to a tube containing 0.5 ml sterile 1× phosphate-buffered saline (PBS). The septum was removed from the port using a sterile scalpel and forceps, cut into small pieces, and transferred to a separate tube containing 0.5 ml sterile 1× PBS. Cells attached to the titanium body of the port were scratched in 100 μl 1× PBS and transferred to the same tube as the septum. Biofilm that formed on the septum and in the lumen of the catheter was extracted by vigorously vortexing the tubes for 1 min, followed by transfer to an ultrasonic water bath (44 to 48 kHz; NEYtech Ultrasonik) for 5 min. Bacterial suspensions from the tubes were then mixed to analyze total CFU/ml/TIVAP. The bacterial suspension was then serially diluted, plated on agar plates, and incubated at 37°C for colony counts.
In vivo ALT.
We had previously shown that treatment of TIVAP colonized by biofilm with ALT alone can lead to systemic infection, thereby causing death of the animals (9). Thus, in this study, ALT was always used in conjunction with systemic vancomycin (50 mg/kg, for MSSA, MRSA, and S. epidermidis) or gentamicin (30 mg/kg, for Gram-negative bacteria) subcutaneous injections (50, 55). The efficacy of gentamicin (5 mg/ml), EDTA (30 mg/ml), ethanol (70%), and gentamicin-EDTA (5 mg/ml to 30 mg/ml) lock therapy was evaluated. All lock solutions were prepared in sterile distilled water. The 3-day-old biofilm formed inside the implanted TIVAP was locked with 200 μl of the above-mentioned antibiotics following 2 types of regimen. First was a 5-day ALT regimen during which the old lock was replaced by a new one every 24 h for 5 days in conjunction with systemic treatment for 5 days. We also assessed a 1-day ALT regimen with a single instillation of ALT dwelling for 7 days in conjunction with 1 day of systemic treatment. We monitored biofilm clearance by luminescence imaging, and rats were sacrificed after day 7 of the last ALT instillation for estimating viable cell counts and electron microscopy analyses. Immunosuppressed rats were sacrificed on day 3 post-ALT instillation. Rats with a colonized TIVAP but receiving PBS ALT were used as controls.
Statistical analysis.
Results for CFU are means ± standard deviations. Statistical differences were evaluated using one-way analysis of variance (ANOVA) (Tukey multiple-comparison test), included in Graphpad Prism version 5.0c. The treatment groups were considered statistically different if P values were lower than 0.05.
RESULTS
Gentamicin-EDTA efficacy against S. aureus in vivo catheter-associated biofilms.
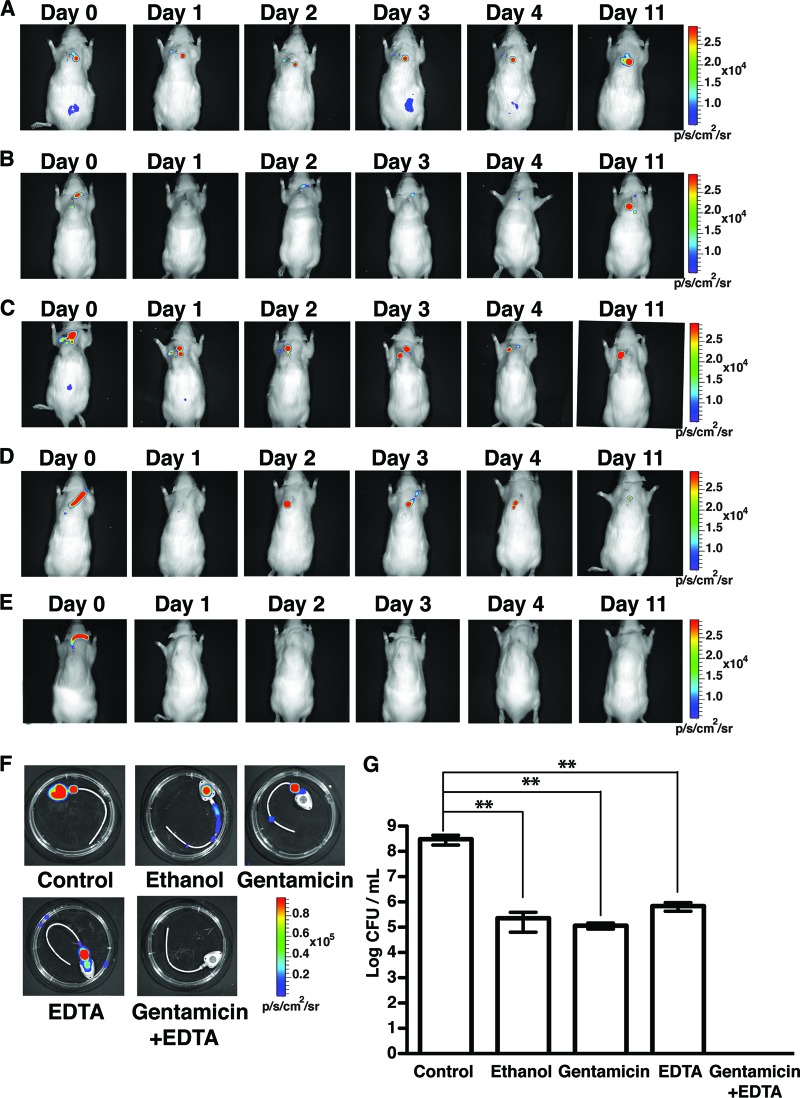
To evaluate the in vivo efficacy of tetrasodium EDTA as a potential adjuvant in ALT, we tested different gentamicin-based lock solutions against bioluminescent methicillin-susceptible S. aureus (MSSA) biofilms growing in TIVAP implanted in rats (n = 5 rats for each treatment). In addition to solutions containing gentamicin alone, EDTA alone, or combined gentamicin-EDTA, we evaluated 70% ethanol, which was shown to be an effective antibacterial lock agent both in vitro and in vivo (12, 24). We first used ALT instillations renewed every 24 h for 5 days in conjunction with systemic vancomycin injections (see Materials and Methods), and we monitored in vivo bacterial clearance as a function of luminescence. While luminescence could still be detected in rats with PBS