Abstract
Insulin secretion from pancreatic β-cells is tightly regulated by glucose and other nutrients, hormones, and neural factors. The exocytosis of insulin granules is triggered by an elevation of the cytoplasmic Ca2+ concentration ([Ca2+]i) and is further amplified by cyclic AMP (cAMP). Cyclic AMP is formed primarily in response to glucoincretin hormones and other Gs-coupled receptor agonists, but generation of the nucleotide is critical also for an optimal insulin secretory response to glucose. Nutrient and receptor stimuli trigger oscillations of the cAMP concentration in β-cells. The oscillations arise from variations in adenylyl cyclase-mediated cAMP production and phosphodiesterase-mediated degradation, processes controlled by factors like cell metabolism and [Ca2+]i. Protein kinase A and the guanine nucleotide exchange factor Epac2 mediate the actions of cAMP in β-cells and operate at multiple levels to promote exocytosis and pulsatile insulin secretion. The cAMP signaling system contains important targets for pharmacological improvement of insulin secretion in type 2 diabetes.
Keywords: Epac2, insulin secretion, oscillations, protein kinase A
Introduction
The pancreatic β-cells are adapted to respond to changes in the extracellular concentrations of glucose and other nutrients as well as hormones and neurotransmitters by releasing appropriate amounts of insulin to promote the uptake and storage of glucose in liver, muscle, and fat. Functional defects in the β-cells may lead to glucose intolerance and eventually clinically manifest diabetes mellitus. Insulin, like many other hormones, is released in pulses with a period of approximately 3–6 minutes (1-3). The pulses are important for the action of insulin on the targets, in particular the liver, probably by preventing down-regulation of the insulin receptors. The pulsatile pattern of insulin secretion arises from an endogenous rhythmicity of the individual pancreatic β-cell that involves several intracellular messengers, including ATP, Ca2+, phospholipid-derived messengers, and cyclic AMP (cAMP) (2,4,5).
There is consensus that glucose stimulates insulin secretion via its metabolism and ATP/ADP-dependent closure of ATP-sensitive K+ channels (KATP channels), which leads to membrane depolarization and opening of voltage-gated Ca2+ channels (6,7). The resulting increase of the cytoplasmic Ca2+ concentration ([Ca2+]i) triggers exocytosis of insulin secretory granules. Cyclic variations in cell metabolism and ATP/ADP ratio are thought to underlie the oscillations of [Ca2+]i and pulsatile insulin secretion (2,4,8,9). Additional signals generated by glucose metabolism are important for a proper insulin secretory response by amplifying exocytosis at steps distal to the elevation of [Ca2+]i, but their identities have not been elucidated (7).
In addition to Ca2+, which is the primary triggering signal, cAMP is the most important regulator of exocytosis in β-cells. Cyclic AMP is a ubiquitous intracellular messenger involved in the regulation of a wide range of processes in many types of cells. The first indication that the nucleotide is involved in the β-cell secretory response came from the observation that cAMP-generating glucagon promotes insulin secretion (10), and other studies soon confirmed a link between cAMP and insulin release (11–14). It is now well established that cAMP promotes secretion at multiple levels, such as by increasing electrical activity and [Ca2+]i signaling, by recruiting granules and by acting directly on the exocytosis machinery (previously reviewed in (15)). Cyclic AMP is also important for β-cell function by stimulating e.g. insulin synthesis, cell differentiation and proliferation, and by protecting the cells from apoptosis (reviewed in (16,17)).
The action of cAMP in β-cells is primarily linked to effects of certain hormones, in particular the glucoincretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which are released from intestinal L- and K-cells, respectively, to reduce postprandial glucose levels by enhancing insulin secretion. Cyclic AMP generation by receptor agonists has been reported to be required for normal glucose-responsiveness (18–20). However, as will be discussed in detail below, glucose also stimulates cAMP production in the absence of neuro-hormonal inputs. The effects of the nucleotide are mediated by protein kinase A (PKA) and exchange protein directly activated by cAMP (Epac), also known as cAMP-dependent guanine nucleotide exchange factor (Figure 1) (21). Despite the importance of cAMP in β-cell function, it is only recently that it has become possible to investigate the intracellular dynamics of the messenger. This review summarizes recent advances in our understanding of cAMP signaling dynamics in the context of insulin secretion.
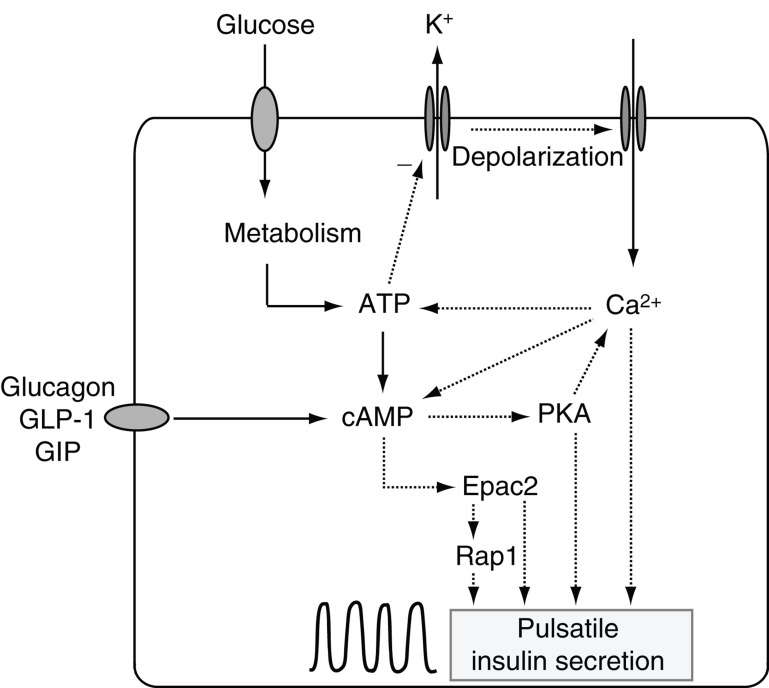
Figure 1.
Cyclic AMP signaling in insulin secretion. Schematic drawing of a β-cell and the involvement of cAMP in insulin secretion stimulated by glucose and amplified by hormones. Glucose metabolism generates ATP, which inhibits ATP-sensitive K+ channels and causes voltage-dependent Ca2+ influx. Elevation of [Ca2+]i triggers exocytotic release of insulin granules. ATP also promotes formation of cAMP, which amplifies secretion via Epac2 and protein kinase A (PKA). Activation of Gs-coupled receptors by e.g. glucagon, GLP-1, or GIP leads to cAMP formation and enhancement of insulin release. Cyclic variations in metabolism, [Ca2+]i and cAMP concentration caused by incompletely understood feedback circuits result in pulsatile insulin secretion.
Cyclic AMP generation by adenylyl cyclases
Cyclic AMP is formed exclusively from ATP via adenylyl cyclases (ACs). The classical pathway for cAMP generation involves activation of transmembrane ACs by Gs-coupled receptors. There are nine isoforms of transmembrane ACs with different regulatory properties (22). Most, if not all, of these are expressed in pancreatic islets and insulinoma cells (23–25). Early studies identified a close link between cAMP and Ca2+ (26–28), and particular attention has therefore been paid to AC isoforms regulated by this ion. The activities of AC1 and AC8 are stimulated by Ca2+ or Ca2+/calmodulin, and, despite relatively low expression, AC8 is functionally important by integrating G-protein and Ca2+ signals in β-cells (25). Recent studies also provided evidence that AC8 is required for GLP-1 generation of [Ca2+]i signals (29). AC8 is preferentially found in raft-like domains of the plasma membrane where it interacts with the A-kinase anchoring protein AKAP79/150 and is regulated by store-operated Ca2+ entry (22,30–32). Although β-cells indeed exhibit store-operated Ca2+ influx, it is quantitatively minor compared to voltage-dependent Ca2+ entry (33), and its importance for AC regulation in β-cells is uncertain.
The more abundantly expressed AC5 and AC6 isoforms are inhibited by Ca2+ and by PKA-mediated phosphorylation. Little is known about their functional importance, but the regulatory properties indicate involvement in feedback inhibition of cAMP formation. Several ACs isoforms are regulated by protein kinase C, and there is evidence for a stimulatory effect of the kinase on AC activity in mouse islets (34–36). Recent data indicate that β-cell AC activity can be directly regulated by cell metabolism, probably via ATP (37). Apart from the nine transmembrane ACs there is a structurally distinct soluble AC (sAC) which at least is expressed in INS-1 cells (29,38) and which has been implicated in glucose-induced cAMP generation (38). In being regulated by Ca2+, HCO3 -, and physiological concentrations of ATP (39–41), sAC seems well suited as a metabolic sensor, but functional evaluation of sAC is complicated by side-effects of the commonly used inhibitor KH7 (38,42). The importance of sAC for β-cell function therefore remains to be clarified.
Cyclic AMP degradation by phosphodiesterases
The intracellular cAMP level is determined by a balance between cAMP production by ACs and degradation by cyclic nucleotide phosphodiesterases (PDEs). The PDEs constitute a large family of enzymes which catalyze the hydrolysis of cAMP and/or cGMP to 5′-AMP and 5′-GMP. There are 11 sub-families with >50 isoforms differing in structure, regulation, and substrate preferences (43). The role of PDEs in islets has previously been reviewed (44,45). The PDE1, PDE3, and PDE4 families are generally regarded as most important for cAMP regulation in islet cells. Pancreatic islets were early found to have Ca2+/calmodulin-sensitive PDE activity (46–48), and later studies have identified PDE1C as a prominent isoform (49–51). Pharmacological inhibition or genetic down-regulation of PDE1 thus enhances glucose-stimulated insulin secretion both from insulinoma cells and pancreatic islets (49,51).
PDE3 is a membrane-associated dual-specificity isoform degrading both cAMP and cGMP with kinetic properties that result in cGMP-inhibition of cAMP degradation. PDE3B is expressed in β-cells and is probably quantitatively the most important PDE in islets, constituting up to 70% of the total PDE activity in some studies (45,52). The enzyme is activated by glucose, insulin, and cAMP via changes in protein kinase A- and B-mediated phosphorylation (53). PDE3B is a major regulator of cAMP at sites important for insulin secretion. Overexpression of PDE3B in β-cells or insulinoma cells consequently reduces insulin secretion (54,55), whereas genetic down-regulation or pharmacological inhibition of the enzyme amplifies secretion (51,52,56,57), probably by regulating the most distal steps of granule fusion (58). Moreover, IGF-1-induced attenuation of insulin secretion is mediated by activation of PDE3B (56).