ALT, ethanol, gentamicin, or EDTA alone, no signal was captured from rats treated with the gentamicin-EDTA lock (Fig. 1A to F). These results were confirmed by the bacterial count. While a high load of MSSA (8.6 log CFU/ml) was recovered from TIVAP of rats with PBS ALT (Fig. 1G), gentamicin or EDTA ALT alone reduced MSSA CFU recovered from TIVAP biofilms to 4.3 to 5.3 log CFU/ml and 4.2 to 6.1 log CFU/ml, respectively (Fig. 1G). Similarly, 70% ethanol ALT could clear biofilm in only 1 of 5 rats; in the remaining 4 rats, 4.2 to 5.9 log CFU/ml were still recovered from TIVAP 7 days post-ALT treatment (Fig. 1G). Although gentamicin or EDTA alone could not completely eradicate MSSA biofilm, the absence of viable cell counts from TIVAP 7 days post-gentamicin-EDTA ALT confirmed its antibiofilm efficacy (Fig. 1G).
Fig 1.
Gentamicin-EDTA ALT completely eradicates MSSA biofilm from the implanted TIVAP. ALT was instilled in TIVAP of immunocompetent rats (day 0) and was associated with systemic vancomycin to treat MSSA biofilm colonization (number of rats used for each treatment, 5). ALT was renewed every 24 h for 5 days, and its efficacy was monitored as photon emissions. Results from a representative animal are shown. (A) Control rats with PBS ALT. (B) 70% ethanol ALT. (C) 5 mg/ml gentamicin ALT. (D) 30 mg/ml EDTA alone. (E) Combined gentamicin (5 mg/ml) and EDTA (30 mg/ml) ALT. In panels A to E, representative experiments are shown. (F) Rats were sacrificed after 7 days of treatment, and TIVAP were harvested and monitored for photon emissions. (G) Bacterial cells from TIVAP were harvested and plated on TSB agar for counts of CFU/ml. Results for CFU are means ± standard deviations. Statistical analysis was done using one-way analysis of variance (ANOVA) with Graphpad Prism version 5.0c. A P value of <0.05 was considered significant. **, P ≤ 0.009.
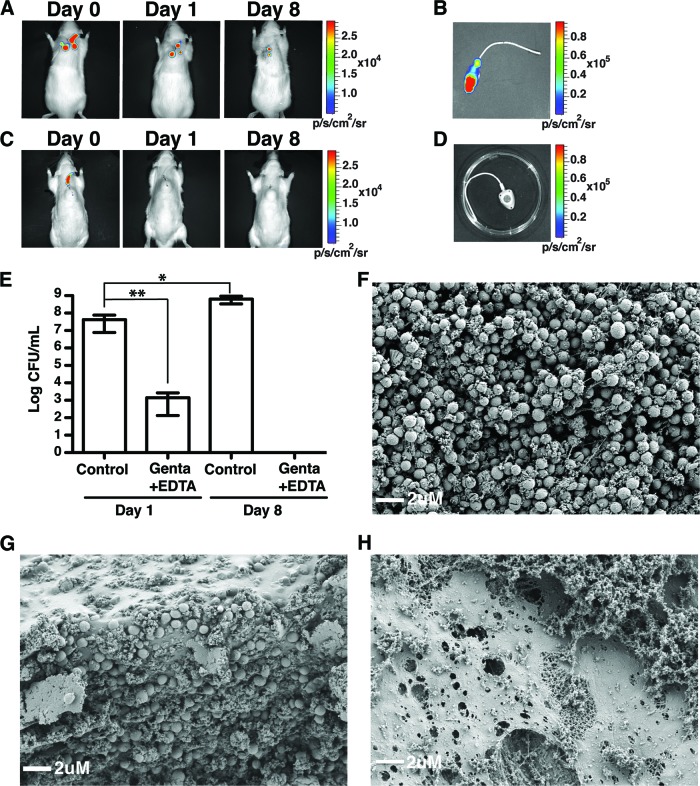
While our results demonstrated the in vivo efficacy of a 5-day regimen of gentamicin-EDTA ALT treatment against MSSA biofilms, we also sought to determine whether gentamicin-EDTA ALT would reduce the length and frequency of ALT treatment. For this, we applied a single instillation of gentamicin-EDTA lock solution in conjunction with systemic vancomycin injections on a 3-day-old in vivo MSSA biofilm (1-day regimen). After 1 day, we observed an absence of luminescence and an ∼4.2-log reduction in bacterial CFU/ml compared to that in rats with PBS ALT (day 1 in Fig. 2A, C, and E). Moreover, analysis of TIVAP 7 days after single gentamicin-EDTA ALT with catheters left to dwell for 7 days (day 8) showed complete removal of bacteria from the catheters (day 8 in Fig. 2A, C, D, and E). Although a decrease in bioluminescence was observed, rats with PBS ALT displayed a 2-log increase in bacterial colonization (Fig. 2B and E). Indeed, bioluminescence will not detect bacteria that are viable but are either dormant or growing anaerobically in TIVAP (9). Furthermore, the absence of bacteria in gentamicin-EDTA-treated TIVAP was confirmed by scanning electron microscopy. While TIVAP extracted from rats treated with either gentamicin or EDTA alone showed the presence of biofilms with a dense bacterial population (Fig. 2F and G), TIVAP from rats treated with gentamicin-EDTA ALT displayed only a meshwork of host-derived fibrin-like material and the absence of bacteria (Fig. 2H).