PDE4 is present in islets and insulin-secreting cells (51,52,59), but studies with inhibitors have yielded conflicting results. While the PDE4 family-selective inhibitor rolipram lacked effect on glucose-induced insulin secretion from islets (52,59), secretion was enhanced in both INS-1 cells and rat islets after selective pharmacological inhibition of the enzyme with roflumilast or L-826,141 and by siRNA-mediated knock-down of PDE4C (51).
Recent studies have also identified members of the PDE7, PDE8, PDE10, and PDE11 families in rodent and human islets and insulin-secreting cell lines (50,51,53). These PDE isoforms probably constitute a relatively small fraction of the total PDE activity in β-cells but may nevertheless play important functional roles. For example, pharmacological inhibition of PDE10A (60) and knock-down of PDE8B (50) potentiate insulin secretion from rat islets, and the latter isoform was recently implicated in cAMP oscillations and pulsatile insulin secretion from MIN6 cells (61).
Cyclic AMP signaling triggered by neuro-hormonal stimuli
Several Gs-coupled receptor agonists, including glucagon, GLP-1, GIP, pituitary adenylyl cyclase-activating polypeptide (PACAP), and ACTH, are known to enhance glucose-stimulated insulin secretion, effects which correlate with their ability to increase cAMP in β-cells (62–64). On the contrary, Gi-coupled agonists like adrenaline, noradrenaline, somatostatin, galanin, ghrelin, and melatonin suppress insulin secretion, in part by reducing cAMP (65–68).
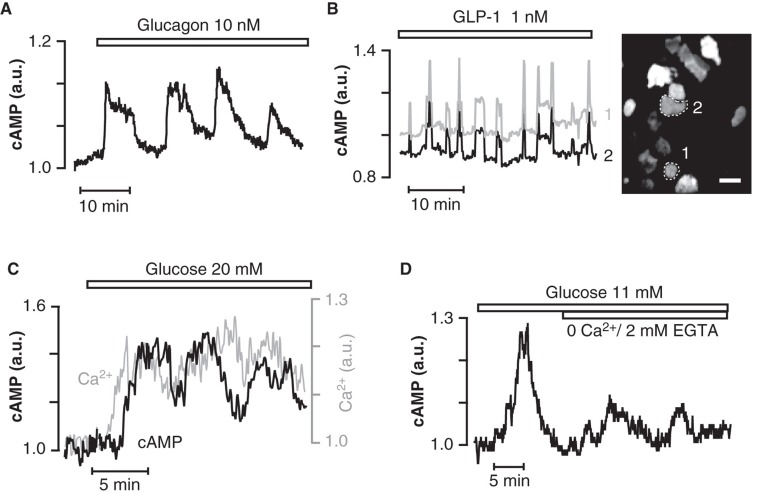
Measurements of single-cell cAMP dynamics beneath the plasma membrane have revealed that the β-cell cAMP response to glucagon and GLP-1 is oscillatory in both rat insulinoma cells (69) and primary mouse β-cells within intact islets (Figure 2) (42). Higher GLP-1 concentrations increase the time-average cAMP by prolonging the periods of cAMP elevation until the oscillations are replaced by stable elevation. The GLP-1-induced cAMP oscillations in insulinoma cells are synchronized with oscillations of [Ca2+]i and abolished upon removal of the ion from the extracellular medium, consistent with a close connection between the two messengers (69). Such co-ordination of the triggering [Ca2+]i and amplifying cAMP signals, which provides distinct stimulation of exocytosis, has been reproduced in modeling studies (70,71). However, elevated [Ca2+]i is not necessary for the cAMP response to Gs-coupled receptor agonists, since both glucagon and GLP-1 can trigger cAMP oscillations in mouse islets at sub-stimulatory glucose concentrations (42). The cAMP oscillations are synchronized among different β-cells within the islet, reinforcing the idea that β-cells are functionally coupled (72,73).
Figure 2.
Cyclic AMP oscillations in hormone- and glucose-stimulated β-cells. Total internal reflection fluorescence (TIRF) microscopy recordings of the sub-membrane cAMP concentration in mouse β-cells within intact pancreatic islets. A, B: Cyclic AMP oscillations evoked by 10 nM glucagon and 1 nM GLP-1 in β-cells exposed to 3 mM glucose. Oscillations are synchronized among different β-cells within the islet as illustrated by graphs from the numbered cells in the TIRF image (B). C, D: Elevation of the glucose concentration from 3 to 11 or 20 mM evokes co-ordinated oscillations of cAMP and Ca2+ beneath the plasma membrane. The cAMP oscillations are amplified by Ca2+ but are maintained also when Ca2+ entry is prevented (D).
Glucose-induced cAMP signaling
Glucose has long been recognized to increase the cAMP content of pancreatic β-cells (26,74–76), an effect regarded to be secondary to elevation of [Ca2+]i (26–28). Since the magnitude was modest and cAMP alone was unable to stimulate secretion, the interest for cAMP as a messenger in glucose-stimulated insulin secretion declined. From experiments demonstrating that purified β-cells have lower cAMP content, glucose-induced cAMP formation, and insulin secretion than intact islets and that the cAMP content and insulin secretion are restored by addition of glucagon or glucagon-releasing α-cells, it was suggested that cAMP has a permissive role in insulin secretion and that the main effect of glucose is to amplify cAMP formation by glucagon (77,78).
Glucose has indeed been found to amplify hormone-induced elevations of cAMP, an effect attributed to the elevation of [Ca2+]i (25). When it became possible to measure cAMP dynamics at the single-cell level it was shown that glucose also induces pronounced increases of cAMP in both clonal β-cells (37,79) and isolated primary mouse β-cells (37) devoid of paracrine influences. Landa et al. (79) observed that the glucose effect is strictly Ca2+-dependent and mimicked by depolarizing agents. However, when [Ca2+]i oscillations are evoked by a combination of high glucose and tetraethylammonium the very pronounced peaks of [Ca2+]i coincide with nadirs of cAMP, probably reflecting activation of Ca2+-sensitive PDEs (79,80). Measurements of cAMP in the sub-plasmamembrane space showed that glucose not only increases the cAMP levels, but that the cAMP concentration often oscillates in synchrony with [Ca2+]i with a periodicity of 2–10 minutes (Figure 2) (37). In intact islets of Langerhans these oscillatory responses become synchronized among neighboring β-cells (42), and the co-ordinated cAMP and Ca2+ signals are critical for generating pulsatile insulin secretion. The glucose-induced cAMP elevation is amplified by Ca2+, but low-amplitude oscillations remain also after removal of extracellular Ca2+ or inhibition of voltage-dependent Ca2+ influx (37,42). Glucose also triggers cAMP elevation and often with oscillations under conditions when [Ca2+]i is clamped by high K+ in the presence of the KATP channel-opener diazoxide, and a similar effect is observed with the mitochondrial substrate α-ketoisokaproic acid (37). Glucose-induced elevation of cAMP independent of Ca2+ has also been reported in β-cells from mice transgenically expressing a FRET-based cAMP indicator (81). Together, these data provide strong evidence that cell metabolism is a potent stimulator of cAMP production.
The mechanisms by which metabolism stimulates cAMP formation are unknown. Since cAMP is formed from ATP it seems likely that its concentration directly regulates AC activity. In support for this idea, lowering of sub-membrane ATP consumption by Na+/K+-ATPase inhibition was found to trigger cAMP elevation, and ATP stimulates cAMP formation in permeabilized MIN6 β-cells (37). A problem with the hypothesis is that the in vitro-Km for ATP of the islet ACs is ∼0.3 mM (82), which is an order of magnitude below the ATP concentration believed to prevail in the cytoplasm. On the other hand, affinities in vitro may not properly reflect the ATP dependence in living cells. The soluble AC has a higher Km for ATP (40), and experiments in INS-1 cells have indicated that glucose-induced cAMP production might be mediated by sAC (38). However, in both MIN6 and mouse β-cells the glucose-induced rise of cAMP is completely suppressed by a selective inhibitor of transmembrane ACs. The sAC inhibitor KH7 abolished both cAMP and [Ca2+]i elevations, but this effect could be ascribed to an inhibitory effect on glucose oxidation unrelated to cAMP (42). Further work is required to clarify the mechanisms underlying the stimulation of cAMP production by cell metabolism. Available data obviously cannot exclude that ATP also may have indirect effects.
The cAMP oscillations are driven by variations in AC rather than PDE activity. Partial inhibition of PDEs with an intermediate concentration of IBMX thus induces cAMP oscillations in the presence of a sub-stimulatory glucose concentration, indicating that variations in the rate of cAMP production under basal conditions are balanced by degradation of PDEs (61). Variations in the rate of cAMP degradation do not seem to drive cAMP oscillations since they are prevented by an AC inhibitor. PDEs are obviously crucial for lowering cAMP levels during each oscillation cycle, but no isoform alone is responsible for this effect. Use of PDE-selective pharmacological inhibitors identified PDE3 and PDE1 as most important for shaping glucose-induced cAMP oscillations in clonal MIN6 and primary mouse β-cells. In addition, siRNA-mediated knock-down of the IBMX-insensitive PDE8B in MIN6 cells was found to perturb both cAMP oscillations and pulsatile insulin secretion (61).
Does cAMP account for the metabolic amplification of glucose-induced insulin secretion? The observations that glucose metabolism promotes cAMP accumulation (37,81) and that ATP can stimulate exocytosis at distal steps in a PKA-dependent fashion (83) are consistent with such an action of cAMP. On the other hand, with the observations that PKA is not involved in the amplifying pathway, that the correlation between cAMP and insulin secretion is sometimes poor, and that cAMP is ineffective in enhancing Ca2+-dependent secretion in the absence of glucose, it has been concluded that cAMP is not the main metabolic amplification signal (84–86). However, the studies have not taken into account that conventional measurements of average cAMP will underestimate the levels reached during the peaks of oscillations, in particular if the changes primarily occur in a specific sub-compartment. Moreover, these studies are typically based on insulin secretion evoked by high concentrations of K+, which may involve a different pool of granules than that induced by glucose (87). Further studies seem required to clarify if cAMP is or contributes to the metabolic amplifying signal or whether the two pathways are distinct and operate in parallel.
Role of PKA in insulin secretion
PKA is a major effector of cAMP in β-cells, and the kinase is involved in mediating the stimulatory effects of the incretin hormones and other cAMP-elevating agents on insulin secretion. Many proteins have been identified as targets for PKA phosphorylation (reviewed in (15,88)). Anchoring of the kinase to specific sub-cellular localizations via A-kinase anchoring proteins is important for its actions on insulin secretion (89–93). PKA is highly dynamic, and cAMP oscillations have been found to be directly translated into oscillations of enzyme activity (80). The oscillations may contribute to keep signaling locally restricted. This idea is supported by the observation that brief elevations of cAMP do not provide sufficient time for the PKA catalytic subunits to diffuse through the nuclear pores and enter the nucleus, which requires prolonged cAMP elevations (69,80,94).
Cyclic AMP has long been known to promote β-cell electrical activity and Ca2+ signaling (95–97). The enhancement of [Ca2+]i signals involves both voltage-dependent entry and intracellular mobilization (98–101) and can largely be explained by PKA phosphorylation of voltage-gated channels (102,103), KATP channels (18,104), and IP3 receptors (101,105). Effects of GLP-1 on intracellular Ca2+ stores have also been suggested to involve the Ca2+-mobilizing messengers cyclic ADP ribose and nicotinic acid adenine dinucleotide phosphate (106) and Ca2+-induced Ca2+ release via ryanodine receptors (107). These mechanisms were reported to involve both PKA and Epac.
Cyclic AMP also stimulates exocytosis by actions distal to the elevation of Ca2+ (102,108–110). PKA is involved in sensitizing the secretory machinery to Ca2+ (111). PKA also increases secretory vesicle mobility and accounts for replenishment of the readily releasable granule pool (112–114), in particular by increasing the number of granules which are highly sensitive to Ca2+ (115,116).
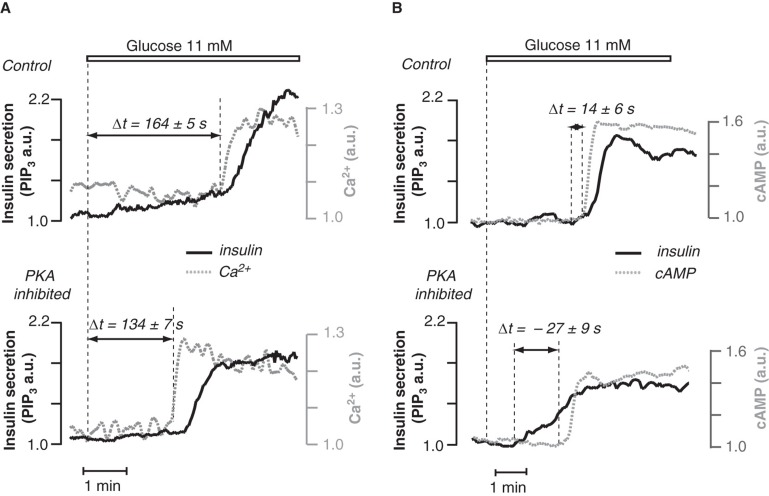
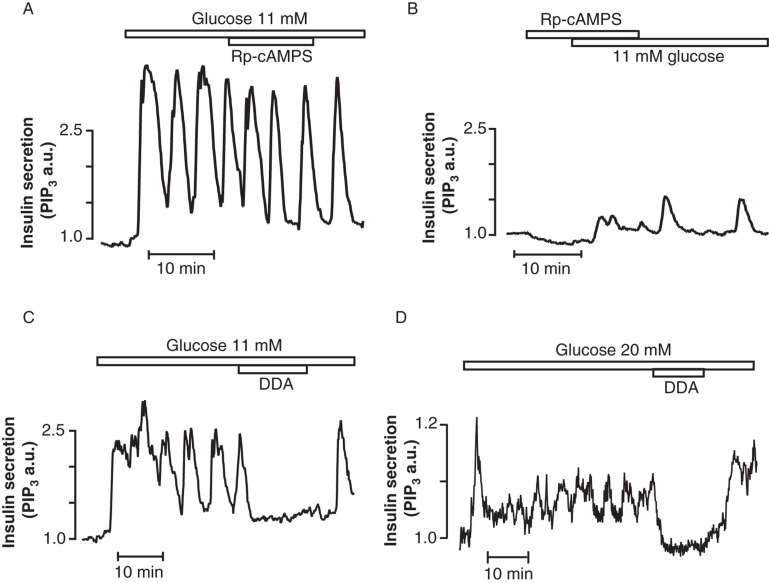
Despite the undisputed importance of PKA in mediating cAMP signals on exocytosis, inhibitors of PKA have surprisingly small effects on glucose-stimulated insulin secretion from rat islets (117,118). The explanation may be that PKA is primarily important during initiation of insulin secretion, as shown by time-resolved measurements of insulin release from single β-cells using two-photon excitation imaging with polar tracers (119). A detailed analysis of cAMP action in glucose-stimulated MIN6 cells demonstrated that PKA controls the magnitude of the secretory response by affecting the co-ordination of Ca2+ and cAMP signals (Figure 3) (120). Although PKA promotes Ca2+ entry via voltage-dependent channels (102,121), inhibition of the kinase neither suppressed the glucose-induced [Ca2+]i response nor the glucose-induced cAMP elevation. Instead, inhibition of PKA accelerated glucose-induced membrane depolarization, such that the resulting [Ca2+]i elevation triggered exocytosis before the amplifying cAMP signal was manifested (120). One potential explanation for this effect is that both the channel-forming Kir6.2 and sulphonylurea receptor-1 (SUR1) subunits of the KATP channel under basal conditions are phosphorylated by PKA at sites that increase channel activity (122,123). The regulation of KATP channels by PKA is complex, and whether phosphorylation is activating or inactivating depends e.g. on the levels of intracellular ADP (124). This intricate regulation may perhaps explain the apparent paradox that GLP-1 stimulates β-cell depolarization by closing KATP channels via a PKA-dependent mechanism (18,125). PKA is thus required for establishing an initial insulin response to glucose stimulation, but PKA inhibitors lack effects on already established pulsatile insulin secretion (Figure 4) or on secretion triggered by Ca2+. The latter observations indicate that the cAMP-dependence of glucose-induced insulin secretion is mediated mainly by effectors other than PKA.
Figure 3.
Temporal relationship between glucose-induced Ca2+ and cAMP signals and insulin secretion. A: TIRF microscopy recordings of sub-membrane Ca2+ concentration (dotted curves) and the insulin secretory response (solid curves) in MIN6 β-cells show that inhibition of PKA markedly shortens the delay between glucose stimulation and the initial Ca2+ elevation triggering secretion. B: Simultaneous recordings of cAMP (dotted curve) and insulin secretion (solid curve) showing that PKA inhibition shifts the timing such that secretion, which normally follows the amplifying cAMP signal, instead precedes the cAMP elevation.
Figure 4.
Cyclic AMP dependence of glucose-induced pulsatile insulin secretion. TIRF microscopy recordings of the insulin secretory response from individual MIN6 β-cells. A: The PKA inhibitor Rp-8-CPT-cAMPS (100 µM) barely affects pulsatile insulin secretion triggered by glucose. B: In contrast, if added prior to glucose stimulation, the inhibitor markedly suppresses the subsequent secretory response. C, D: Glucose-induced pulsatile insulin secretion critically depends on cAMP generation as 50 µM of the AC inhibitor dideoxyadenosine (DDA) inhibits secretion in both MIN6 (C), and primary mouse pancreatic β-cells (D).
Role of Epac in insulin secretion
While PKA was long regarded as the only cAMP effector in β-cells, it was evident that some cAMP effects on exocytosis are independent of the kinase (113). It was soon discovered that the PKA-independent effects of cAMP on exocytosis are mediated by Epac (126), a guanine nucleotide exchange factor for the Rap family of small GTPases (21). The role of Epac in insulin secretion has previously been reviewed (15,127–129). There are two Epac isoforms, Epac1 and Epac2, which are expressed in pancreatic islets (130–132), but it is mainly Epac2 that has been implicated in exocytosis. Of the three splice variants of Epac2, β-cells only express the full-length version (133).
Epac-specific cyclic nucleotide analogues have been found to amplify glucose-induced insulin secretion from INS-1 cells and from mouse and human islets (131,132,134). Although the specific activator does not activate PKA, its effect on human islets is prevented by inhibitors of PKA, indicating that PKA has a permissive role for insulin secretion in human islets. Capacitance measurements have demonstrated that Epac2 accounts for the rapid cAMP-dependent potentiation of exocytosis and that PKA has slower effects (113,114). Epac has also been reported to recruit granules to the plasma membrane (87,135) and together with PKA to stimulate granule-granule fusion events (135). The small GTPase Rap1 has been found to link activation of Epac2 to stimulation of insulin secretion, probably by stimulating the recruitment of secretory granules to the membrane, but the detailed mechanism of action has not been clarified (87). One possibility is that Rap1 activates Vav2 and Tiam (87), guanine nucleotide exchange factors for the small GTPases Cdc42 and Rac, which regulate insulin secretion via modulation of the actin cytoskeleton (136,137). Another alternative is that Rap1 stimulates mobilization of intracellular Ca2+ via activation of phospholipase C-ε (138). In support of the latter idea knock-out of phospholipase C-ε has been found to disrupt Epac-selective potentiation of insulin secretion (139). It has been suggested that cAMP stimulates intracellular Ca2+ mobilization primarily via Epac activation of ryanodine receptors (140,141), but this conclusion has been questioned (105). The study by Dyachok et al. (105) is instead consistent with the idea that cAMP-stimulated Ca2+ mobilization mainly occurs via a phospholipase C–IP3-mediated mechanism.
Epac was originally found to interact with the SUR1 subunit of the KATP channel (126). This interaction may result in modification of the ATP-sensitivity of the channel (142). Interestingly, the PKA-independent component of cAMP-stimulated secretion is absent in SUR1-/- mice (114), suggesting that interaction between Epac and SUR1 is important for granule priming. Epac2 has also been found to bind to the Rab3-binding protein Rim2 (126,143,144), and this interaction is important for the stimulatory effect of incretin hormones on insulin secretion. Also the Ca2+-binding protein Piccolo, a neural active zone protein, is expressed in β-cells and interacts with Epac2 (145,146). In addition, interaction between Epac2 and the t-SNARE protein SNAP25 may be a prerequisite for the fast PKA-independent effects of cAMP on exocytosis (147). Whether these actions of Epac are mediated by Rap1 or not has not been determined.