Fig 2.
Gentamicin-EDTA ALT reduces time to eradicate MSSA biofilm in vivo. A gentamicin-EDTA lock solution was instilled in MSSA-colonized TIVAP of immunocompetent rats (n = 3) in conjunction with systemic vancomycin treatment. Rats were sacrificed either 1 day or 7 days (day 8) after a single instillation and monitored as photon emissions. Results from a representative animal are shown. Bacterial cells were harvested from the TIVAP on day 1 or day 8 and plated for counts of CFU/ml. (A) Control rats with PBS ALT. (B) TIVAP harvested from control rats. (C) Gentamicin-EDTA-instilled rats. (D) TIVAP harvested from gentamicin-EDTA-treated rats. (E) Bacteria were harvested from TIVAP and plated on TSB agar for CFU/ml. Eradication of in vivo TIVAP-associated MSSA biofilm was confirmed by scanning electron microscopy (SEM). (F) Gentamicin-ALT-treated TIVAP. (G) EDTA-ALT-treated TIVAP. (H) Gentamicin-EDTA-ALT-treated TIVAP. Results for CFU are means ± standard deviations. Statistical analysis was done using one-way analysis of variance (ANOVA) with Graphpad Prism version 5.0c. A P value of <0.05 was considered significant. **, P ≤ 0.009; *, P ≤ 0.09.
These results demonstrated that the use of a single instillation of gentamicin-EDTA antibiotic lock solution successfully eradicated catheter-associated biofilms formed in vivo by MSSA.
One-shot gentamicin-EDTA ALT is effective in immunosuppressed animals.
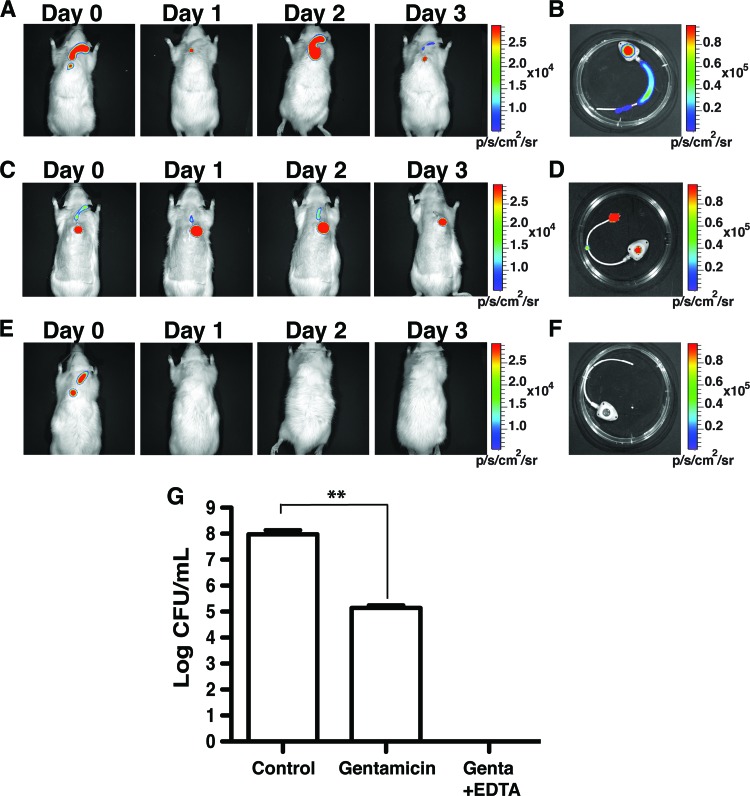
Immunosuppressed patients are highly susceptible to bloodstream infections associated with central venous catheter colonization (46). In order to evaluate whether gentamicin-EDTA ALT might also be useful in immunosuppressed hosts, TIVAP-implanted rats were treated with cyclophosphamide prior to inoculating them with 102 CFU/100 μl of MSSA in the TIVAP (number of rats [n] = 3). TIVAP-associated biofilms that developed after 3 days were treated with a single instillation of PBS, gentamicin, or gentamicin-EDTA lock in conjunction with systemic treatment. We showed that rats with PBS ALT displayed luminescent signals corresponding to ∼7.9 log CFU/ml and died by day 3 despite concomitant systemic treatment with vancomycin (Fig. 3A, B, and G). Moreover, rats (n = 3) that received only gentamicin ALT survived but continued to display ∼5.1 log CFU/ml in the lumen of TIVAP collected 3 days after ALT (Fig. 3C, D, and G). In contrast, immunosuppressed rats (n = 3) treated with a single instillation of a gentamicin-EDTA lock in conjunction with systemic treatment showed 100% survival, and complete eradication of MSSA biofilm from TIVAP was noted 3 days after treatment (Fig. 3E, F, and G). These results therefore demonstrated the in vivo efficacy of the gentamicin-EDTA lock solution in both immunocompetent and immunosuppressed animals.