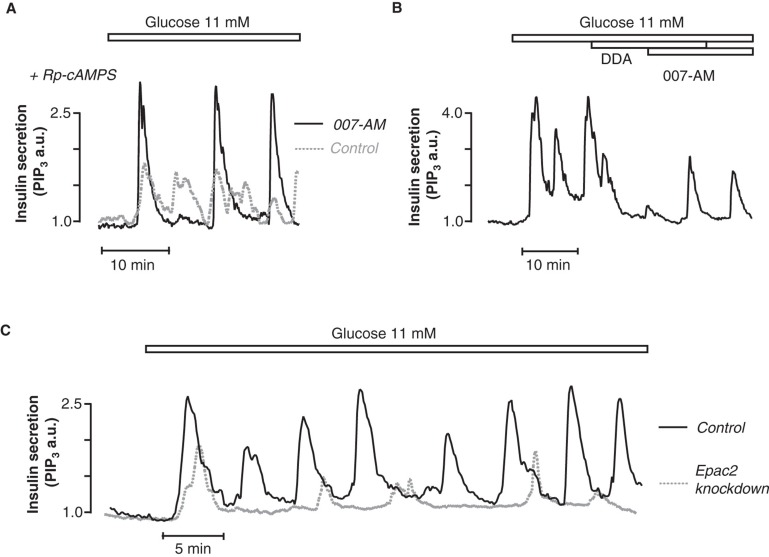
Recent observations indicate that Epac2, in addition to mediating the amplification of insulin release by incretins and other cAMP-elevating agents, is involved in glucose generation of pulsatile insulin secretion (120). Thus, studies in MIN6 cells demonstrated that an Epac-selective cAMP analogue restored not only the initial glucose-induced insulin secretion suppressed by PKA inhibition, but also subsequent pulsatile secretion perturbed by adenylyl cyclase inhibition (Figure 5). Conversely, when the expression of Epac2 was knocked down by siRNA there was a marked reduction of both the initial and subsequent pulsatile insulin secretion. These findings contrast with results from Epac2 knock-out mouse islets where the glucose response is not significantly decreased despite markedly reduced cAMP amplification of glucose-induced insulin exocytosis (87,148). This discrepancy may reflect an inherent difference between MIN6 cells and mouse islets or that compensatory mechanisms are differently activated by the knock-down and knock-out strategies. However, there is no information on secretion dynamics from the knock-out islets, and secretion was either measured in static incubation experiments (148) or estimated by imaging of single exocytosis events (87). It has been reported that Epac regulates exocytosis of small synaptic-like vesicles rather than that of insulin-containing dense-core granules (149). However, this conclusion is supported neither by granule-imaging of knock-out mouse β-cells (87) nor by studies of the autocrine effects of insulin in MIN6 cells (120).
Figure 5.
Involvement of Epac in glucose-induced pulsatile insulin secretion. TIRF microscopy recordings of the insulin secretory response from individual MIN6 β-cells. A, B: The Epac-selective cAMP analogue 8-pCPT-2’-O-Me-cAMP-AM (007-AM, 1 µM) restores the magnitude of insulin secretion initiated by glucose in the presence of the PKA inhibitor Rp-8-CPT-cAMPS (A), as well as that of established glucose-induced insulin pulses suppressed by AC inhibition with dideoxyadenosine (DDA) (B). C: Knock-down of Epac2 with siRNA reduces the magnitude of both initial and subsequent pulsatile insulin secretion in response to glucose.
The sulphonylurea class of anti-diabetic drugs, which depolarize the β-cell by inhibiting KATP channel conductance after binding to the SUR1 subunit of the channel, was recently found to directly bind and activate Epac2 (148). This observation has gained support from another study, which even identified an Epac2 mutation that abolished the sulphonylurea interaction (150), whereas other studies have failed to demonstrate a direct interaction between Epac2 and sulphonylurea (151,152). A link between sulphonylureas and Epac2 activation is supported by suppression of the insulin secretory response to sulphonylureas in Epac2-knock-out mice (148). From the available data it is not clear whether the activation of Epac2 by sulphonylureas is direct or indirect, mediated for example by an increase of cAMP. Even if no overall elevation of cAMP was detected in the study by Zhang et al. (148), sulphonylureas may interact with PDEs (153,154) and thereby increase cAMP in local sub-compartments, which might be sufficient for Epac2 activation. Future studies will establish the nature of the link between sulphonylureas and Epac2.
Cyclic AMP signaling in type 2 diabetes
Type 2 diabetes is characterized by loss of first phase and impaired second phase insulin release (155) with disappearance of the regular pulsatile secretory pattern (156). It is now broadly accepted that the disease develops as a result of β-cell dysfunction (157,158). It is not known whether cAMP generation is impaired in β-cells from patients with type 2 diabetes. However, several aberrations in diabetic subjects may be envisaged to affect β-cell cAMP handling, such as the reduced incretin effect and alterations of glucagon secretion (159,160). Single nucleotide polymorphisms that correlate with fasting blood glucose and type 2 diabetes have been identified in genes linked to cAMP signaling, including the GIP receptor, the α2 adrenergic receptor and AC5 (161–163). Alterations in β-cell cAMP signaling have been reported from several animal models of diabetes. Decreased glucose-induced cAMP generation and insulin secretion were thus found in diabetic Chinese hamsters (164), neonatal streptozotocin diabetic rats (165), and GK rats (166), and β-cell function was regained by treatment with cAMP-elevating agents (165–167). In some animal models of type 2 diabetes there are increased basal cAMP levels and exaggerated responses to AC activators, which may be linked to an increased expression of several AC isoforms (24,166,168–170) and decreased expression of PDEs (166). Altered cAMP handling has also been found after prolonged culture of β-cells in high glucose (29,171). INS-1E cells exposed to 20 mM glucose for 3 days showed reduced cAMP accumulation in response to forskolin and IBMX. Microarray analysis of gene expression showed several changes in the cAMP-signaling pathways, including a reduction of AC8, a finding confirmed also in rat and human islets (29).
Recently developed treatment strategies for type 2 diabetes are based on mechanisms that increase β-cell cAMP levels (reviewed in (172–176). The most successful approaches are based on activation of β-cell ACs via incretin hormones, but the rapid degradation of the hormones via dipeptidylpeptidase-4 (DPP4) is a problem. However, inhibitors of DPP4 increase the availability of endogenous circulating GLP-1 and GIP, and stable incretin hormone analogues as well as the GLP-1 mimetic exendin-4 have successfully been employed in diabetes treatment. The fatty acid receptor GPR119, a Gs-coupled receptor predominantly expressed in islets, has also been identified as a promising drug target (174,177). Strategies based on inhibition of PDEs seem less promising due to low tissue specificity.
Conclusions and future perspectives
Nearly half a century after the discovery of the link between cAMP and insulin secretion, cAMP signaling in β-cells is still a topic for intense research. Methodological advances in the past few years have provided novel insights into the spatio-temporal dynamics of the messenger and the regulation of its downstream effectors. It has become increasingly clear that the cAMP concentration often show complex spatio-temporal patterns that contribute to the versatility and specificity of the signaling. There are yet many unresolved questions. Future studies will clarify the detailed molecular organization of the local cAMP signaling circuits and the precise mechanisms by which PKA and Epac potentiate insulin secretion, particularly in human β-cells. Moreover, potential defects in cAMP handling of β-cells from diabetic human islet donors need to be explored. Cyclic AMP is important also for the release of other pancreatic islet hormones. Clarification of the intricate interplay between the different endocrine cell types in the islet is a prerequisite for fully understanding normal β-cell function and the pathophysiology of impaired hormone secretion in diabetes as well as for improving treatment strategies.
 Winner of the Eric K. Fernström Award for young investigators 2011 at the Medical Faculty of Uppsala University.
Winner of the Eric K. Fernström Award for young investigators 2011 at the Medical Faculty of Uppsala University.
Acknowledgements
I wish to express my gratitude to all present and past members of my laboratory as well as to colleagues within and outside the department for valuable discussions. The work in my laboratory is supported by grants from the European Foundation for the Study of Diabetes/MSD, Family Ernfors Foundation, Novo Nordisk Foundation, Swedish Diabetes Association, and the Swedish Research Council.
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- 1.Pørksen N. The in vivo regulation of pulsatile insulin secretion. Diabetologia. 2002;45:3–20. doi: 10.1007/s125-002-8240-x. [DOI] [PubMed] [Google Scholar]
- 2.Hellman B. Pulsatility of insulin release—a clinically important phenomenon. Ups J Med Sci. 2009;114:193–205. doi: 10.3109/03009730903366075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitz O, Rungby J, Edge L, Juhl CB. On high-frequency insulin oscillations. Ageing Res Rev. 2008;7:301–5. doi: 10.1016/j.arr.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Tengholm A, Gylfe E. Oscillatory control of insulin secretion. Mol Cell Endocrinol. 2009;297:58–72. doi: 10.1016/j.mce.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Gilon P, Ravier MA, Jonas JC, Henquin JC. Control mechanisms of the oscillations of insulin secretion in vitro and in vivo. Diabetes. 2002;51:S144–51. doi: 10.2337/diabetes.51.2007.s144. [DOI] [PubMed] [Google Scholar]
- 6.Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic β-cell. Prog Biophys Mol Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 7.Henquin JC. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia. 2009;52:739–51. doi: 10.1007/s00125-009-1314-y. [DOI] [PubMed] [Google Scholar]
- 8.Bertram R, Sherman A, Satin LS. Metabolic and electrical oscillations: partners in controlling pulsatile insulin secretion. Am J Physiol Endocrinol Metab. 2007;293:E890–900. doi: 10.1152/ajpendo.00359.2007. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy RT, Kauri LM, Dahlgren GM, Jung SK. Metabolic oscillations in β-cells. Diabetes. 2002;51:S152–61. doi: 10.2337/diabetes.51.2007.s152. [DOI] [PubMed] [Google Scholar]
- 10.Samols E, Marri G, Marks V. Promotion of insulin secretion by glucagon. Lancet. 1965;2:415–16. doi: 10.1016/s0140-6736(65)90761-0. [DOI] [PubMed] [Google Scholar]
- 11.Sussman KE, Vaughan GD. Insulin release after ACTH, glucagon and adenosine-3'-5'-phosphate (cyclic AMP) in the perfused isolated rat pancreas. Diabetes. 1967;16:449–54. doi: 10.2337/diab.16.7.449. [DOI] [PubMed] [Google Scholar]
- 12.Malaisse WJ, Malaisse-Lagae F, Mayhew D. A possible role for the adenylcyclase system in insulin secretion. J Clin Invest. 1967;46:1724–34. doi: 10.1172/JCI105663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turtle JR, Littleton GK, Kipnis DM. Stimulation of insulin secretion by theophylline. Nature. 1967;213:727–8. doi: 10.1038/213727a0. [DOI] [PubMed] [Google Scholar]
- 14.Turtle JR, Kipnis DM. An adrenergic receptor mechanism for the control of cyclic 3'5' adenosine monophosphate synthesis in tissues. Biochem Biophys Res Commun. 1967;28:797–802. doi: 10.1016/0006-291x(67)90388-9. [DOI] [PubMed] [Google Scholar]
- 15.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–42. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 16.Furman B, Ong WK, Pyne NJ. Cyclic AMP signaling in pancreatic islets. Adv Exp Med Biol. 2010;654:281–304. doi: 10.1007/978-90-481-3271-3_13. [DOI] [PubMed] [Google Scholar]
- 17.Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12:141–51. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holz GG, 4th, Kuhtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7–37) Nature. 1993;361:362–5. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuit FC. Factors determining the glucose sensitivity and glucose responsiveness of pancreatic beta cells. Horm Res. 1996;46:99–106. doi: 10.1159/000185004. [DOI] [PubMed] [Google Scholar]
- 20.Fujimoto W, Miki T, Ogura T, Zhang M, Seino Y, Satin LS, et al. Niflumic acid-sensitive ion channels play an important role in the induction of glucose-stimulated insulin secretion by cyclic AMP in mice. Diabetologia. 2009;52:863–72. doi: 10.1007/s00125-009-1306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol. 2010;50:355–75. doi: 10.1146/annurev.pharmtox.010909.105714. [DOI] [PubMed] [Google Scholar]
- 22.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 23.Leech CA, Castonguay MA, Habener JF. Expression of adenylyl cyclase subtypes in pancreatic β-cells. Biochem Biophys Res Commun. 1999;254:703–6. doi: 10.1006/bbrc.1998.9906. [DOI] [PubMed] [Google Scholar]
- 24.Guenifi A, Portela-Gomes GM, Grimelius L, Efendic S, Abdel-Halim SM. Adenylyl cyclase isoform expression in non-diabetic and diabetic Goto-Kakizaki (GK) rat pancreas. Evidence for distinct overexpression of type-8 adenylyl cyclase in diabetic GK rat islets. Histochem Cell Biol. 2000;113:81–9. doi: 10.1007/s004180050010. [DOI] [PubMed] [Google Scholar]
- 25.Delmeire D, Flamez D, Hinke SA, Cali JJ, Pipeleers D, Schuit F. Type VIII adenylyl cyclase in rat beta cells: coincidence signal detector/generator for glucose and GLP-1. Diabetologia. 2003;46:1383–93. doi: 10.1007/s00125-003-1203-8. [DOI] [PubMed] [Google Scholar]
- 26.Charles MA, Lawecki J, Pictet R, Grodsky GM. Insulin secretion. Interrelationships of glucose, cyclic adenosine 3:5-monophosphate, and calcium. J Biol Chem. 1975;250:6134–40. [PubMed] [Google Scholar]
- 27.Valverde I, Vandermeers A, Anjaneyulu R, Malaisse WJ. Calmodulin activation of adenylate cyclase in pancreatic islets. Science. 1979;206:225–7. doi: 10.1126/science.225798. [DOI] [PubMed] [Google Scholar]
- 28.Sharp GW, Wiedenkeller DE, Kaelin D, Siegel EG, Wollheim CB. Stimulation of adenylate cyclase by Ca2+ and calmodulin in rat islets of Langerhans: explanation for the glucose-induced increase in cyclic AMP levels. Diabetes. 1980;29:74–7. doi: 10.2337/diab.29.1.74. [DOI] [PubMed] [Google Scholar]
- 29.Roger B, Papin J, Vacher P, Raoux M, Mulot A, Dubois M, et al. Adenylyl cyclase 8 is central to glucagon-like peptide 1 signalling and effects of chronically elevated glucose in rat and human pancreatic beta cells. Diabetologia. 2011;54:390–402. doi: 10.1007/s00125-010-1955-x. [DOI] [PubMed] [Google Scholar]
- 30.Willoughby D, Everett KL, Halls ML, Pacheco J, Skroblin P, Vaca L, et al. Direct binding between Orai1 and AC8 mediates dynamic interplay between Ca2+ and cAMP signaling. Sci Signal. 2012;5:ra29. doi: 10.1126/scisignal.2002299. [DOI] [PubMed] [Google Scholar]
- 31.Martin AC, Willoughby D, Ciruela A, Ayling LJ, Pagano M, Wachten S, et al. Capacitative Ca2+ entry via Orai1 and stromal interacting molecule 1 (STIM1) regulates adenylyl cyclase type 8. Mol Pharmacol. 2009;75:830–42. doi: 10.1124/mol.108.051748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willoughby D, Masada N, Wachten S, Pagano M, Halls ML, Everett KL, et al. AKAP79/150 interacts with AC8 and regulates Ca2+-dependent cAMP synthesis in pancreatic and neuronal systems. J Biol Chem. 2010;285:20328–42. doi: 10.1074/jbc.M110.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu YJ, Gylfe E. Store-operated Ca2+ entry in insulin-releasing pancreatic β-cells. Cell Calcium. 1997;22:277–86. doi: 10.1016/s0143-4160(97)90066-x. [DOI] [PubMed] [Google Scholar]
- 34.Tian Y, Laychock SG. Protein kinase C and calcium regulation of adenylyl cyclase in isolated rat pancreatic islets. Diabetes. 2001;50:2505–13. doi: 10.2337/diabetes.50.11.2505. [DOI] [PubMed] [Google Scholar]
- 35.Thams P, Capito K, Hedeskov CJ. Stimulation by glucose of cyclic AMP accumulation in mouse pancreatic islets is mediated by protein kinase C. Biochem J. 1988;253:229–34. doi: 10.1042/bj2530229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bozem M, Nenquin M, Henquin JC. The ionic, electrical, and secretory effects of protein kinase C activation in mouse pancreatic B-cells: studies with a phorbol ester. Endocrinology. 1987;121:1025–33. doi: 10.1210/endo-121-3-1025. [DOI] [PubMed] [Google Scholar]
- 37.Dyachok O, Idevall-Hagren O, Sågetorp J, Tian G, Wuttke A, Arrieumerlou C, et al. Glucose-induced cyclic AMP oscillations regulate pulsatile insulin secretion. Cell Metab. 2008;8:26–37. doi: 10.1016/j.cmet.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Ramos LS, Zippin JH, Kamenetsky M, Buck J, Levin LR. Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells. J Gen Physiol. 2008;132:329–38. doi: 10.1085/jgp.200810044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–8. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 40.Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem. 2003;278:15922–6. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- 41.Jaiswal BS, Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc Natl Acad Sci USA. 2003;100:10676–81. doi: 10.1073/pnas.1831008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian G, Sandler S, Gylfe E, Tengholm A. Glucose- and hormone-induced cAMP oscillations in α- and β-cells within intact pancreatic islets. Diabetes. 2011;60:1535–43. doi: 10.2337/db10-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 44.Pyne NJ, Furman BL. Cyclic nucleotide phosphodiesterases in pancreatic islets. Diabetologia. 2003;46:1179–89. doi: 10.1007/s00125-003-1176-7. [DOI] [PubMed] [Google Scholar]
- 45.Degerman E, Ahmad F, Chung YW, Guirguis E, Omar B, Stenson L, et al. From PDE3B to the regulation of energy homeostasis. Curr Opin Pharmacol. 2011;11:676–82. doi: 10.1016/j.coph.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugden MC, Ashcroft SJ. Cyclic nucleotide phosphodiesterase of rat pancreatic islets. Effects of Ca2+, calmodulin and trifluoperazine. Biochem J. 1981;197:459–64. doi: 10.1042/bj1970459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lipson LG, Oldham SB. The role of calmodulin in insulin secretion: the presence of a calmodulin-stimulatable phosphodiesterase in pancreatic islets of normal and pregnant rats. Life Sci. 1983;32:775–80. doi: 10.1016/0024-3205(83)90312-0. [DOI] [PubMed] [Google Scholar]
- 48.Capito K, Hedeskov CJ, Thams P. Cyclic AMP phosphodiesterase activity in mouse pancreatic islets. Effects of calmodulin and phospholipids. Acta Endocrinol (Copenh) 1986;111:533–8. doi: 10.1530/acta.0.1110533. [DOI] [PubMed] [Google Scholar]
- 49.Han P, Werber J, Surana M, Fleischer N, Michaeli T. The calcium/calmodulin-dependent phosphodiesterase PDE1C down-regulates glucose-induced insulin secretion. J Biol Chem. 1999;274:22337–44. doi: 10.1074/jbc.274.32.22337. [DOI] [PubMed] [Google Scholar]
- 50.Dov A, Abramovitch E, Warwar N, Nesher R. Diminished phosphodiesterase-8B potentiates biphasic insulin response to glucose. Endocrinology. 2008;149:741–8. doi: 10.1210/en.2007-0968. [DOI] [PubMed] [Google Scholar]
- 51.Waddleton D, Wu W, Feng Y, Thompson C, Wu M, Zhou YP, et al. Phosphodiesterase 3 and 4 comprise the major cAMP metabolizing enzymes responsible for insulin secretion in INS-1 (832/13) cells and rat islets. Biochem Pharmacol. 2008;76:884–93. doi: 10.1016/j.bcp.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 52.Parker JC, VanVolkenburg MA, Ketchum RJ, Brayman KL, Andrews KM. Cyclic AMP phosphodiesterases of human and rat islets of Langerhans: contributions of types III and IV to the modulation of insulin secretion. Biochem Biophys Res Commun. 1995;217:916–23. doi: 10.1006/bbrc.1995.2858. [DOI] [PubMed] [Google Scholar]
- 53.Heimann E, Jones HA, Resjö S, Manganiello VC, Stenson L, Degerman E. Expression and regulation of cyclic nucleotide phosphodiesterases in human and rat pancreatic islets. PLoS One. 2010;5:e14191. doi: 10.1371/journal.pone.0014191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Härndahl L, Jing XJ, Ivarsson R, Degerman E, Ahren B, Manganiello VC, et al. Important role of phosphodiesterase 3B for the stimulatory action of cAMP on pancreatic β-cell exocytosis and release of insulin. J Biol Chem. 2002;277:37446–55. doi: 10.1074/jbc.M205401200. [DOI] [PubMed] [Google Scholar]
- 55.Härndahl L, Wierup N, Enerbäck S, Mulder H, Manganiello VC, Sundler F, et al. β-cell-targeted overexpression of phosphodiesterase 3B in mice causes impaired insulin secretion, glucose intolerance, and deranged islet morphology. J Biol Chem. 2004;279:15214–22. doi: 10.1074/jbc.M308952200. [DOI] [PubMed] [Google Scholar]