Fig 3.
Gentamicin-EDTA/ALT eradicates MSSA TIVAP-associated biofilm in immunosuppressed rats. TIVAP-implanted and immunosuppressed rats (n = 3 for each treatment) were contaminated with MSSA and allowed to form biofilm for 3 days prior to ALT instillation and vancomycin systemic antibiotic injection. Treatment efficacy was monitored as photon emissions. Results from a representative animal are shown. (A) Control rats with PBS ALT. (B) TIVAP harvested from control rats. (C) Gentamicin-alone-instilled rats. (D) TIVAP harvested from gentamicin-alone-instilled rats. (E) Gentamicin-EDTA-instilled rats. (F) TIVAP harvested from gentamicin-EDTA-instilled rats. (G) Bacteria were harvested from TIVAP and plated on TSB agar for counts of CFU/ml. Genta, gentamicin. Results for CFU are means ± standard deviations. Statistical analysis was done using one-way analysis of variance (ANOVA) with Graphpad Prism version 5.0c. A P value of <0.05 was considered significant. **, P ≤ 0.003.
Efficacy of gentamicin-EDTA ALT against S. epidermidis and methicillin-resistant S. aureus.
In addition to MSSA infections, staphylococcus-associated biofilm infections may also be due to methicillin-resistant (MRSA) strains, while S. epidermidis is the most commonly reported bacterium in catheter-related infections (9, 25, 31). To test the efficacy of a single instillation of gentamicin-EDTA against S. epidermidis and MRSA, we used two clinical bioluminescent strains of S. epidermidis (Xen43) and MRSA (Xen31). These two strains are poorly luminescent in vivo and therefore did not enable us to noninvasively monitor biofilm colonization in implanted TIVAP; however, both of them led to formation of in vivo biofilm (Fig. 4 and reference 9). Although a single instillation of gentamicin-EDTA (1-day ALT regimen) eradicated S. epidermidis biofilm, ∼3 log CFU/ml bacteria could still be recovered from TIVAP-associated MRSA biofilms (number of animals, 3) (Fig. 4). However, using a 5-day ALT regimen, we demonstrated that in vivo TIVAP-associated MRSA biofilms could be successfully eradicated with gentamicin-EDTA ALT, compared with gentamicin-treated biofilms, where ∼6.2 log CFU/ml bacteria were recovered (number of animals, 3) (Fig. 4).
Fig 4.
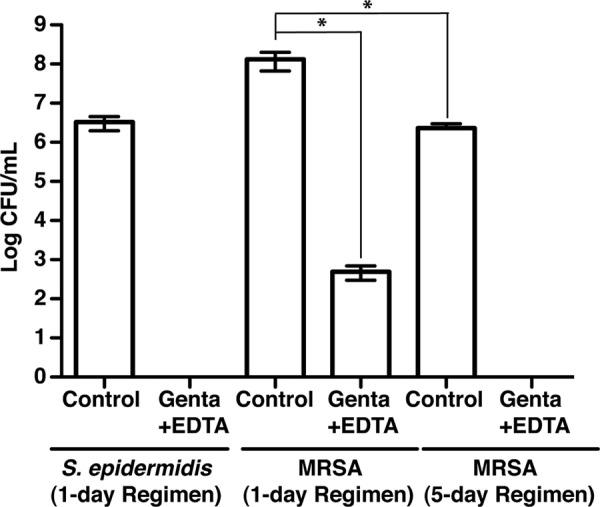
Gentamicin-EDTA ALT eradicates S. epidermidis and MRSA biofilms. Three-day-old S. epidermidis or MRSA TIVAP-associated biofilm in immunocompetent rats was treated by a 1-day or a 5-day regimen of gentamicin-EDTA ALT in conjunction with systemic vancomycin (number of animals, 3 for each treatment). Rats were sacrificed 8 days post-ALT, TIVAP was removed aseptically, and harvested cells were plated on TSB agar plates for counts of CFU/ml. Results for CFU are means ± standard deviations. Statistical analysis was done using one-way analysis of variance (ANOVA) with Graphpad Prism version 5.0c. A P value of <0.05 was considered significant. *, P ≤ 0.02.
The gentamicin-EDTA lock solution is efficient against Gram-negative bacteria.
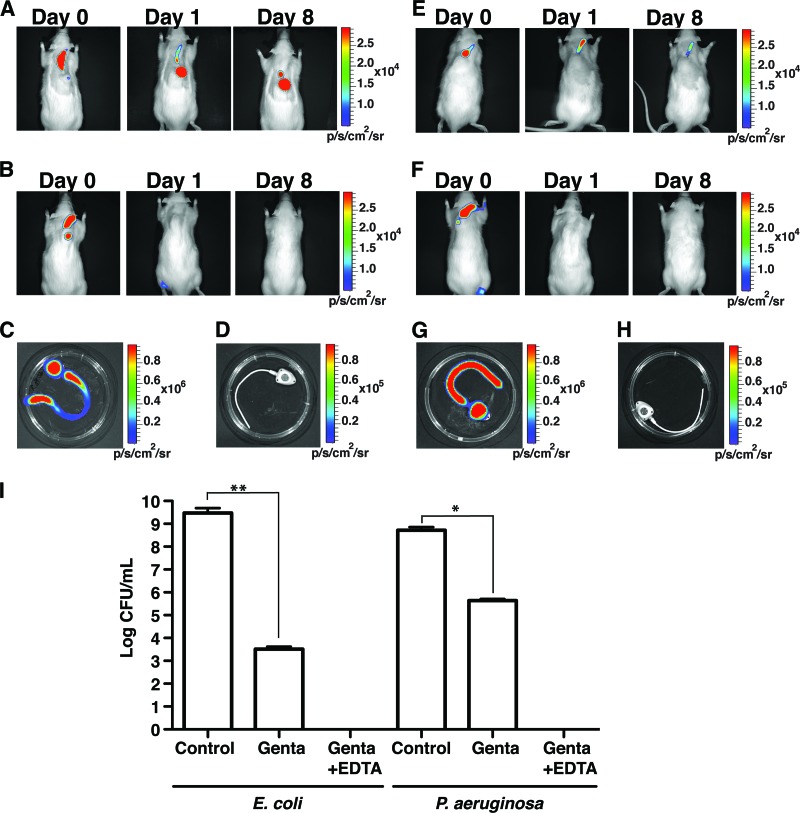
Although a high treatment success rate has been recently shown in the case of Gram-negative bacteria catheter-related bloodstream infections (21), current guidelines suggest the removal of catheters colonized by Gram-negative pathogens having a propensity for biofilm formation, such as P. aeruginosa (31). To test the potential of a combined gentamicin-EDTA lock against frequent catheter-associated Gram-negative pathogens such as E. coli and P. aeruginosa, we used a single instillation of gentamicin-EDTA ALT 3 days after inoculation with E. coli or P. aeruginosa, alongside systemic gentamicin treatment. We observed complete eradication of E. coli and P. aeruginosa TIVAP-associated biofilms using the combined gentamicin-EDTA lock solution, as indicated by the absence of luminescent signals within 24 h of treatment (Fig. 5A to D and E to H). Moreover, no bacteria were recovered from the catheters harvested after 7 days posttreatment, while control rats continued to display ∼9.5 log CFU/ml for E. coli and ∼8.7 log CFU/ml for P. aeruginosa (Fig. 5I).
Fig 5.
The gentamicin-EDTA lock solution is also effective against Gram-negative bacteria. Three-day-old E. coli or P. aeruginosa TIVAP-associated biofilm in immunocompetent rats was treated by a 1-day regimen of gentamicin-EDTA ALT in conjunction with systemic gentamicin (number of animals, 3 for each treatment). Treatment efficacy was monitored as photon emissions. Results from a representative animal are shown. (A) TIVAP-implanted control rats (with PBS ALT) with E. coli colonization. (B) E. coli-colonized TIVAP-implanted rats with gentamicin-EDTA ALT. (C) TIVAP from control rats with E. coli colonization. (D) TIVAP harvested from E. coli-colonized and gentamicin-EDTA-instilled rats. (E) TIVAP-implanted control rats (with PBS ALT) with P. aeruginosa colonization. (F) P. aeruginosa-colonized TIVAP-implanted rats with gentamicin-EDTA ALT. (G) TIVAP from control rats with P. aeruginosa colonization. (H) TIVAP harvested from P. aeruginosa-colonized and gentamicin-EDTA-instilled rats. (I) Rats were sacrificed 8 days post-ALT, TIVAP were aseptically removed, and harvested cells were plated on LB agar (E. coli or P. aeruginosa) plates for counts of CFU/ml. Genta, gentamicin. Results for CFU are means ± standard deviations. Statistical analysis was done using one-way analysis of variance (ANOVA) with Graphpad Prism version 5.0c. A P value of <0.05 was considered significant. **, P < 0.001; *, P < 0.01.
Taken together, these results demonstrate the potential of a gentamicin-EDTA combination as a broad-spectrum antibiofilm lock solution, not only against Gram-positive but also against Gram-negative catheter-associated biofilm-forming pathogens.
DISCUSSION
Significant progress has been made in clinical handling of central venous catheters, but the development of pathogenic biofilms remains a major problem with severe clinical implications (31, 38). In the case of intermittently used devices, ALT is a widely used strategy recommended for prevention or cure of intraluminal catheter-associated biofilms (31, 41). Although current ALT have significant effects on catheter handling, the use of adjuvant molecules is under investigation at present to potentiate existing antibiotic treatment against biofilms for preventing and curing catheter-related bloodstream infections (CRBSI) (31, 41).
Recent in vitro studies demonstrated that metal chelators such as EDTA and citrate, which bind to metal cations such as Ca2+, Fe3+, and Mg2+, act as both anticoagulant and antibiofilm agents and therefore enhance the antimicrobial effect of antibiotics (3, 6, 36, 54).
In the present study, using our previously optimized rat model with an implanted TIVAP, we evaluated the in vivo efficacy of the anticoagulant chelator tetrasodium EDTA, in combination with gentamicin, as a potential curative antibiotic lock solution (9). For initial evaluation of EDTA as an adjuvant to the gentamicin lock solution compared to gentamicin alone, EDTA alone, or ethanol, we chose S. aureus catheter colonization, for which catheter removal is mandatory (17, 20). We showed that the gentamicin-EDTA combination was the most effective lock solution compared to gentamicin alone, EDTA alone, or ethanol (70%). Since we previously demonstrated the risk of systemic infection when ALT was used alone, we always used systemic antibiotics alongside ALT (9). We chose systemic vancomycin, even in cases of methicillin-susceptible Staphylococcus spp., in order to compare the effects of ALT between these different strains without having a bias related to various systemic treatments. We also wanted to reproduce the first 24 or 48 h of treatment, during which the antibiotic susceptibility pattern is not determined and clinicians have to deal with Gram-positive health care-associated bloodstream infections.