- 56.Zhao AZ, Zhao H, Teague J, Fujimoto W, Beavo JA. Attenuation of insulin secretion by insulin-like growth factor 1 is mediated through activation of phosphodiesterase 3B. Proc Natl Acad Sci USA. 1997;94:3223–8. doi: 10.1073/pnas.94.7.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi YH, Park S, Hockman S, Zmuda-Trzebiatowska E, Svennelid F, Haluzik M, et al. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice. J Clin Invest. 2006;116:3240–51. doi: 10.1172/JCI24867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walz HA, Wierup N, Vikman J, Manganiello VC, Degerman E, Eliasson L, et al. β-cell PDE3B regulates Ca2+-stimulated exocytosis of insulin. Cell Signal. 2007;19:1505–13. doi: 10.1016/j.cellsig.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 59.Shafiee-Nick R, Pyne NJ, Furman BL. Effects of type-selective phosphodiesterase inhibitors on glucose-induced insulin secretion and islet phosphodiesterase activity. Br J Pharmacol. 1995;115:1486–92. doi: 10.1111/j.1476-5381.1995.tb16641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cantin LD, Magnuson S, Gunn D, Barucci N, Breuhaus M, Bullock WH, et al. PDE-10A inhibitors as insulin secretagogues. Bioorg Med Chem Lett. 2007;17:2869–73. doi: 10.1016/j.bmcl.2007.02.061. [DOI] [PubMed] [Google Scholar]
- 61.Tian G, Sågetorp J, Xu Y, Shuai H, Degerman E, Tengholm A. Role of phosphodiesterases in the shaping of sub-plasma membrane cAMP oscillations and pulsatile insulin secretion. J Cell Sci. 2012 doi: 10.1242/jcs.107201. in press, doi:10.1242/jcs.107201. [DOI] [PubMed] [Google Scholar]
- 62.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Filipsson K, Kvist-Reimer M, Ahren B. The neuropeptide pituitary adenylate cyclase-activating polypeptide and islet function. Diabetes. 2001;50:1959–69. doi: 10.2337/diabetes.50.9.1959. [DOI] [PubMed] [Google Scholar]
- 64.Al-Majed HT, Jones PM, Persaud SJ, Sugden D, Huang GC, Amiel S, et al. ACTH stimulates insulin secretion from MIN6 cells and primary mouse and human islets of Langerhans. J Endocrinol. 2004;180:155–66. doi: 10.1677/joe.0.1800155. [DOI] [PubMed] [Google Scholar]
- 65.Sharp GW. Mechanisms of inhibition of insulin release. Am J Physiol. 1996;271:C1781–99. doi: 10.1152/ajpcell.1996.271.6.C1781. [DOI] [PubMed] [Google Scholar]
- 66.Dezaki K, Damdindorj B, Sone H, Dyachok O, Tengholm A, Gylfe E, et al. Ghrelin attenuates cAMP-PKA signaling to evoke insulinostatic cascade in islet β-cells. Diabetes. 2011;60:2315–24. doi: 10.2337/db11-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Renström E, Ding WG, Bokvist K, Rorsman P. Neurotransmitter-induced inhibition of exocytosis in insulin-secreting β cells by activation of calcineurin. Neuron. 1996;17:513–22. doi: 10.1016/s0896-6273(00)80183-x. [DOI] [PubMed] [Google Scholar]
- 68.Peschke E, Mühlbauer E. New evidence for a role of melatonin in glucose regulation. Best Pract Res Clin Endocrinol Metab. 2010;24:829–41. doi: 10.1016/j.beem.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 69.Dyachok O, Isakov Y, Sågetorp J, Tengholm A. Oscillations of cyclic AMP in hormone-stimulated insulin-secreting β-cells. Nature. 2006;439:349–52. doi: 10.1038/nature04410. [DOI] [PubMed] [Google Scholar]
- 70.Takeda Y, Amano A, Noma A, Nakamura Y, Fujimoto S, Inagaki N. Systems analysis of GLP-1 receptor signaling in pancreatic β-cells. Am J Physiol Cell Physiol. 2011;301:C792–803. doi: 10.1152/ajpcell.00057.2011. [DOI] [PubMed] [Google Scholar]
- 71.Fridlyand LE, Harbeck MC, Roe MW, Philipson LH. Regulation of cAMP dynamics by Ca2+ and G protein-coupled receptors in the pancreatic β-cell: a computational approach. Am J Physiol Cell Physiol. 2007;293:C1924–33. doi: 10.1152/ajpcell.00555.2006. [DOI] [PubMed] [Google Scholar]
- 72.Ravier MA, Guldenagel M, Charollais A, Gjinovci A, Caille D, Sohl G, et al. Loss of connexin36 channels alters b-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes. 2005;54:1798–807. doi: 10.2337/diabetes.54.6.1798. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Q, Galvanovskis J, Abdulkader F, Partridge CJ, Göpel SO, Eliasson L, et al. Cell coupling in mouse pancreatic β-cells measured in intact islets of Langerhans. Philos Transact A Math Phys Eng Sci. 2008;366:3503–23. doi: 10.1098/rsta.2008.0110. [DOI] [PubMed] [Google Scholar]
- 74.Grill V, Cerasi E. Activation by glucose of adenyl cyclase in pancreatic islets of the rat. FEBS Lett. 1973;33:311–14. doi: 10.1016/0014-5793(73)80218-2. [DOI] [PubMed] [Google Scholar]
- 75.Sharp GW. The adenylate cyclase-cyclic AMP system in islets of Langerhans and its role in the control of insulin release. Diabetologia. 1979;16:287–96. doi: 10.1007/BF01223617. [DOI] [PubMed] [Google Scholar]
- 76.Hellman B, Idahl LÅ, Lernmark Å, Täljedal IB. The pancreatic b-cell recognition of insulin secretagogues: does cyclic AMP mediate the effect of glucose? Proc Natl Acad Sci USA. 1974;71:3405–9. doi: 10.1073/pnas.71.9.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pipeleers DG, Schuit FC, in't Veld PA, Maes E, Hooghe-Peters EL, Van de Winkel M, et al. Interplay of nutrients and hormones in the regulation of insulin release. Endocrinology. 1985;117:824–33. doi: 10.1210/endo-117-3-824. [DOI] [PubMed] [Google Scholar]
- 78.Schuit FC, Pipeleers DG. Regulation of adenosine 3',5'-monophosphate levels in the pancreatic B cell. Endocrinology. 1985;117:834–40. doi: 10.1210/endo-117-3-834. [DOI] [PubMed] [Google Scholar]
- 79.Landa LR, Jr, Harbeck M, Kaihara K, Chepurny O, Kitiphongspattana K, Graf O, et al. Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 β-cell line. J Biol Chem. 2005;280:31294–302. doi: 10.1074/jbc.M505657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ni Q, Ganesan A, Aye-Han NN, Gao X, Allen MD, Levchenko A, et al. Signaling diversity of PKA achieved via a Ca2+-cAMP-PKA oscillatory circuit. Nat Chem Biol. 2011;7:34–40. doi: 10.1038/nchembio.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim JW, Roberts CD, Berg SA, Caicedo A, Roper SD, Chaudhari N. Imaging cyclic AMP changes in pancreatic islets of transgenic reporter mice. PLoS One. 2008;3:e2127. doi: 10.1371/journal.pone.0002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davis B, Lazarus NR. Insulin release from mouse islets. Effect of glucose and hormones on adenylate cyclase. Biochem J. 1972;129:373–9. doi: 10.1042/bj1290373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takahashi N, Kadowaki T, Yazaki Y, Ellis-Davies GC, Miyashita Y, Kasai H. Post-priming actions of ATP on Ca2+-dependent exocytosis in pancreatic beta cells. Proc Natl Acad Sci USA. 1999;96:760–5. doi: 10.1073/pnas.96.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gembal M, Detimary P, Gilon P, Gao ZY, Henquin JC. Mechanisms by which glucose can control insulin release independently from its action on adenosine triphosphate-sensitive K+ channels in mouse B cells. J Clin Invest. 1993;91:871–80. doi: 10.1172/JCI116308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yajima H, Komatsu M, Schermerhorn T, Aizawa T, Kaneko T, Nagai M, et al. cAMP enhances insulin secretion by an action on the ATP-sensitive K+ channel-independent pathway of glucose signaling in rat pancreatic islets. Diabetes. 1999;48:1006–12. doi: 10.2337/diabetes.48.5.1006. [DOI] [PubMed] [Google Scholar]
- 86.Sato Y, Henquin JC. The K+-ATP channel-independent pathway of regulation of insulin secretion by glucose: in search of the underlying mechanism. Diabetes. 1998;47:1713–21. doi: 10.2337/diabetes.47.11.1713. [DOI] [PubMed] [Google Scholar]
- 87.Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, et al. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA. 2007;104:19333–8. doi: 10.1073/pnas.0707054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jones PM, Persaud SJ. Protein kinases, protein phosphorylation, and the regulation of insulin secretion from pancreatic β-cells. Endocr Rev. 1998;19:429–61. doi: 10.1210/edrv.19.4.0339. [DOI] [PubMed] [Google Scholar]
- 89.Lester LB, Langeberg LK, Scott JD. Anchoring of protein kinase A facilitates hormone-mediated insulin secretion. Proc Natl Acad Sci USA. 1997;94:14942–7. doi: 10.1073/pnas.94.26.14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lester LB, Faux MC, Nauert JB, Scott JD. Targeted protein kinase A and PP-2B regulate insulin secretion through reversible phosphorylation. Endocrinology. 2001;142:1218–27. doi: 10.1210/endo.142.3.8023. [DOI] [PubMed] [Google Scholar]
- 91.Fraser ID, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, Dean RA, et al. A novel lipid-anchored A-kinase anchoring protein facilitates cAMP-responsive membrane events. EMBO J. 1998;17:2261–72. doi: 10.1093/emboj/17.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Josefsen K, Lee YC, Thams P, Efendic S, Nielsen JH. AKAP 18 α and γ have opposing effects on insulin release in INS-1E cells. FEBS Lett. 2010;584:81–5. doi: 10.1016/j.febslet.2009.10.086. [DOI] [PubMed] [Google Scholar]
- 93.Faruque OM, Le-Nguyen D, Lajoix AD, Vives E, Petit P, Bataille D, et al. Cell-permeable peptide-based disruption of endogenous PKA-AKAP complexes: a tool for studying the molecular roles of AKAP-mediated PKA subcellular anchoring. Am J Physiol Cell Physiol. 2009;296:C306–16. doi: 10.1152/ajpcell.00216.2008. [DOI] [PubMed] [Google Scholar]
- 94.Dyachok O, Sågetorp J, Isakov Y, Tengholm A. cAMP oscillations restrict protein kinase A redistribution in insulin-secreting cells. Biochem Soc Trans. 2006;34:498–501. doi: 10.1042/BST0340498. [DOI] [PubMed] [Google Scholar]
- 95.Gylfe E, Hellman B. Calcium and pancreatic β-cell function: modification of 45Ca fluxes by methylxanthines and dibutyryl cyclic-AMP. Biochem Med. 1981;26:365–76. doi: 10.1016/0006-2944(81)90012-0. [DOI] [PubMed] [Google Scholar]