Ethanol alone or in combination is reported to be effective against in vitro biofilms as well as decreasing CRBSI and the need for catheter replacement in clinical trials (22, 24, 33). However, under our experimental conditions, it was able to reduce the biofilm but could not completely eradicate it even after 5 consecutive ALT replacements. This suggests that reducing the bacterial load in the TIVAP might suffice for weakening bacterial biofilms, thus enabling the host immune system or systemic antibiotics to control CRBSI, as shown in clinical studies using ethanol. While treatments that do not completely eradicate biofilms efficiently reduce the incidence of CRBSI, therapy such as gentamicin-EDTA might totally eradicate biofilms colonizing the catheters and would thus have greater potential for curing biofilm-related infections.
The recurrence of biofilm-associated infections due to the presence of highly antibiotic-tolerant bacteria within biofilms is one of the major challenges for catheter management in the clinical setting (13, 26, 27). The existence of such highly tolerant biofilm bacteria was demonstrated in our model by the fact that they could sustain very high concentrations (up to 1,700× the MIC) of gentamicin. Gentamicin-EDTA proved to be a potential lock solution able to cure these highly tolerant biofilms and eradicate persistent bacteria, thereby preventing recurrence of Gram-positive as well as Gram-negative (see below) bacterial biofilms on TIVAP. Moreover, currently proposed ALT regimens are used for up to 14 days, resulting in reduced access to the device, which could cause distress in patients with limited venous access (31). Development of a rapid and efficient ALT would enable earlier access to the long-term catheter, improving patient outcome. We showed that a single instillation of gentamicin-EDTA ALT, left to dwell for 7 days, effectively eradicated biofilms formed by MSSA and S. epidermidis, thus probably reducing the possibility of recurrence. It is noteworthy that the MRSA strain used in this study is gentamicin resistant, like 3% of MSSA and 11% of MRSA in hospital-acquired S. aureus infections in Texas (23). Nevertheless, use of a 5-day ALT regimen led to total eradication of in vivo TIVAP-associated MRSA biofilms, suggesting that the gentamicin-EDTA lock solution could still be used in this setting. As EDTA was earlier shown to disrupt biofilm through metal chelation, we speculate that the bacteria released would be more susceptible to the direct antibacterial effect of EDTA (see Table 1) (35). Besides, free-swimming bacteria may have been killed by gentamicin that still could have some efficacy at such a high concentration.
A reduction in the catheter-associated bacterial biofilm load may not be sufficient to cure CRBSI in patients with impaired immune systems (46). In our in vivo study, a single instillation of the gentamicin-EDTA lock was effective at curing immunosuppressed rats with TIVAP-related MSSA infection, in conjunction with systemic vancomycin treatment. Rapid clearance of the pathogen is of clinical importance, especially in immunocompromised and critically ill patients. Thus, gentamicin-EDTA might, in the future, prove to be of great value in clinical settings.
Furthermore, we demonstrated the efficacy of the gentamicin-EDTA antibiotic lock solution against biofilms of E. coli and P. aeruginosa pathogenic strains. Although multiresistance associated with Gram-negative pathogens is of some concern, these bacteria are often overlooked in studies using catheter-related infections (8, 21, 43). In the case of P. aeruginosa CRBSI, treatment failures are frequent and conservative management is often excluded (31). Gentamicin-EDTA could be an excellent candidate lock for such patients, as it extends the possibility of conservative therapy.
In summary, we conclude that gentamicin-EDTA effectively eradicated the in vivo TIVAP-associated biofilms of all tested strains. Gentamicin and ethanol were also effective at reducing MSSA biofilm but were not able to completely eradicate the biofilm from implanted TIVAP. In light of these results, we believe that the gentamicin-EDTA lock deserves further exploration for use in clinical practice.
ACKNOWLEDGMENTS
We are grateful to Brigitte Arbeille and Claude Lebos (LBCME, Faculté de Médecine de Tours) for their help in performing electronic microscopy.
This work was supported by grants from the Institut Pasteur and, in part, from the Institut Mérieux-Institut Pasteur collaborative research program. D.L. was supported by a grant from the AXA Research Fund.
Footnotes
Published ahead of print 1 October 2012
REFERENCES
- 1. Ahmad NM, Rojtman AD. 2010. Successful treatment of daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus bacteremia with the addition of rifampin to daptomycin. Ann. Pharmacother. 44:918–921 [DOI] [PubMed] [Google Scholar]
- 2. Allon M. 2007. Current management of vascular access. Clin. J. Am. Soc. Nephrol. 2:786–800 [DOI] [PubMed] [Google Scholar]
- 3. Betjes MG, van Agteren M. 2004. Prevention of dialysis catheter-related sepsis with a citrate-taurolidine-containing lock solution. Nephrol. Dial. Transplant. 19:1546–1551 [DOI] [PubMed] [Google Scholar]
- 4. Bleyer AJ, Mason L, Russell G, Raad II, Sherertz RJ. 2005. A randomized, controlled trial of a new vascular catheter flush solution (minocycline-EDTA) in temporary hemodialysis access. Infect. Control Hosp. Epidemiol. 26:520–524 [DOI] [PubMed] [Google Scholar]
- 5. Blot SI, et al. 2005. Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Clin. Infect. Dis. 41:1591–1598 [DOI] [PubMed] [Google Scholar]
- 6. Bookstaver PB, Williamson JC, Tucker BK, Raad II, Sherertz RJ. 2009. Activity of novel antibiotic lock solutions in a model against isolates of catheter-related bloodstream infections. Ann. Pharmacother. 43:210–219 [DOI] [PubMed] [Google Scholar]
- 7. Carratala J, et al. 1999. Randomized, double-blind trial of an antibiotic-lock technique for prevention of gram-positive central venous catheter-related infection in neutropenic patients with cancer. Antimicrob. Agents Chemother. 43:2200–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang L, Tsai JS, Huang SJ, Shih CC. 2003. Evaluation of infectious complications of the implantable venous access system in a general oncologic population. Am. J. Infect. Control 31:34–39 [DOI] [PubMed] [Google Scholar]
- 9. Chauhan A, et al. 2012. A rat model of central venous catheter to study establishment of long-term bacterial biofilm and related acute and chronic infections. PLoS One 7:e37281 doi:10.1371/journal.pone.0037281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. CLSI 2009. Methods for dilution of antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—eighth edition. CLSI document M07-A8. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 11. Console G, et al. 2007. Clinical and economic effects of central venous catheters on oncology patient care. J. Chemother. 19:309–314 [DOI] [PubMed] [Google Scholar]
- 12. Dannenberg C, Bierbach U, Rothe A, Beer J, Korholz D. 2003. Ethanol-lock technique in the treatment of bloodstream infections in pediatric oncology patients with broviac catheter. J. Pediatr. Hematol. Oncol. 25:616–621 [DOI] [PubMed] [Google Scholar]
- 13. Darouiche RO. 2004. Treatment of infections associated with surgical implants. N. Engl. J. Med. 350:1422–1429 [DOI] [PubMed] [Google Scholar]
- 14. Del Pozo JL, et al. 2009. Effectiveness of teicoplanin versus vancomycin lock therapy in the treatment of port-related coagulase-negative staphylococci bacteraemia: a prospective case-series analysis. Int. J. Antimicrob. Agents 34:482–485 [DOI] [PubMed] [Google Scholar]
- 15. Donlan RM. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8:881–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Droste JC, Jeraj HA, MacDonald A, Farrington K. 2003. Stability and in vitro efficacy of antibiotic-heparin lock solutions potentially useful for treatment of central venous catheter-related sepsis. J. Antimicrob. Chemother. 51:849–855 [DOI] [PubMed] [Google Scholar]
- 17. Fernandez-Hidalgo N, et al. 2006. Antibiotic-lock therapy for long-term intravascular catheter-related bacteraemia: results of an open, non-comparative study. J. Antimicrob. Chemother. 57:1172–1180 [DOI] [PubMed] [Google Scholar]
- 18. Fortun J, et al. 2006. Treatment of long-term intravascular catheter-related bacteraemia with antibiotic-lock therapy. J. Antimicrob. Chemother. 58:816–821 [DOI] [PubMed] [Google Scholar]
- 19. Foucault M-L, Thomas L, Goussard S, Branchini BR, Grillot-Courvalin C. 2010. In vivo bioluminescence imaging for the study of intestinal colonization by Escherichia coli in mice. Appl. Environ. Microbiol. 76:264–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fowler VG, Jr, et al. 2005. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin. Infect. Dis. 40:695–703 [DOI] [PubMed] [Google Scholar]
- 21. Funalleras G, et al. 2011. Effectiveness of antibiotic-lock therapy for long-term catheter-related bacteremia due to Gram-negative bacilli: a prospective observational study. Clin. Infect. Dis. 53:e129–e132 [DOI] [PubMed] [Google Scholar]
- 22. Ghannoum MA, Isham N, Jacobs MR. 2011. Antimicrobial activity of B-Lock against bacterial and Candida spp. causing catheter-related bloodstream infections. Antimicrob. Agents Chemother. 55:4430–4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hulten KG, et al. 2010. Hospital-acquired Staphylococcus aureus infections at Texas Children's Hospital, 2001–2007. Infect. Control Hosp. Epidemiol. 31:183–190 [DOI] [PubMed] [Google Scholar]
- 24. Jones BA, et al. 2010. Efficacy of ethanol locks in reducing central venous catheter infections in pediatric patients with intestinal failure. J. Pediatr. Surg. 45:1287–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lafrance JP, et al. 2010. Vascular access-related bloodstream infections in First Nations, community and teaching Canadian dialysis units, and other centre-level predictors. Nephron Clin. Pract. 114:c204–c212 [DOI] [PubMed] [Google Scholar]
- 26. Lewis K. 2007. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 5:48–56 [DOI] [PubMed] [Google Scholar]