- 96.Henquin JC, Meissner HP. The ionic, electrical, and secretory effects of endogenous cyclic adenosine monophosphate in mouse pancreatic B cells: studies with forskolin. Endocrinology. 1984;115:1125–34. doi: 10.1210/endo-115-3-1125. [DOI] [PubMed] [Google Scholar]
- 97.Eddlestone GT, Oldham SB, Lipson LG, Premdas FH, Beigelman PM. Electrical activity, cAMP concentration, and insulin release in mouse islets of Langerhans. Am J Physiol. 1985;248:C145–53. doi: 10.1152/ajpcell.1985.248.1.C145. [DOI] [PubMed] [Google Scholar]
- 98.Prentki M, Glennon MC, Geschwind JF, Matschinsky FM, Corkey BE. Cyclic AMP raises cytosolic Ca2+ and promotes Ca2+ influx in a clonal pancreatic β-cell line (HIT T-15) FEBS Lett. 1987;220:103–7. doi: 10.1016/0014-5793(87)80884-0. [DOI] [PubMed] [Google Scholar]
- 99.Prentki M, Matschinsky FM. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987;67:1185–248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- 100.Grapengiesser E, Gylfe E, Hellman B. Three types of cytoplasmic Ca2+ oscillations in stimulated pancreatic β-cells. Arch Biochem Biophys. 1989;268:404–7. doi: 10.1016/0003-9861(89)90602-4. [DOI] [PubMed] [Google Scholar]
- 101.Liu YJ, Grapengiesser E, Gylfe E, Hellman B. Crosstalk between the cAMP and inositol trisphosphate-signalling pathways in pancreatic β-cells. Arch Biochem Biophys. 1996;334:295–302. doi: 10.1006/abbi.1996.0458. [DOI] [PubMed] [Google Scholar]
- 102.Ämmälä C, Ashcroft FM, Rorsman P. Calcium-independent potentiation of insulin release by cyclic AMP in single β-cells. Nature. 1993;363:356–8. doi: 10.1038/363356a0. [DOI] [PubMed] [Google Scholar]
- 103.Kanno T, Suga S, Wu J, Kimura M, Wakui M. Intracellular cAMP potentiates voltage-dependent activation of L-type Ca2+ channels in rat islet β-cells. Pflügers Arch. 1998;435:578–80. doi: 10.1007/s004240050556. [DOI] [PubMed] [Google Scholar]
- 104.Gromada J, Ma X, Hoy M, Bokvist K, Salehi A, Berggren PO, et al. ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1-/- mouse α-cells. Diabetes. 2004;53:S181–9. doi: 10.2337/diabetes.53.suppl_3.s181. [DOI] [PubMed] [Google Scholar]
- 105.Dyachok O, Gylfe E. Ca2+-induced Ca2+ release via inositol 1,4,5-trisphosphate receptors is amplified by protein kinase A and triggers exocytosis in pancreatic β-cells. J Biol Chem. 2004;279:45455–61. doi: 10.1074/jbc.M407673200. [DOI] [PubMed] [Google Scholar]
- 106.Kim BJ, Park KH, Yim CY, Takasawa S, Okamoto H, Im MJ, et al. Generation of nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose by glucagon-like peptide-1 evokes Ca2+ signal that is essential for insulin secretion in mouse pancreatic islets. Diabetes. 2008;57:868–78. doi: 10.2337/db07-0443. [DOI] [PubMed] [Google Scholar]
- 107.Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, Li WH, et al. A cAMP and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release in mouse pancreatic β cells. J Physiol. 2005;566:173–88. doi: 10.1113/jphysiol.2005.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jones PM, Fyles JM, Howell SL. Regulation of insulin secretion by cAMP in rat islets of Langerhans permeabilised by high-voltage discharge. FEBS Lett. 1986;205:205–9. doi: 10.1016/0014-5793(86)80898-5. [DOI] [PubMed] [Google Scholar]
- 109.Gillis KD, Misler S. Enhancers of cytosolic cAMP augment depolarization-induced exocytosis from pancreatic B-cells: evidence for effects distal to Ca2+ entry. Pflugers Arch. 1993;424:195–7. doi: 10.1007/BF00374612. [DOI] [PubMed] [Google Scholar]
- 110.Tamagawa T, Niki H, Niki A. Insulin release independent of a rise in cytosolic free Ca2+ by forskolin and phorbol ester. FEBS Lett. 1985;183:430–2. doi: 10.1016/0014-5793(85)80825-5. [DOI] [PubMed] [Google Scholar]
- 111.Skelin M, Rupnik M. cAMP increases the sensitivity of exocytosis to Ca2+ primarily through protein kinase A in mouse pancreatic beta cells. Cell Calcium. 2011;49:89–99. doi: 10.1016/j.ceca.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 112.Hisatomi M, Hidaka H, Niki I. Ca2+/calmodulin and cyclic 3,5' adenosine monophosphate control movement of secretory granules through protein phosphorylation/dephosphorylation in the pancreatic β-cell. Endocrinology. 1996;137:4644–9. doi: 10.1210/endo.137.11.8895328. [DOI] [PubMed] [Google Scholar]
- 113.Renström E, Eliasson L, Rorsman P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J Physiol. 1997;502:105–18. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eliasson L, Ma X, Renström E, Barg S, Berggren PO, Galvanovskis J, et al. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J Gen Physiol. 2003;121:181–97. doi: 10.1085/jgp.20028707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wan QF, Dong Y, Yang H, Lou X, Ding J, Xu T. Protein kinase activation increases insulin secretion by sensitizing the secretory machinery to Ca2+ . J Gen Physiol. 2004;124:653–62. doi: 10.1085/jgp.200409082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang Y, Gillis KD. A highly Ca2+-sensitive pool of granules is regulated by glucose and protein kinases in insulin-secreting INS-1 cells. J Gen Physiol. 2004;124:641–51. doi: 10.1085/jgp.200409081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Persaud SJ, Jones PM, Howell SL. Glucose-stimulated insulin secretion is not dependent on activation of protein kinase A. Biochem Biophys Res Commun. 1990;173:833–9. doi: 10.1016/s0006-291x(05)80862-9. [DOI] [PubMed] [Google Scholar]
- 118.Harris TE, Persaud SJ, Jones PM. Pseudosubstrate inhibition of cyclic AMP-dependent protein kinase in intact pancreatic islets: effects on cyclic AMP-dependent and glucose-dependent insulin secretion. Biochem Biophys Res Commun. 1997;232:648–51. doi: 10.1006/bbrc.1997.6344. [DOI] [PubMed] [Google Scholar]
- 119.Hatakeyama H, Kishimoto T, Nemoto T, Kasai H, Takahashi N. Rapid glucose sensing by protein kinase A for insulin exocytosis in mouse pancreatic islets. J Physiol. 2006;570:271–82. doi: 10.1113/jphysiol.2005.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Idevall-Hagren O, Barg S, Gylfe E, Tengholm A. cAMP mediators of pulsatile insulin secretion from glucose-stimulated single β-cells. J Biol Chem. 2010;285:23007–18. doi: 10.1074/jbc.M109.095992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kanno T, Suga S, Wu J, Kimura M, Wakui M. Intracellular cAMP potentiates voltage-dependent activation of L-type Ca2+ channels in rat islet β-cells. Pflügers Arch. 1998;435:578–80. doi: 10.1007/s004240050556. [DOI] [PubMed] [Google Scholar]
- 122.Beguin P, Nagashima K, Nishimura M, Gonoi T, Seino S. PKA-mediated phosphorylation of the human KATP channel: separate roles of Kir6.2 and SUR1 subunit phosphorylation. EMBO J. 1999;18:4722–32. doi: 10.1093/emboj/18.17.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lin YF, Jan YN, Jan LY. Regulation of ATP-sensitive potassium channel function by protein kinase A-mediated phosphorylation in transfected HEK293 cells. EMBO J. 2000;19:942–55. doi: 10.1093/emboj/19.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Light PE, Manning Fox JE, Riedel MJ, Wheeler MB. Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A- and ADP-dependent mechanism. Mol Endocrinol. 2002;16:2135–44. doi: 10.1210/me.2002-0084. [DOI] [PubMed] [Google Scholar]
- 125.Gromada J, Brock B, Schmitz O, Rorsman P. Glucagon-like peptide-1: regulation of insulin secretion and therapeutic potential. Basic Clin Pharmacol Toxicol. 2004;95:252–62. doi: 10.1111/j.1742-7843.2004.t01-1-pto950502.x. [DOI] [PubMed] [Google Scholar]
- 126.Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, et al. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol. 2000;2:805–11. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- 127.Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol. 2006;577:5–15. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Leech CA, Chepurny OG, Holz GG. Epac2-dependent rap1 activation and the control of islet insulin secretion by glucagon-like peptide-1. Vitam Horm. 2010;84:279–302. doi: 10.1016/B978-0-12-381517-0.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Holz GG. Epac: a new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic β-cell. Diabetes. 2004;53:5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Leech CA, Holz GG, Chepurny O, Habener JF. Expression of cAMP-regulated guanine nucleotide exchange factors in pancreatic β-cells. Biochem Biophys Res Commun. 2000;278:44–7. doi: 10.1006/bbrc.2000.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kelley GG, Chepurny OG, Schwede F, Genieser HG, Leech CA, Roe MW, et al. Glucose-dependent potentiation of mouse islet insulin secretion by Epac activator 8-pCPT-2'-O-Me-cAMP-AM. Islets. 2009;1:260–5. doi: 10.4161/isl.1.3.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chepurny OG, Kelley GG, Dzhura I, Leech CA, Roe MW, Dzhura E, et al. PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2'-O-Me-cAMP-AM in human islets of Langerhans. Am J Physiol Endocrinol Metab. 2010;298:E622–33. doi: 10.1152/ajpendo.00630.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Niimura M, Miki T, Shibasaki T, Fujimoto W, Iwanaga T, Seino S. Critical role of the N-terminal cyclic AMP-binding domain of Epac2 in its subcellular localization and function. J Cell Physiol. 2009;219:652–8. doi: 10.1002/jcp.21709. [DOI] [PubMed] [Google Scholar]
- 134.Chepurny OG, Leech CA, Kelley GG, Dzhura I, Dzhura E, Li X, et al. Enhanced Rap1 activation and insulin secretagogue properties of an acetoxymethyl ester of an Epac-selective cyclic AMP analog in rat INS-1 cells: studies with 8-pCPT-2'-O-Me-cAMP-AM. J Biol Chem. 2009;284:10728–36. doi: 10.1074/jbc.M900166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kwan EP, Gao X, Leung YM, Gaisano HY. Activation of exchange protein directly activated by cyclic adenosine monophosphate and protein kinase A regulate common and distinct steps in promoting plasma membrane exocytic and granule-to-granule fusions in rat islet beta cells. Pancreas. 2007;35:e45–54. doi: 10.1097/mpa.0b013e318073d1c9. [DOI] [PubMed] [Google Scholar]