- 27. Lewis K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mack D, et al. 2002. Differential expression of methicillin resistance by different biofilm-negative Staphylococcus epidermidis transposon mutant classes. Antimicrob. Agents Chemother. 46:178–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maki DG, Kluger DM, Crnich CJ. 2006. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin. Proc. 81:1159–1171 [DOI] [PubMed] [Google Scholar]
- 30. McIntyre CW, Hulme LJ, Taal M, Fluck RJ. 2004. Locking of tunneled hemodialysis catheters with gentamicin and heparin. Kidney Int. 66:801–805 [DOI] [PubMed] [Google Scholar]
- 31. Mermel LA, et al. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 49:1–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O'Grady NP. 2002. Applying the science to the prevention of catheter-related infections. J. Crit. Care 17:114–121 [DOI] [PubMed] [Google Scholar]
- 33. Oliveira C, Nasr A, Brindle M, Wales PW. 2012. Ethanol locks to prevent catheter-related bloodstream infections in parenteral nutrition: a meta-analysis. Pediatrics 129:318–329 [DOI] [PubMed] [Google Scholar]
- 34. Parra-Ruiz J, Vidaillac C, Rose WE, Rybak MJ. 2010. Activities of high-dose daptomycin, vancomycin, and moxifloxacin alone or in combination with clarithromycin or rifampin in a novel in vitro model of Staphylococcus aureus biofilm. Antimicrob. Agents Chemother. 54:4329–4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raad II, et al. 2008. The role of chelators in preventing biofilm formation and catheter-related bloodstream infections. Curr. Opin. Infect. Dis. 21:385–392 [DOI] [PubMed] [Google Scholar]
- 36. Raad II, et al. 2008. Role of ethylene diamine tetra-acetic acid (EDTA) in catheter lock solutions: EDTA enhances the antifungal activity of amphotericin B lipid complex against Candida embedded in biofilm. Int. J. Antimicrob. Agents 32:515–518 [DOI] [PubMed] [Google Scholar]
- 37. Raad II, et al. 1994. The relationship between the thrombotic and infectious complications of central venous catheters. JAMA 271:1014–1016 [PubMed] [Google Scholar]
- 38. Raad I. 1998. Intravascular-catheter-related infections. Lancet 351:893–898 [DOI] [PubMed] [Google Scholar]
- 39. Raad I, Hanna H, Dvorak T, Chaiban G, Hachem R. 2007. Optimal antimicrobial catheter lock solution, using different combinations of minocycline, EDTA, and 25-percent ethanol, rapidly eradicates organisms embedded in biofilm. Antimicrob. Agents Chemother. 51:78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramphal R, et al. 2008. Control of Pseudomonas aeruginosa in the lung requires the recognition of either lipopolysaccharide or flagellin. J. Immunol. 181:586–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rijnders BJ, Van Wijngaerden E, Vandecasteele SJ, Stas M, Peetermans WE. 2005. Treatment of long-term intravascular catheter-related bacteraemia with antibiotic lock: randomized, placebo-controlled trial. J. Antimicrob. Chemother. 55:90–94 [DOI] [PubMed] [Google Scholar]
- 42. Scott RD. 2009. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf [Google Scholar]
- 43. Seifert H. 1997. Catheter-related infections due to gram-negative bacilli, p 255–284 In Seifert H, Jansen B, Farr BM. (ed), Catheter-related infections. Marcel Dekker, New York, NY [Google Scholar]
- 44. Shanks RM, Sargent JL, Martinez RM, Graber ML, O'Toole GA. 2006. Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrol. Dial. Transplant. 21:2247–2255 [DOI] [PubMed] [Google Scholar]
- 45. Stewart PS, Costerton JW. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138 [DOI] [PubMed] [Google Scholar]
- 46. Sydnor ER, Perl TM. 2011. Hospital epidemiology and infection control in acute-care settings. Clin. Microbiol. Rev. 24:141–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Timsit JF, et al. 2011. New challenges in the diagnosis, management, and prevention of central venous catheter-related infections. Semin. Respir. Crit. Care Med. 32:139–150 [DOI] [PubMed] [Google Scholar]
- 48. Tyler LS, Rehder TL, Davis RB. 1981. Effect of gentamicin on heparin activity. Am. J. Hosp. Pharm. 38:537–540 [PubMed] [Google Scholar]
- 49. Vandijck DM, et al. 2008. Daily cost of antimicrobial therapy in patients with intensive care unit-acquired, laboratory-confirmed bloodstream infection. Int. J. Antimicrob. Agents 31:161–165 [DOI] [PubMed] [Google Scholar]
- 50. Van Praagh AD, et al. 2011. Daptomycin antibiotic lock therapy in a rat model of staphylococcal central venous catheter biofilm infections. Antimicrob. Agents Chemother. 55:4081–4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Venkatesh M, Rong L, Raad I, Versalovic J. 2009. Novel synergistic antibiofilm combinations for salvage of infected catheters. J. Med. Microbiol. 58:936–944 [DOI] [PubMed] [Google Scholar]
- 52. Vescia S, et al. 2008. Management of venous port systems in oncology: a review of current evidence. Ann. Oncol. 19:9–15 [DOI] [PubMed] [Google Scholar]
- 53. Vuong C, Kocianova S, Yu J, Jagath Kadurugamuwa L, Otto M. 2008. Development of real-time in vivo imaging of device-related Staphylococcus epidermidis infection in mice and influence of animal immune status on susceptibility to infection. J. Infect. Dis. 198:258–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weijmer MC, et al. 2005. Randomized, clinical trial comparison of trisodium citrate 30% and heparin as catheter-locking solution in hemodialysis patients. J. Am. Soc. Nephrol. 16:2769–2777 [DOI] [PubMed] [Google Scholar]
- 55. Zuluaga AF, Agudelo M, Cardeno JJ, Rodriguez CA, Vesga O. 2010. Determination of therapeutic equivalence of generic products of gentamicin in the neutropenic mouse thigh infection model. PLoS One 5:e10744 doi:10.1371/journal.pone.0010744 [DOI] [PMC free article] [PubMed] [Google Scholar]