- 136.Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis—roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci. 2009;122:893–903. doi: 10.1242/jcs.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kowluru A. Small G proteins in islet b-cell function. Endocr Rev. 2010;31:52–78. doi: 10.1210/er.2009-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dzhura I, Chepurny OG, Kelley GG, Leech CA, Roe MW, Dzhura E, et al. Epac2-dependent mobilization of intracellular Ca2+ by GLP-1 receptor agonist Exendin-4 is disrupted in β-cells of PLC-ϵ knockout mice. J Physiol. 2010;588:4871–89. doi: 10.1113/jphysiol.2010.198424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dzhura I, Chepurny OG, Leech CA, Roe MW, Dzhura E, Xu X, et al. Phospholipase C-ϵ links Epac2 activation to the potentiation of glucose-stimulated insulin secretion from mouse islets of Langerhans. Islets. 2011;3:121–8. doi: 10.4161/isl.3.3.15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kang G, Chepurny OG, Holz GG. cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic β-cells. J Physiol. 2001;536:375–85. doi: 10.1111/j.1469-7793.2001.0375c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, et al. Epac-selective cAMP analog 8-pCPT-2'-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic β-cells. J Biol Chem. 2003;278:8279–85. doi: 10.1074/jbc.M211682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kang G, Leech CA, Chepurny OG, Coetzee WA, Holz GG. Role of the cAMP sensor Epac as a determinant of KATP channel ATP sensitivity in human pancreatic β-cells and rat INS-1 cells. J Physiol. 2008;586:1307–19. doi: 10.1113/jphysiol.2007.143818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yasuda T, Shibasaki T, Minami K, Takahashi H, Mizoguchi A, Uriu Y, et al. Rim2α determines docking and priming states in insulin granule exocytosis. Cell Metab. 2010;12:117–29. doi: 10.1016/j.cmet.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 144.Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, Yano H, et al. Critical role of cAMP-GEFII–Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem. 2001;276:46046–53. doi: 10.1074/jbc.M108378200. [DOI] [PubMed] [Google Scholar]
- 145.Fujimoto K, Shibasaki T, Yokoi N, Kashima Y, Matsumoto M, Sasaki T, et al. Piccolo, a Ca2+ sensor in pancreatic beta-cells. Involvement of cAMP-GEFII.Rim2.Piccolo complex in cAMP-dependent exocytosis. J Biol Chem. 2002;277:50497–502. doi: 10.1074/jbc.M210146200. [DOI] [PubMed] [Google Scholar]
- 146.Shibasaki T, Sunaga Y, Fujimoto K, Kashima Y, Seino S. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. J Biol Chem. 2004;279:7956–61. doi: 10.1074/jbc.M309068200. [DOI] [PubMed] [Google Scholar]
- 147.Vikman J, Svensson H, Huang YC, Kang Y, Andersson SA, Gaisano HY, et al. Truncation of SNAP-25 reduces the stimulatory action of cAMP on rapid exocytosis in insulin-secreting cells. Am J Physiol Endocrinol Metab. 2009;297:E452–61. doi: 10.1152/ajpendo.90585.2008. [DOI] [PubMed] [Google Scholar]
- 148.Zhang CL, Katoh M, Shibasaki T, Minami K, Sunaga Y, Takahashi H, et al. The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science. 2009;325:607–10. doi: 10.1126/science.1172256. [DOI] [PubMed] [Google Scholar]
- 149.Hatakeyama H, Takahashi N, Kishimoto T, Nemoto T, Kasai H. Two cAMP-dependent pathways differentially regulate exocytosis of large dense-core and small vesicles in mouse β-cells. J Physiol. 2007;582:1087–98. doi: 10.1113/jphysiol.2007.135228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Herbst KJ, Coltharp C, Amzel LM, Zhang J. Direct activation of Epac by sulfonylurea is isoform selective. Chem Biol. 2011;18:243–51. doi: 10.1016/j.chembiol.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tsalkova T, Gribenko AV, Cheng X. Exchange protein directly activated by cyclic AMP isoform 2 is not a direct target of sulfonylurea drugs. Assay Drug Dev Technol. 2011;9:88–91. doi: 10.1089/adt.2010.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rehmann H. Epac2: a sulfonylurea receptor? Biochem Soc Trans. 2012;40:6–10. doi: 10.1042/BST20110640. [DOI] [PubMed] [Google Scholar]
- 153.Brooker G, Fichman M. Chlorpropamide and tolbutamide inhibition of adenosine 3'5' cyclic monophosphate phosphodiesterase. Biochem Biophys Res Commun. 1971;42:824–8. doi: 10.1016/0006-291x(71)90504-3. [DOI] [PubMed] [Google Scholar]
- 154.Goldfine ID, Perlman R, Roth J. Inhibition of cyclic 3',5'-AMP phosphodiesterase in islet cells and other tissues by tolbutamide. Nature. 1971;234:295–7. doi: 10.1038/234295a0. [DOI] [PubMed] [Google Scholar]
- 155.Hosker JP, Rudenski AS, Burnett MA, Matthews DR, Turner RC. Similar reduction of first- and second-phase B-cell responses at three different glucose levels in type II diabetes and the effect of glicazide therapy. Metabolism. 1989;38:767–72. doi: 10.1016/0026-0495(89)90064-4. [DOI] [PubMed] [Google Scholar]
- 156.Lang DA, Matthews DR, Burnett M, Turner RC. Brief, irregular oscillations of basal plasma insulin and glucose concentrations in diabetic man. Diabetes. 1981;30:435–9. doi: 10.2337/diab.30.5.435. [DOI] [PubMed] [Google Scholar]
- 157.Mari A, Tura A, Natali A, Laville M, Laakso M, Gabriel R, et al. Impaired beta cell glucose sensitivity rather than inadequate compensation for insulin resistance is the dominant defect in glucose intolerance. Diabetologia. 2010;53:749–56. doi: 10.1007/s00125-009-1647-6. [DOI] [PubMed] [Google Scholar]
- 158.Kahn SE, Zraika S, Utzschneider KM, Hull RL. The beta cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia. 2009;52:1003–12. doi: 10.1007/s00125-009-1321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nauck M, Stockmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- 160.Unger RH. Glucagon physiology and pathophysiology in the light of new advances. Diabetologia. 1985;28:574–8. doi: 10.1007/BF00281991. [DOI] [PubMed] [Google Scholar]
- 161.Rosengren AH, Jokubka R, Tojjar D, Granhall C, Hansson O, Li DQ, et al. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science. 2010;327:217–20. doi: 10.1126/science.1176827. [DOI] [PubMed] [Google Scholar]
- 162.Saxena R, Hivert MF, Langenberg C, Tanaka T, Pankow JS, Vollenweider P, et al. Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet. 2010;42:142–8. doi: 10.1038/ng.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–16. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rabinovitch A, Renold AE, Cerasi E. Decreased cyclic AMP and insulin responses to glucose in pancreatic islets of diabetic Chinese hamsters. Diabetologia. 1976;12:581–7. doi: 10.1007/BF01220634. [DOI] [PubMed] [Google Scholar]
- 165.Dachicourt N, Serradas P, Giroix MH, Gangnerau MN, Portha B. Decreased glucose-induced cAMP and insulin release in islets of diabetic rats: reversal by IBMX, glucagon, GIP. Am J Physiol. 1996;271:E725–32. doi: 10.1152/ajpendo.1996.271.4.E725. [DOI] [PubMed] [Google Scholar]
- 166.Dolz M, Movassat J, Bailbe D, Le Stunff H, Giroix MH, Fradet M, et al. cAMP-secretion coupling is impaired in diabetic GK/Par rat beta-cells: a defect counteracted by GLP-1. Am J Physiol Endocrinol Metab. 2011;301:E797–806. doi: 10.1152/ajpendo.00652.2010. [DOI] [PubMed] [Google Scholar]
- 167.Abdel-Halim SM, Guenifi A, Khan A, Larsson O, Berggren PO, Östenson CG, et al. Impaired coupling of glucose signal to the exocytotic machinery in diabetic GK rats: a defect ameliorated by cAMP. Diabetes. 1996;45:934–40. doi: 10.2337/diab.45.7.934. [DOI] [PubMed] [Google Scholar]
- 168.Abdel-Halim SM, Guenifi A, He B, Yang B, Mustafa M, Hojeberg B, et al. Mutations in the promoter of adenylyl cyclase (AC)-III gene, overexpression of AC-III mRNA, and enhanced cAMP generation in islets from the spontaneously diabetic GK rat model of type 2 diabetes. Diabetes. 1998;47:498–504. doi: 10.2337/diabetes.47.3.498. [DOI] [PubMed] [Google Scholar]
- 169.Frayon S, Pessah M, Giroix MH, Mercan D, Boissard C, Malaisse WJ, et al. Gαolf identification by RT-PCR in purified normal pancreatic B cells and in islets from rat models of non-insulin-dependent diabetes. Biochem Biophys Res Commun. 1999;254:269–72. doi: 10.1006/bbrc.1998.9791. [DOI] [PubMed] [Google Scholar]
- 170.Portela-Gomes GM, Abdel-Halim SM. Overexpression of Gs proteins and adenylyl cyclase in normal and diabetic islets. Pancreas. 2002;25:176–81. doi: 10.1097/00006676-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 171.Dubois M, Vacher P, Roger B, Huyghe D, Vandewalle B, Kerr-Conte J, et al. Glucotoxicity inhibits late steps of insulin exocytosis. Endocrinology. 2007;148:1605–14. doi: 10.1210/en.2006-1022. [DOI] [PubMed] [Google Scholar]
- 172.Furman B, Pyne N, Flatt P, O'Harte F. Targeting β-cell cyclic 3'5' adenosine monophosphate for the development of novel drugs for treating type 2 diabetes mellitus. A review. J Pharm Pharmacol. 2004;56:1477–92. doi: 10.1211/0022357044805. [DOI] [PubMed] [Google Scholar]
- 173.Thompson PE, Manganiello V, Degerman E. Re-discovering PDE3 inhibitors—new opportunities for a long neglected target. Curr Top Med Chem. 2007;7:421–36. doi: 10.2174/156802607779941224. [DOI] [PubMed] [Google Scholar]
- 174.Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8:369–85. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- 175.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–9. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- 176.Knop FK, Vilsboll T, Holst JJ. Incretin-based therapy of type 2 diabetes mellitus. Curr Protein Pept Sci. 2009;10:46–55. doi: 10.2174/138920309787315158. [DOI] [PubMed] [Google Scholar]
- 177.Ohishi T, Yoshida S. The therapeutic potential of GPR119 agonists for type 2 diabetes. Expert Opin Investig Drugs. 2012;21:321–8. doi: 10.1517/13543784.2012.657797. [DOI] [PubMed] [Google Scholar]